Abstract
Background
Harmane (1-methyl-9H-pyrido[3,4-b]indole), a neurotoxin, may be an environmental risk factor for essential tremor (ET). Harmane and related chemicals are toxic to the cerebellum. Whether it is through this mechanism (cerebellar toxicity) that harmane leads to ET is unknown. Impaired olfaction may be a feature of cerebellar disease.
Objective
To determine whether blood harmane concentrations correlate with olfactory test scores in patients with ET.
Methods
Blood harmane concentrations were quantified using high performance liquid chromatography. Odor identification testing was performed with the University of Pennsylvania Smell Identification Test (UPSIT).
Results
In 83 ET cases, higher log blood harmane concentration was correlated with lower UPSIT score (rho = −0.46, p < 0.001). 25/40 (62.5%) cases with high log blood harmane concentration (based on a median split) had low UPSIT scores (based on a median split) vs. 12/43 (27.9%) ET cases with low log blood harmane concentration (adjusted OR 4.04, 95% CI 1.42 – 11.50, p = 0.009). When compared with the low log blood harmane tertile, the odds of olfactory dysfunction were 2.64 times higher in cases in the middle tertile and 10.95 times higher in cases in the high tertile. In 69 control subjects, higher log blood harmane concentration was not correlated with lower UPSIT score (rho = 0.12, p = 0.32).
Conclusions
Blood harmane concentrations were correlated with UPSIT scores in ET cases but not controls. These analyses set the stage for postmortem studies to further explore the role of harmane as a cerebellar toxin in ET.
Keywords: essential tremor, beta-carboline alkaloid, harmane, cerebellum, epidemiology, olfaction
Introduction
Essential tremor (ET), characterized by action tremor of the hands, is a widespread late-life neurological disease that is present in 4.0% of individuals aged ≥ 40 years and 8.7% in individuals in their ninth decade (Benito-Leon et al., 2003; Dogu et al., 2003). It is one of the most prevalent neurological disorders (Benito-Leon et al., 2003; Dogu et al., 2003). Aside from action tremor, ET patients exhibit other signs, including cognitive impairment (Benito-Leon et al., 2006), and gait ataxia and incoordination (Singer et al., 1994). Treatment options are limited (Zesiewicz et al., 2005).
The pathophysiology of ET is not well-understood, but clinical (Singer et al., 1994) and imaging (Bucher et al., 1997; Jenkins et al., 1993; Pagan et al., 2003) studies have demonstrated cerebellar abnormalities and pathological studies have recently revealed several features of cerebellar degeneration in many ET cases (Louis et al., 2007a; Louis et al., 2006; Shill H, 2007).
Harmane (1-methyl-9H-pyrido[3,4-b]indole) and other dietary β-carboline alkaloids are potent tremor-producing neurotoxins (McKenna, 1996; Zetler et al., 1972). These alkaloids, including harmane, are present in a variety of foods (especially meats but also in plant-derived foods [e.g., coffee, flour, cocao, tomatoes]) (Herraiz, 2004; Pfau and Skog, 2004). β-carboline alkaloids can produce marked cerebellar damage in exposed laboratory animals (Milner et al., 1995; O'Hearn and Molliver, 1997). Interestingly, higher blood harmane concentrations have been reported in ET patients compared to controls (Louis et al., 2005; Louis et al., 2002b), raising the possibility that harmane is an environmental/dietary risk factor for ET. Moreover, in ET patients, higher blood harmane concentrations have been correlated with evidence of impaired cerebellar metabolism in a magnetic resonance spectroscopic imaging study (Louis et al., 2007b), suggesting that harmane may perhaps be acting as a cerebellar toxin in patients with ET. However, this has not been definitively established.
Olfactory dysfunction occurs in a number of neurological diseases (Doty et al., 1988; Doty et al., 1987). Olfaction is mildly impaired in several studies of ET in which patients exhibit lower University of Pennsylvania Smell Identification Test (UPSIT) scores (Applegate and Louis, 2005; Louis et al., 2002a; Louis and Jurewicz, 2003). Olfactory dysfunction occurs in patients with cerebellar diseases (Connelly et al., 2003; Velazquez-Perez et al., 2006) and there is considerable evidence that the cerebellum itself may play a role in central olfactory processing (Connelly et al., 2003; Deiss and Baudoin, 1997; Sobel et al., 1998; Velazquez-Perez et al., 2006).
To further test the model that harmane, through cerebellar toxicity, leads to ET, we hypothesized that we would find a correlation between blood harmane concentrations and UPSIT scores in ET cases and furthermore, that such a correlation would not be detected among a group of controls. Our overarching goal in this study was to further explore the emerging links between this dietary neurotoxin and ET.
Methods
Participants
Recruitment began in July, 2000 and has continued to present (September, 2007). All ET cases were enrolled in a study of the environmental epidemiology of ET at Columbia-University Medical Center (CUMC). ET cases were identified from two sources: (1) a computerized billing database at CUMC, and (2) advertisements to members of the International Essential Tremor Foundation. All cases had received a diagnosis of ET from their treating neurologist and all lived within two hours driving distance of CUMC in New York, New Jersey, and Connecticut. The office records of all identified patients were reviewed and patients with diagnoses or physical signs of dystonia, Parkinson’s disease (PD), or spinocerebellar ataxia were excluded. Control subjects were recruited during the same time period. Controls were identified using random digit telephone dialing within a defined set of telephone area codes that were represented by the cases (e.g., 212, 201, 203, 516, 718, 914) within the New York Metropolitan area. Controls were frequency-matched to cases based on gender, race (White, African-American, Hispanic, Asian, Other), and current age (5 year intervals). The CUMC Internal Review Board approved of all study procedures and written informed consent was obtained at the time of enrollment.
