Abstract
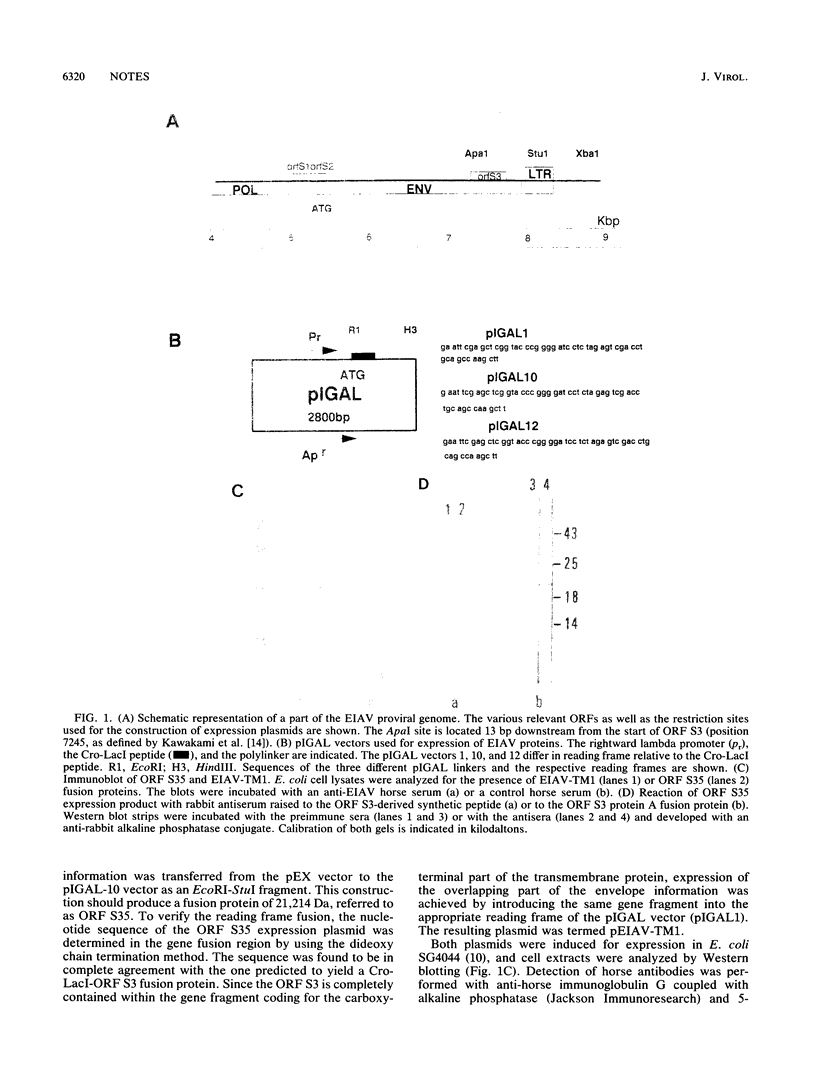
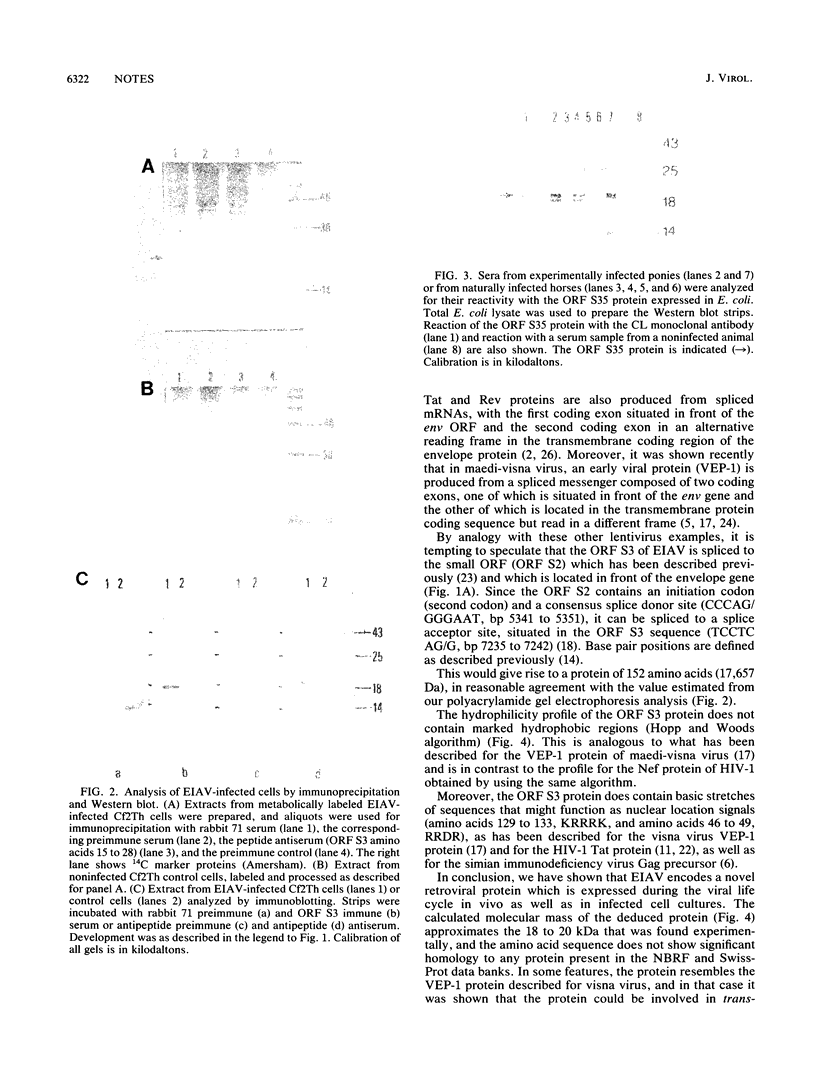
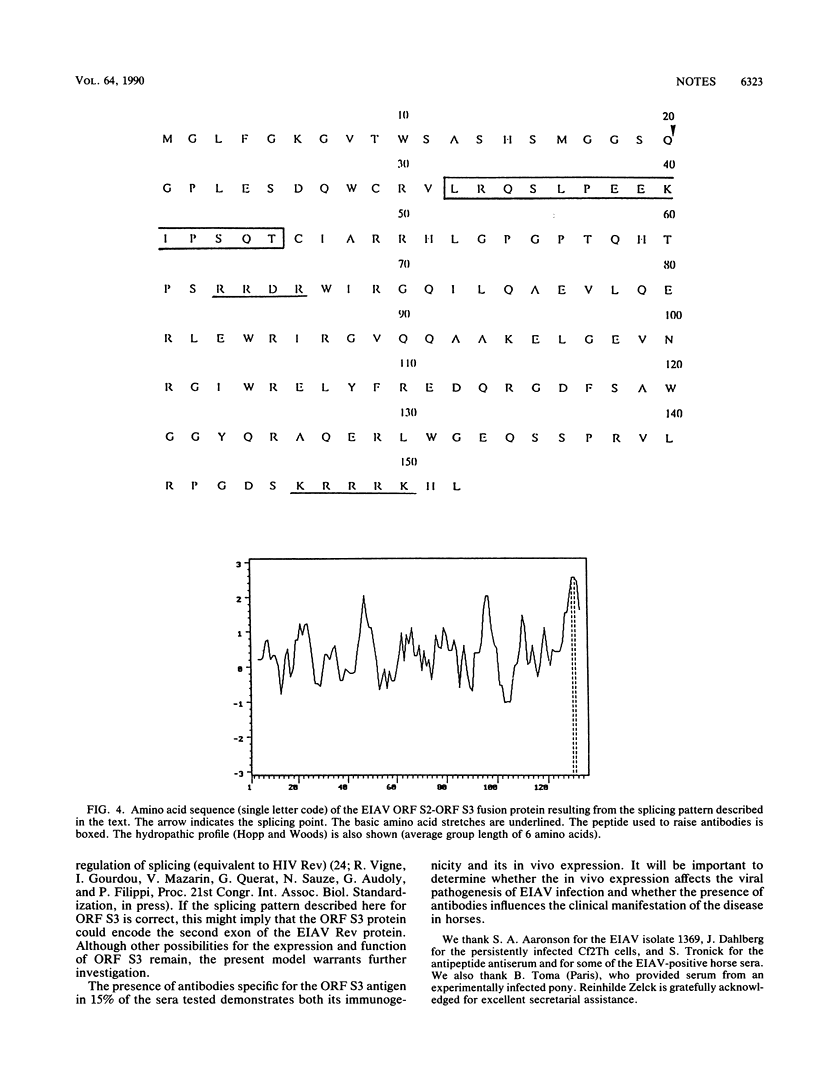
The genome of equine infectious anemia virus (EIAV) contains several small open reading frames (ORFs), the importance of which in the development of the virus is not clear. We investigated the possibility that the largest of these ORFs (ORF S3) is expressed during the course of the viral infection. The ORF S3 information was expressed in Escherichia coli, and the antigen was used to raise monospecific antiserum. A 20-kDa protein expressed in cells producing EIAV was identified as the gene product of ORF S3. Furthermore, sera from EIAV-infected animals specifically recognized this protein, indicating that the ORF S3 antigen is expressed in vivo as well. A model for the expression of this new viral antigen is presented. The proposed splicing pattern is similar to that of the VEP-1 protein of maedi-visna-virus, which tempts us to speculate that ORF S3 defines the second exon of the EIAV Rev protein.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldovini A., Debouck C., Feinberg M. B., Rosenberg M., Arya S. K., Wong-Staal F. Synthesis of the complete trans-activation gene product of human T-lymphotropic virus type III in Escherichia coli: demonstration of immunogenicity in vivo and expression in vitro. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6672–6676. doi: 10.1073/pnas.83.18.6672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arya S. K., Gallo R. C. Three novel genes of human T-lymphotropic virus type III: immune reactivity of their products with sera from acquired immune deficiency syndrome patients. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2209–2213. doi: 10.1073/pnas.83.7.2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu I. M., Yaniv A., Dahlberg J. E., Gazit A., Skuntz S. F., Tronick S. R., Aaronson S. A. Nucleotide sequence evidence for relationship of AIDS retrovirus to lentiviruses. 1985 Sep 26-Oct 2Nature. 317(6035):366–368. doi: 10.1038/317366a0. [DOI] [PubMed] [Google Scholar]