ET cases and controls were screened for cognitive impairment using the 10-minute Telephone Interview for Cognitive Status (Brandt J, 1988). This was done to minimize the enrollment of individuals with dementia and, therefore, potentially invalid medical histories. Eight individuals (one case and seven controls) with cognitive impairment (score < 30 of 41) were excluded prior to enrollment.
Clinical Evaluation
All case and control subjects were evaluated in person by a trained tester who administered clinical questionnaires and performed a videotaped examination. Most evaluations were performed in the late morning or early afternoon, making fasting levels impractical. Data suggest that plasma concentrations of harmane do not change significantly during the day (Rommelspacher et al., 1991). In one study (Rommelspacher et al., 1991), human subjects ingested food or ethanol, and plasma harmane concentrations were measured hourly for eight hours. The concentration remained stable. The same investigators also demonstrated that variability in concentration was minimal over a longer (three week) period (Rommelspacher et al., 1991). Our own data indicate that log blood harmane concentration is not correlated with the time latency since last food consumption (rho = −0.097, p = 0.49 [52 subjects]).
The tester collected demographic information (age in years, gender, race [non-Hispanic white vs. others], years of education, number of rooms in home) and clinical information (tremor duration in years, years since last hospitalization, tobacco use including current cigarette smoking, names and dosages of all current medications). Medical co-morbidity was assessed with the Cumulative Illness Rating Scale, in which the severity of medical problems (0 [none] - 3 [severe]) was rated in 14 bodily systems and a Cumulative Illness Rating Scale score was assigned (range = 0 – 42) to each subject (Linn et al., 1968).
The tester videotaped a tremor examination in all subjects (Louis et al., 1997; Louis et al., 2002b), and each of 12 videotaped action tremor items was rated by Dr. Louis on a scale from 0 to 3, resulting in a total tremor score (range = 0 – 36 [maximum]). The diagnosis of ET was confirmed by Dr. Louis using published diagnostic criteria (moderate or greater amplitude tremor during three activities or a head tremor in the absence of PD, dystonia or another neurological disorder)(Louis et al., 1998a; Louis et al., 1997; Louis et al., 2002b). None of the final sample of 83 ET cases or 69 control subjects had PD or dystonia.
Blood Harmane Concentrations
During the clinical evaluation, phlebotomy was performed. Blood concentrations of harmane were measured blinded to any clinical information. Harmane concentrations in blood were quantified by a well-established high performance liquid chromatography (HPLC) method in this group in our previous studies (Zheng et al., 2000). Briefly, one volume (9 – 12 ml) of whole blood was digested with NaOH, extracted with ethyl acetate and methyl-t-butyl ether (2:98, V:V), and reconstructed in methanol. Harmane was separated and quantified by HPLC with a fluorescence detector at an excitation wavelength of 300 nm and an emission wavelength of 435 nm. The intraday precision, measured as a coefficient of variation at 25 ng/ml, was 6.7%. The interday precision was 7.3% (Guan et al., 2001; Zheng et al., 2000).
Olfactory Testing
Odor identification testing was performed with the UPSIT (Sensonics Inc., Haddon Heights NJ) in which 40 microencapsulated odors were presented using a four-item forced choice word format (Doty et al., 1988; Doty et al., 1992). Testing was not performed if participants had active upper respiratory tract infections or allergies or were current smokers. ET cases with tremor that was severe enough to compromise their ability to scratch the odor panel or hold it to their nose were assisted by the tester, although this was an extremely rare occurrence. The UPSIT is highly reliable and sensitive to a variety of olfactory deficits. The scores range from 0 to 40 (all odors correctly identified)(Doty et al., 1988; Doty et al., 1992). UPSIT percentiles are based on age and gender norms (Doty et al., 1988; Doty et al., 1992).
Final Sample
509 subjects were enrolled in the study of blood harmane concentration. Of these, 101 (19.8%) did not have their blood drawn (e.g., refused or failed phlebotomy) and 12 (2.4%) had phlebotomy but the quantity of blood was not sufficient. Thirty-five current cigarette smokers were excluded because smoking is associated with a lower UPSIT score (Frye et al., 1990). Of the remaining 361 subjects, 152 (83 cases and 69 controls) were enrolled after the administration of the UPSIT had been added to the study protocol. We compared the base sample (509 subjects) to the final sample (152 subjects used in these analyses), and they were similar in terms of gender (274 [53.8%] vs. 76 [50.0%] women, chi-square [χ2] = 0.69, p = 0.41), years of education (15.2 ± 3.6 years vs. 15.6 ± 3.5 years, t = 1.21, p = 0.23), and race (463 [91.0%] vs. 140 [92.1%] white, χ2 = 0.19, p = 0.66) but on average were 2.7 years older (66.3 ± 14.4 years vs. 63.6 ± 14.2 years, t = 2.03, p = 0.04).
Statistical Analyses
Statistical analyses were performed in SPSS (Version 13.0). We used the published formula (41 - UPSIT), suggested for analysis of UPSIT data (Frye et al., 1990). This score was then log transformed to better normalize the distribution: log (41 – UPSIT). All analyses were performed with UPSIT scores and log transformed scores; we presented the results of the former, which lend themselves to comparisons with other published studies. The empirical distribution of harmane was also skewed; therefore harmane concentrations were logarithmically transformed.
We used Student’s t tests and χ2 tests to compare cases and controls. Pearson’s rho (normally distributed variables) and Spearman’s rho (non-normal distribution) were used to test for correlations. Mann Whitney test was used to test for group differences in UPSIT scores.
Our main analyses were stratified by diagnosis (ET cases, controls). In linear regression analyses, UPSIT score was the dependent variable and log blood harmane concentration was the main independent variable. We considered a number of potential confounders (age in years, gender, race, years of education, Cumulative Illness Rating scale score, number of rooms in home, and years since last hospitalization), and included these in the adjusted regression analyses if they were associated with either log blood harmane concentration or UPSIT score.
In ET cases, log blood harmane concentration and UPSIT scores were stratified into high and low values based on median values. In logistic regression analyses, UPSIT score (low vs. high, based on the median split) was the dependent variable and log blood harmane concentration (low vs. high, based on the median split) was the main independent variable. In other analyses, log blood harmane concentration was also stratified into tertiles. In logistic regression analyses, UPSIT score (low vs. high) was the dependent variable and log blood harmane concentration tertiles were the independent variables; we calculated odds ratios (OR) with 95% Confidence Intervals (CI), comparing the middle and highest tertiles to the lowest (reference) tertile. To test for trend, in a linear regression analysis, UPSIT score was the dependent variable and log blood harmane concentration tertile was the independent variable.
Results
Cases and Controls
The 83 ET cases and 69 controls were similar in terms of demographic characteristics (Table 1). ET cases had a mean ± standard deviation (SD) tremor duration of 20.7 ± 16.4 years, a mean total tremor score of 18.1 ± 6.9, and 30 (36.1%) were taking daily medication to treat their tremor. In the current sample, the transformed UPSIT score was marginally higher and UPSIT percentile was marginally lower (i.e., marginally greater olfactory dysfunction) in ET cases than in controls. Log blood harmane concentration was higher in ET cases than controls (Table 1).
Table 1.
Demographic and clinical characteristics of study subjects
| ET Cases (N = 83) | Controls (N = 69) | |
|---|---|---|
| Age in years | 63.7 ± 13.7 | 63.5 ± 14.9 |
| Female gender | 38 (45.8%) | 38 (55.1%) |
| White Race | 78 (94.0%) | 62 (89.9%) |
| Years of education | 15.7 ± 3.6 | 15.5 ± 3.4 |
| Cumulative Illness Rating Scale Score | 5.0 ± 3.4 | 4.7 ± 3.8 |
| Number of rooms in home | 6.0 ± 2.6 | 6.3 ± 4.3 |
| Years since last hospitalization | 16.5 ± 21.7 | 16.3 ± 18.6 |
| UPSIT score | 30.5 ± 5.3 | 31.7 ± 5.5 |
| Transformed UPSIT score | 0.97 ± 0.22* | 0.90 ± 0.24 |
| UPSIT percentile | 36.9 ± 24.7* | 43.6 ± 24.2 |
| Log blood harmane concentration, g−10/ml | 0.64 ± 0.59*** | 0.32 ± 0.62 |
p < 0.09
p < 0.005 compared with controls.
Using our control sample, we examined the correlates of UPSIT score. Lower UPSIT score (i.e., greater olfactory dysfunction) was associated with older age (rho = −0.45, p < 0.001), fewer years of education (rho = 0.28, p = 0.02), and marginally with higher Cumulative Illness Rating Scale score (rho = −0.19, p = 0.12). It was not associated with years since last hospitalization (rho = 0.17, p = 0.35), number of rooms in home (rho = 0.11, p = 0.37) or race. There was a marginal association with gender (median = 32 in men and 34 in women, Mann Whitney z = 1.42, p = 0.16). In controls, log blood harmane concentration was not associated with age (rho = 0.03, p = 0.79), years of education (rho = 0.10, p = 0.40), Cumulative Illness Rating Scale score (rho = −0.12, p = 0.32), number of rooms in home (rho = 0.06, p = 0.65), years since last hospitalization (rho = −0.10, p = 0.45), gender (0.37 ± 0.51 g−10/ml in men and 0.28 ± 0.70 g−10/ml in women, t = 0.65, p = 0.52), or race.
Analyses in ET Cases
In ET cases, higher log blood harmane concentration was correlated with lower UPSIT score (rho = −0.46, p < 0.001, Figure 1). Even among 28 cases with ET of shorter tremor duration (< 10 years, mean ± SD = 5.4 ± 2.6 years), the correlation was robust (rho = −0.59, p = 0.001). In a linear regression analysis, higher log blood harmane concentration was associated with lower UPSIT score (dependent variable) after adjusting for age in years, gender, years of education, and Cumulative Illness Rating Scale score (beta = −3.41, p = 0.001).
Figure 1.
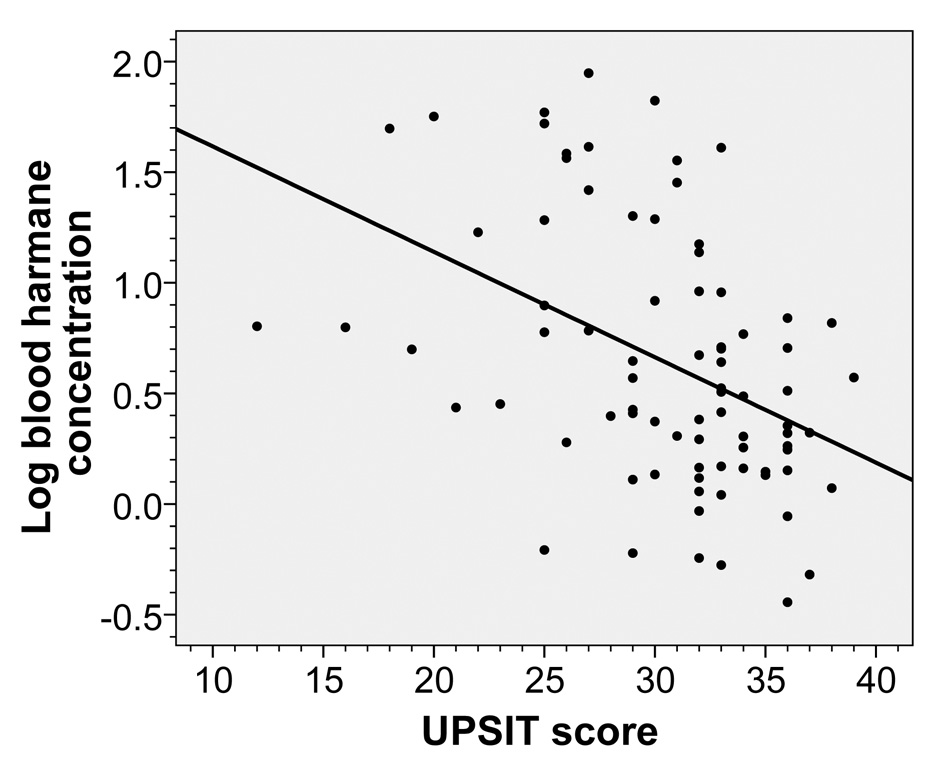
Log blood harmane concentration (g−10/ml) was inversely associated with UPSIT score in ET cases (rho = −0.46, p < 0.001). The fit line is also shown.