- Dasso M. C., Jackson R. J. Efficient initiation of mammalian mRNA translation at a CUG codon. Nucleic Acids Res. 1989 Aug 25;17(16):6485–6497. doi: 10.1093/nar/17.16.6485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis J. L., Clements J. E. Characterization of a cDNA clone encoding the visna virus transactivating protein. Proc Natl Acad Sci U S A. 1989 Jan;86(2):414–418. doi: 10.1073/pnas.86.2.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delchambre M., Gheysen D., Thines D., Thiriart C., Jacobs E., Verdin E., Horth M., Burny A., Bex F. The GAG precursor of simian immunodeficiency virus assembles into virus-like particles. EMBO J. 1989 Sep;8(9):2653–2660. doi: 10.1002/j.1460-2075.1989.tb08405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorn P. L., Derse D. cis- and trans-acting regulation of gene expression of equine infectious anemia virus. J Virol. 1988 Sep;62(9):3522–3526. doi: 10.1128/jvi.62.9.3522-3526.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn S. D. Effects of the modification of transfer buffer composition and the renaturation of proteins in gels on the recognition of proteins on Western blots by monoclonal antibodies. Anal Biochem. 1986 Aug 15;157(1):144–153. doi: 10.1016/0003-2697(86)90207-1. [DOI] [PubMed] [Google Scholar]
- Goh W. C., Rosen C., Sodroski J., Ho D. D., Haseltine W. A. Identification of a protein encoded by the trans activator gene tatIII of human T-cell lymphotropic retrovirus type III. J Virol. 1986 Jul;59(1):181–184. doi: 10.1128/jvi.59.1.181-184.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman S., Gottesman M., Shaw J. E., Pearson M. L. Protein degradation in E. coli: the lon mutation and bacteriophage lambda N and cII protein stability. Cell. 1981 Apr;24(1):225–233. doi: 10.1016/0092-8674(81)90518-3. [DOI] [PubMed] [Google Scholar]
- Green M., Loewenstein P. M. Autonomous functional domains of chemically synthesized human immunodeficiency virus tat trans-activator protein. Cell. 1988 Dec 23;55(6):1179–1188. doi: 10.1016/0092-8674(88)90262-0. [DOI] [PubMed] [Google Scholar]