Log blood harmane concentration and UPSIT scores were stratified into high versus low values based on the medians. Twenty-five (62.5%) of 40 ET cases with high log blood harmane concentration had low UPSIT scores vs. 12 (27.9%) of 43 ET cases with low log blood harmane concentration (OR = 4.31, 95% CI = 1.71 – 10.85, p = 0.002, i.e., the odds of having olfactory dysfunction were four times higher among ET cases with high log blood harmane concentration than ET cases with low log blood harmane concentration). After adjusting for age in years, gender, years of education, and Cumulative Illness Rating Scale score in a logistic regression analysis, results were similar (OR = 4.04, 95% CI = 1.42 – 11.50, p = 0.009).
Log blood harmane concentration was also stratified into tertiles. The mean ± SD UPSIT score in the low log blood harmane tertile = 32.9 ± 3.1, in the middle tertile = 31.1 ± 4.9, and in the high tertile = 27.3 ± 6.0 (difference between low and high tertile = 5.6 UPSIT points, and in a test for trend [linear regression model], p < 0.001)(Figure 2). When compared with the low (reference) log blood harmane tertile, the odds of olfactory dysfunction (based on a median split) were 2.64 times higher in cases in the middle tertile and 10.95 times higher in cases in the high tertile (Table 2). These odds were higher in a logistic regression analysis that adjusted for age in years, gender, years of education, and Cumulative Illness Rating Scale score (Table 2).
Figure 2.
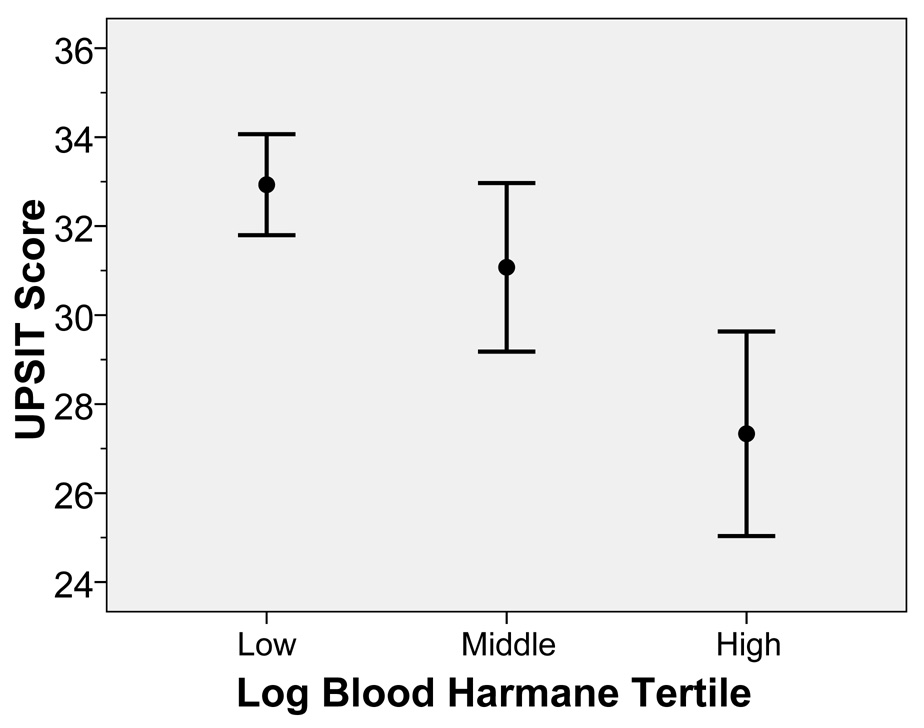
Log blood harmane concentration was also stratified into tertiles in ET cases. The mean ± SD UPSIT score in the low tertile = 32.9 ± 3.1, in the middle tertile = 31.1 ± 4.9, and in the high tertile = 27.3 ± 6.0 (difference between low and high tertile = 5.6 UPSIT points, and in a test for trend, beta = −2.79, p < 0.001). The bar represents two standard errors and the closed circle represents the mean.
Table 2.
Odds of lower UPSIT score in different log blood harmane concentration tertiles among ET cases
| N (%) with olfactory dysfunction* | N (%) without olfactory dysfunction* | Unadjusted OR, 95% CI, p | Adjusted** OR, 95% CI, p | |
|---|---|---|---|---|
| Log blood | ||||
| harmane | ||||
| concentration | ||||
| tertile | ||||
| Low | 6 (20.7%) | 23 (79.3%) | 1 (reference) | 1 (reference) |
| Middle | 11 (40.7%) | 16 (59.3%) | 2.64 (0.81 – 8.59), 0.11 | 3.29 (0.85 – 12.78), 0.086 |
| High | 20 (74.1%) | 7 (25.9%) | 10.95 (3.16 – 38.01), <0.001 | 12.51 (2.83 – 55.23), 0.001 |
Olfactory dysfunction was based on a median split of UPSIT scores into low vs. high values.
Percentages are row percentages.
Adjusted for age in years, gender, years of education, and Cumulative Illness Rating Scale score in a logistic regression analysis.
Analyses in Controls
In controls, higher log blood harmane concentration was not correlated with lower UPSIT score (rho = 0.12, p = 0.32)(Figure 3). In a linear regression analysis, higher log blood harmane concentration was not associated with lower UPSIT score (dependent variable) after adjusting for age in years, gender, years of education, and Cumulative Illness Rating Scale score (beta = 0.74, p = 0.48).
Figure 3.
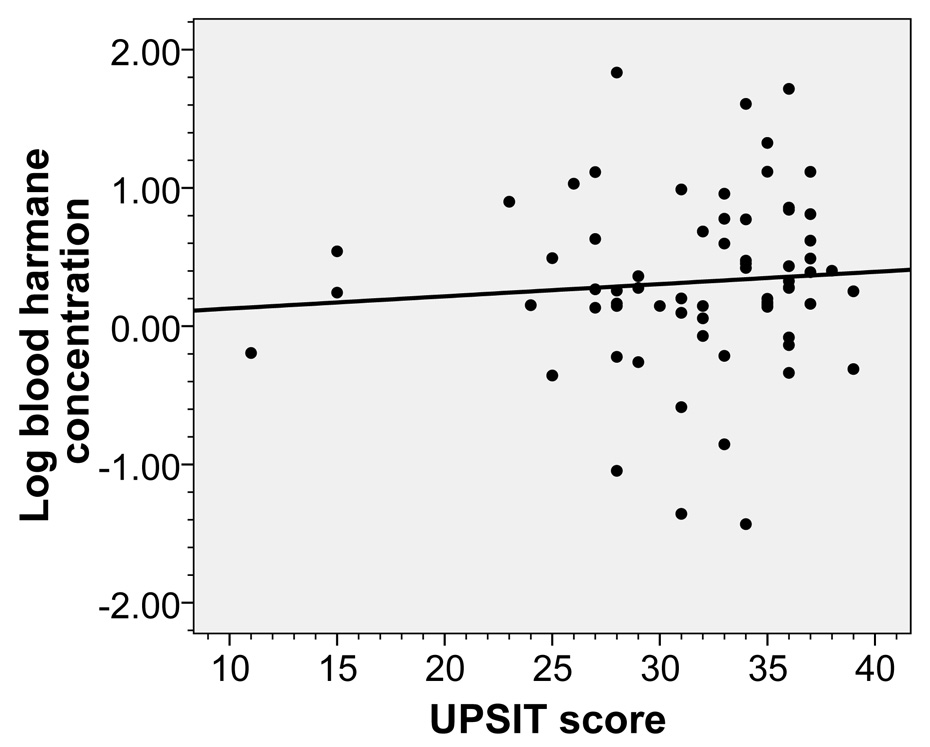
Log blood harmane concentration (g−10/ml) was not associated with UPSIT score in controls (rho = 0.12, p = 0.32). The fit line is also shown.
Discussion
Higher blood harmane concentrations were correlated with lower olfactory test scores. The association was specific to ET cases and was not observed in controls. Harmane is an environmental toxin with potent tremor-producing properties (McKenna, 1996; Zetler et al., 1972). Harmane and other members of its chemical class (e.g., harmaline, harmine and other β-carboline alkaloids) are highly neurotoxic; and it has been known for more than 100 years that administration of these chemicals to a wide variety of laboratory animals produces an intense and generalized action tremor resembling ET (Du et al., 1997). The harmaline model is the traditional animal model for ET and new therapies are tested using exposed animals (Martin et al., 2005). β-carboline alkaloids, and especially harmane, are present in a variety of foods (especially protein-rich foods such as meat but also in plants) and certain cooking practices (e.g., char-broiling) increase the concentrations of these chemicals (Herraiz, 2004; Pfau and Skog, 2004). Once they are ingested and enter the systemic circulation, these chemicals cross the blood brain barrier through an active uptake mechanism and, although related, brain concentrations are higher than those seen in the blood (Anderson et al., 2006; Guan et al., 2001). Some members of this class of chemicals are able to produce toxic damage with marked destruction of cerebellar Purkinje cells (O'Hearn and Molliver, 1997).
Higher blood harmane concentrations have been observed in ET patients in case-control studies (Louis ED, 2008 In Press; Louis et al., 2005; Louis et al., 2002b), raising the possibility that this environmental-dietary toxin is a risk factor for ET. In one recent study of ET patients, higher blood harmane concentrations correlated strongly with metabolic evidence of cerebellar damage on magnetic resonance spectroscopic imaging (Louis et al., 2007b) This finding, of increased blood harmane concentration associated with greater cerebellar neuronal damage, is consistent with the animal study data that β-carboline alkaloids appear to be neurotoxic to the cerebellum (Milner et al., 1995; O'Hearn and Molliver, 1997). We have furthermore shown in the present analyses that blood harmane concentrations are associated with olfactory test scores, which themselves may be another measure of cerebellar dysfunction in ET. Indeed, in the present set of analyses, we found that higher blood harmane concentrations were correlated with lower UPSIT scores among ET cases but not in controls. Postmortem studies are now needed to more directly address the relationship between this toxin and the cerebellum in patients with ET. With postmortem tissue, one could directly assess whether cerebellar harmane concentration is elevated in ET case brains relative to control brains and whether higher blood and cerebellar harmane concentrations are correlated with greater postmortem changes in the cerebellum in ET. The current set of results provides further rationale for conducting such a postmortem study.
Several types of evidence suggest that the cerebellum may be involved in olfactory processing. First, there are reports of olfactory dysfunction in patients with cerebellar diseases (Connelly et al., 2003; Mainland et al., 2005; Tucker, 1911; Velazquez-Perez et al., 2006). Second, the staggerer mouse, which has abnormalities in the olivocerebellar pathway, is hyposmic (Deiss and Baudoin, 1997; Deiss and Baudoin, 1999). Third, recent functional magnetic resonance imaging and positron emission tomography studies show marked odor-induced cerebellar activity (Ferdon and Murphy, 2003; Qureshy et al., 2000; Sobel et al., 1998; Yousem et al., 1997).