- Guyader M., Emerman M., Sonigo P., Clavel F., Montagnier L., Alizon M. Genome organization and transactivation of the human immunodeficiency virus type 2. Nature. 1987 Apr 16;326(6114):662–669. doi: 10.1038/326662a0. [DOI] [PubMed] [Google Scholar]
- Kawakami T., Sherman L., Dahlberg J., Gazit A., Yaniv A., Tronick S. R., Aaronson S. A. Nucleotide sequence analysis of equine infectious anemia virus proviral DNA. Virology. 1987 Jun;158(2):300–312. doi: 10.1016/0042-6822(87)90202-9. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Malmquist W. A., Barnett D., Becvar C. S. Production of equine infectious anemia antigen in a persistently infected cell line. Arch Gesamte Virusforsch. 1973;42(4):361–370. doi: 10.1007/BF01250717. [DOI] [PubMed] [Google Scholar]
- Mazarin V., Gourdou I., Quérat G., Sauze N., Vigne R. Genetic structure and function of an early transcript of visna virus. J Virol. 1988 Dec;62(12):4813–4818. doi: 10.1128/jvi.62.12.4813-4818.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mount S. M. A catalogue of splice junction sequences. Nucleic Acids Res. 1982 Jan 22;10(2):459–472. doi: 10.1093/nar/10.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson B., Abrahmsén L., Uhlén M. Immobilization and purification of enzymes with staphylococcal protein A gene fusion vectors. EMBO J. 1985 Apr;4(4):1075–1080. doi: 10.1002/j.1460-2075.1985.tb03741.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson B., Moks T., Jansson B., Abrahmsén L., Elmblad A., Holmgren E., Henrichson C., Jones T. A., Uhlén M. A synthetic IgG-binding domain based on staphylococcal protein A. Protein Eng. 1987 Feb-Mar;1(2):107–113. doi: 10.1093/protein/1.2.107. [DOI] [PubMed] [Google Scholar]
- Remaut E., Stanssens P., Fiers W. Plasmid vectors for high-efficiency expression controlled by the PL promoter of coliphage lambda. Gene. 1981 Oct;15(1):81–93. doi: 10.1016/0378-1119(81)90106-2. [DOI] [PubMed] [Google Scholar]