In the present sample, olfactory test scores were marginally lower in ET cases than in controls. In our previous studies (Applegate and Louis, 2005; Louis et al., 2002a; Louis and Jurewicz, 2003), the case-control difference was slightly greater, and this might reflect sampling differences between studies.
This study had limitations. First, our final sample of 152 subjects was 2.7 years younger on average than the base sample of 509; however, our data indicate that blood harmane concentration was not associated with age, so this should not have influenced our findings. Second, fasting blood harmane levels were impractical in this study. However, data, including our own, suggest that plasma concentrations of harmane do not change significantly during the day (Rommelspacher et al., 1991). Finally, we did not collect data on reported (i.e., symptomatic) olfactory dysfunction. The strengths of this study include the uniqueness of the question (to our knowledge, no other studies have examined this issue), the collection of both UPSIT and blood harmane concentration data in the same study subjects, collection of data in both cases and a comparison group of controls, and as part of a larger epidemiological study we had the ability to adjust for multiple potential confounding factors.
ET is the most common cause of tremor in humans, affecting large numbers of individuals in every human population, with an estimated 13 million affected individuals in the United States alone (Dogu et al., 2003; Louis et al., 1998b). There is a non-genetic component to the etiology of ET that most likely represents the effects of environmental toxicants and that accounts for a significant proportion of the incidence of this disease in the population (Fabrizio et al., 2007; Jiménez-Jiménez FJ and B., 2007; Salemi et al., 1998). It is commonly stated that 50% of ET cases are non-familial (Louis and Ottman, 1996). Given its population prevalence of 4.0% among persons age 40 and older (Dogu et al., 2003), then this suggests that approximately 2.0% of the population aged ≥ 40 years has a non-familial form of ET (Dogu et al., 2003), yet the environmental correlates for this tremor are just beginning to be explored. The role that the neurotoxin harmane plays in the etiology of ET is not entirely clear, although our research is suggesting that it likely to play a role (Guan et al., 2001; Louis et al., 2005; Louis et al., 2002b; Louis et al., 2007b; Zheng et al., 2000). Further research to more rigorously study this link and clarify its role will yield valuable information about the etiology of one of the most commonly-occurring yet poorly-explored of the neurological diseases.
Acknowledgments
Funding Source: R01 NS39422, P30 ES009089, RR00645 (General Clinical Research Center)(NIH, Bethesda, MD).
Footnotes
Disclosure: The authors report no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Statistical Analyses: The statistical analyses were conducted by Dr. Louis.
References
- Anderson NJ, Tyacke RJ, Husbands SM, Nutt DJ, Hudson AL, Robinson ES. In vitro and ex vivo distribution of [3H]harmane, an endogenous beta-carboline, in rat brain. Neuropharmacology. 2006;50:269–276. doi: 10.1016/j.neuropharm.2005.08.022. [DOI] [PubMed] [Google Scholar]
- Applegate LM, Louis ED. Essential tremor: mild olfactory dysfunction in a cerebellar disorder. Parkinsonism Relat Disord. 2005;11:399–402. doi: 10.1016/j.parkreldis.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Benito-Leon J, Bermejo-Pareja F, Morales JM, Vega S, Molina JA. Prevalence of essential tremor in three elderly populations of central Spain. Mov Disord. 2003;18:389–394. doi: 10.1002/mds.10376. [DOI] [PubMed] [Google Scholar]
- Benito-Leon J, Louis ED, Bermejo-Pareja F. Population-based case-control study of cognitive function in essential tremor. Neurology. 2006;66:69–74. doi: 10.1212/01.wnl.0000192393.05850.ec. [DOI] [PubMed] [Google Scholar]
- Brandt JSM, Folstein M. The telephone interview for cognitive status. Neuropsychi Neuropsychol Beh Neurol. 1988;1 [Google Scholar]
- Bucher SF, Seelos KC, Dodel RC, Reiser M, Oertel WH. Activation mapping in essential tremor with functional magnetic resonance imaging. Ann Neurol. 1997;41:32–40. doi: 10.1002/ana.410410108. [DOI] [PubMed] [Google Scholar]
- Connelly T, Farmer JM, Lynch DR, Doty RL. Olfactory dysfunction in degenerative ataxias. J Neurol Neurosurg Psychiatry. 2003;74:1435–1437. doi: 10.1136/jnnp.74.10.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deiss V, Baudoin C. Hyposmia for butanol and vanillin in mutant staggerer male mice. Physiol Behav. 1997;61:209–213. doi: 10.1016/s0031-9384(96)00402-7. [DOI] [PubMed] [Google Scholar]
- Deiss V, Baudoin C. Olfactory learning abilities in staggerer mutant mice. C R Acad Sci III. 1999;322:467–471. doi: 10.1016/s0764-4469(99)80096-1. [DOI] [PubMed] [Google Scholar]
- Dogu O, Sevim S, Camdeviren H, Sasmaz T, Bugdayci R, Aral M, et al. Prevalence of essential tremor: door-to-door neurologic exams in Mersin Province, Turkey. Neurology. 2003;61:1804–1806. doi: 10.1212/01.wnl.0000099075.19951.8c. [DOI] [PubMed] [Google Scholar]