- Ruben S., Perkins A., Purcell R., Joung K., Sia R., Burghoff R., Haseltine W. A., Rosen C. A. Structural and functional characterization of human immunodeficiency virus tat protein. J Virol. 1989 Jan;63(1):1–8. doi: 10.1128/jvi.63.1.1-8.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushlow K., Olsen K., Stiegler G., Payne S. L., Montelaro R. C., Issel C. J. Lentivirus genomic organization: the complete nucleotide sequence of the env gene region of equine infectious anemia virus. Virology. 1986 Dec;155(2):309–321. doi: 10.1016/0042-6822(86)90195-9. [DOI] [PubMed] [Google Scholar]
- Sargan D. R., Bennet I. D. A transcriptional map of visna virus: definition of the second intron structure suggests a rev-like gene product. J Gen Virol. 1989 Aug;70(Pt 8):1995–2006. doi: 10.1099/0022-1317-70-8-1995. [DOI] [PubMed] [Google Scholar]
- Sherman L., Gazit A., Yaniv A., Kawakami T., Dahlberg J. E., Tronick S. R. Localization of sequences responsible for trans-activation of the equine infectious anemia virus long terminal repeat. J Virol. 1988 Jan;62(1):120–126. doi: 10.1128/jvi.62.1.120-126.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodroski J., Goh W. C., Rosen C., Dayton A., Terwilliger E., Haseltine W. A second post-transcriptional trans-activator gene required for HTLV-III replication. Nature. 1986 May 22;321(6068):412–417. doi: 10.1038/321412a0. [DOI] [PubMed] [Google Scholar]
- Stanley K. K., Luzio J. P. Construction of a new family of high efficiency bacterial expression vectors: identification of cDNA clones coding for human liver proteins. EMBO J. 1984 Jun;3(6):1429–1434. doi: 10.1002/j.1460-2075.1984.tb01988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens R. M., Casey J. W., Rice N. R. Equine infectious anemia virus gag and pol genes: relatedness to visna and AIDS virus. Science. 1986 Feb 7;231(4738):589–594. doi: 10.1126/science.3003905. [DOI] [PubMed] [Google Scholar]
- Terwilliger E., Sodroski J. G., Rosen C. A., Haseltine W. A. Effects of mutations within the 3' orf open reading frame region of human T-cell lymphotropic virus type III (HTLV-III/LAV) on replication and cytopathogenicity. J Virol. 1986 Nov;60(2):754–760. doi: 10.1128/jvi.60.2.754-760.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]