- Doty RL, Deems DA, Stellar S. Olfactory dysfunction in parkinsonism: a general deficit unrelated to neurologic signs, disease stage, or disease duration. Neurology. 1988;38:1237–1244. doi: 10.1212/wnl.38.8.1237. [DOI] [PubMed] [Google Scholar]
- Doty RL, Reyes PF, Gregor T. Presence of both odor identification and detection deficits in Alzheimer's disease. Brain Res Bull. 1987;18:597–600. doi: 10.1016/0361-9230(87)90129-8. [DOI] [PubMed] [Google Scholar]
- Doty RL, Stern MB, Pfeiffer C, Gollomp SM, Hurtig HI. Bilateral olfactory dysfunction in early stage treated and untreated idiopathic Parkinson's disease. J Neurol Neurosurg Psychiatry. 1992;55:138–142. doi: 10.1136/jnnp.55.2.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du W, Aloyo VJ, Harvey JA. Harmaline competitively inhibits [3H]MK-801 binding to the NMDA receptor in rabbit brain. Brain Res. 1997;771:26–29. doi: 10.1016/s0006-8993(97)00606-9. [DOI] [PubMed] [Google Scholar]
- Fabrizio E, Vanacore N, Valente M, Rubino A, Meco G. High prevalence of extrapyramidal signs and symptoms in a group of Italian dental technicians. BMC Neurol. 2007;7:24. doi: 10.1186/1471-2377-7-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferdon S, Murphy C. The cerebellum and olfaction in the aging brain: a functional magnetic resonance imaging study. Neuroimage. 2003;20:12–21. doi: 10.1016/s1053-8119(03)00276-3. [DOI] [PubMed] [Google Scholar]
- Frye RE, Schwartz BS, Doty RL. Dose-related effects of cigarette smoking on olfactory function. Jama. 1990;263:1233–1236. [PubMed] [Google Scholar]
- Guan Y, Louis ED, Zheng W. Toxicokinetics of tremorogenic natural products, harmane and harmine, in male Sprague-Dawley rats. J Toxicol Environ Health A. 2001;64:645–660. doi: 10.1080/152873901753246241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herraiz T. Relative exposure to beta-carbolines norharman and harman from foods and tobacco smoke. Food Addit Contam. 2004;21:1041–1050. doi: 10.1080/02652030400019844. [DOI] [PubMed] [Google Scholar]
- Jenkins IH, Bain PG, Colebatch JG, Thompson PD, Findley LJ, Frackowiak RS, et al. A positron emission tomography study of essential tremor: evidence for overactivity of cerebellar connections. Ann Neurol. 1993;34:82–90. doi: 10.1002/ana.410340115. [DOI] [PubMed] [Google Scholar]
- Jiménez-Jiménez FJ dT-HM, Alonso-Navarro H, Ayuso-Peralta L, Arévalo-Serrano J, Ballesteros-Barranco A, Puertas I, Jabbour-Wadih T, Barcenilla B. Environmental risk factors for essential tremor. Eur Neurol. 2007;58:106–113. doi: 10.1159/000103646. [DOI] [PubMed] [Google Scholar]
- Linn BS, Linn MW, Gurel L. Cumulative illness rating scale. J Am Geriatr Soc. 1968;16:622–626. doi: 10.1111/j.1532-5415.1968.tb02103.x. [DOI] [PubMed] [Google Scholar]
- Louis ED, Bromley SM, Jurewicz EC, Watner D. Olfactory dysfunction in essential tremor: a deficit unrelated to disease duration or severity. Neurology. 2002a;59:1631–1633. doi: 10.1212/01.wnl.0000033798.85208.f2. [DOI] [PubMed] [Google Scholar]
- Louis ED, Faust PL, Vonsattel JP, Honig LS, Rajput A, Robinson CA, et al. Neuropathological changes in essential tremor: 33 cases compared with 21 controls. Brain. 2007a;130:3297–3307. doi: 10.1093/brain/awm266. [DOI] [PubMed] [Google Scholar]
- Louis ED, Ford B, Lee H, Andrews H. Does a screening questionnaire for essential tremor agree with the physician's examination? Neurology. 1998a;50:1351–1357. doi: 10.1212/wnl.50.5.1351. [DOI] [PubMed] [Google Scholar]
- Louis ED, Jurewicz EC. Olfaction in essential tremor patients with and without isolated rest tremor. Mov Disord. 2003;18:1387–1389. doi: 10.1002/mds.10603. [DOI] [PubMed] [Google Scholar]
- Louis EDJW, Pellegrino KM, Rios E, Factor-Litvak P, Henchcliffe C, Zheng W. Elevated blood harmane (1-methyl-9h-pyrido[3,4-b]indole) concentrations in essential tremor. Neurotoxicology. 2008 doi: 10.1016/j.neuro.2007.12.001. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis ED, Ottman R. How familial is familial tremor? The genetic epidemiology of essential tremor. Neurology. 1996;46:1200–1205. doi: 10.1212/wnl.46.5.1200. [DOI] [PubMed] [Google Scholar]
- Louis ED, Ottman R, Ford B, Pullman S, Martinez M, Fahn S, et al. The Washington Heights-Inwood Genetic Study of Essential Tremor: methodologic issues in essential-tremor research. Neuroepidemiology. 1997;16:124–133. doi: 10.1159/000109681. [DOI] [PubMed] [Google Scholar]
- Louis ED, Ottman R, Hauser WA. How common is the most common adult movement disorder? estimates of the prevalence of essential tremor throughout the world. Mov Disord. 1998b;13:5–10. doi: 10.1002/mds.870130105. [DOI] [PubMed] [Google Scholar]
- Louis ED, Vonsattel JP, Honig LS, Lawton A, Moskowitz C, Ford B, et al. Essential tremor associated with pathologic changes in the cerebellum. Arch Neurol. 2006;63:1189–1193. doi: 10.1001/archneur.63.8.1189. [DOI] [PubMed] [Google Scholar]
- Louis ED, Zheng W, Applegate L, Shi L, Factor-Litvak P. Blood harmane concentrations and dietary protein consumption in essential tremor. Neurology. 2005;65:391–396. doi: 10.1212/01.wnl.0000172352.88359.2d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis ED, Zheng W, Jurewicz EC, Watner D, Chen J, Factor-Litvak P, et al. Elevation of blood beta-carboline alkaloids in essential tremor. Neurology. 2002b;59:1940–1944. doi: 10.1212/01.wnl.0000038385.60538.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis ED, Zheng W, Mao X, Shungu DC. Blood harmane is correlated with cerebellar metabolism in essential tremor: a pilot study. Neurology. 2007b;69:515–520. doi: 10.1212/01.wnl.0000266663.27398.9f. [DOI] [PubMed] [Google Scholar]
- Mainland JD, Johnson BN, Khan R, Ivry RB, Sobel N. Olfactory impairments in patients with unilateral cerebellar lesions are selective to inputs from the contralesional nostril. J Neurosci. 2005;25:6362–6371. doi: 10.1523/JNEUROSCI.0920-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin FC, Thu Le A, Handforth A. Harmaline-induced tremor as a potential preclinical screening method for essential tremor medications. Mov Disord. 2005;20:298–305. doi: 10.1002/mds.20331. [DOI] [PubMed] [Google Scholar]
- McKenna DJ. Plant hallucinogens: springboards for psychotherapeutic drug discovery. Behav Brain Res. 1996;73:109–116. doi: 10.1016/0166-4328(96)00079-4. [DOI] [PubMed] [Google Scholar]
- Milner TE, Cadoret G, Lessard L, Smith AM. EMG analysis of harmaline-induced tremor in normal and three strains of mutant mice with Purkinje cell degeneration and the role of the inferior olive. J Neurophysiol. 1995;73:2568–2577. doi: 10.1152/jn.1995.73.6.2568. [DOI] [PubMed] [Google Scholar]
- O'Hearn E, Molliver ME. The olivocerebellar projection mediates ibogaine-induced degeneration of Purkinje cells: a model of indirect, trans-synaptic excitotoxicity. J Neurosci. 1997;17:8828–8841. doi: 10.1523/JNEUROSCI.17-22-08828.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagan FL, Butman JA, Dambrosia JM, Hallett M. Evaluation of essential tremor with multi-voxel magnetic resonance spectroscopy. Neurology. 2003;60:1344–1347. doi: 10.1212/01.wnl.0000065885.15875.0d. [DOI] [PubMed] [Google Scholar]
- Pfau W, Skog K. Exposure to beta-carbolines norharman and harman. J Chromatogr B Analyt Technol Biomed Life Sci. 2004;802:115–126. doi: 10.1016/j.jchromb.2003.10.044. [DOI] [PubMed] [Google Scholar]
- Qureshy A, Kawashima R, Imran MB, Sugiura M, Goto R, Okada K, et al. Functional mapping of human brain in olfactory processing: a PET study. J Neurophysiol. 2000;84:1656–1666. doi: 10.1152/jn.2000.84.3.1656. [DOI] [PubMed] [Google Scholar]
- Rommelspacher H, Schmidt LG, May T. Plasma norharman (beta-carboline) levels are elevated in chronic alcoholics. Alcohol Clin Exp Res. 1991;15:553–559. doi: 10.1111/j.1530-0277.1991.tb00559.x. [DOI] [PubMed] [Google Scholar]
- Salemi G, Aridon P, Calagna G, Monte M, Savettieri G. Population-based case-control study of essential tremor. Ital J Neurol Sci. 1998;19:301–305. doi: 10.1007/BF00713856. [DOI] [PubMed] [Google Scholar]
- Shill HAC, Sabbagh M, Connor D, Caviness J, Beach T. Pathological changes in prospectively-ascertained essential tremor subjects. Neurology. 2007;68 doi: 10.1212/01.wnl.0000310425.76205.02. [DOI] [PubMed] [Google Scholar]
- Singer C, Sanchez-Ramos J, Weiner WJ. Gait abnormality in essential tremor. Mov Disord. 1994;9:193–196. doi: 10.1002/mds.870090212. [DOI] [PubMed] [Google Scholar]
- Sobel N, Prabhakaran V, Hartley CA, Desmond JE, Zhao Z, Glover GH, et al. Odorant-induced and sniff-induced activation in the cerebellum of the human. J Neurosci. 1998;18:8990–9001. doi: 10.1523/JNEUROSCI.18-21-08990.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker B. Report of a case of tumor of the ponto-cerebellar angle on the left side of the brain with bilateral loss of smell and disturbance of taste. Old Dominion J Med Surg. 1911;13:327–334. [Google Scholar]
- Velazquez-Perez L, Fernandez-Ruiz J, Diaz R, Gonzalez RP, Ochoa NC, Cruz GS, et al. Spinocerebellar ataxia type 2 olfactory impairment shows a pattern similar to other major neurodegenerative diseases. J Neurol. 2006;253:1165–1169. doi: 10.1007/s00415-006-0183-2. [DOI] [PubMed] [Google Scholar]
- Yousem DM, Williams SC, Howard RO, Andrew C, Simmons A, Allin M, et al. Functional MR imaging during odor stimulation: preliminary data. Radiology. 1997;204:833–838. doi: 10.1148/radiology.204.3.9280268. [DOI] [PubMed] [Google Scholar]
- Zesiewicz TA, Elble R, Louis ED, Hauser RA, Sullivan KL, Dewey RB, Jr, et al. Practice parameter: therapies for essential tremor: report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2005;64:2008–2020. doi: 10.1212/01.WNL.0000163769.28552.CD. [DOI] [PubMed] [Google Scholar]
- Zetler G, Singbartl G, Schlosser L. Cerebral pharmacokinetics of tremor-producing harmala and iboga alkaloids. Pharmacology. 1972;7:237–248. doi: 10.1159/000136294. [DOI] [PubMed] [Google Scholar]
- Zheng W, Wang S, Barnes LF, Guan Y, Louis ED. Determination of harmane and harmine in human blood using reversed-phased high-performance liquid chromatography and fluorescence detection. Anal Biochem. 2000;279:125–129. doi: 10.1006/abio.1999.4456. [DOI] [PMC free article] [PubMed] [Google Scholar]


