Abstract
The role of progestins in combined hormone therapy is the inhibition of uterine epithelial cell proliferation. The Women’s Health Initiative study provided evidence for an increased risk of breast cancer in women treated with conjugated equine estrogens plus the synthetic progestin medroxyprogesterone acetate (MPA), compared with conjugated equine estrogens-only treatment. These findings continue to be discussed, and it remains to be clarified whether the results obtained for MPA in the Women’s Health Initiative study are directly applicable to other progestins used in hormone therapy. In this study we compared in a mouse model the effects of the synthetic progestins, MPA, and drospirenone in two major target organs: the uterus and mammary gland. As quantitative measures of progestin activity, we analyzed maintenance of pregnancy, ductal side branching in the mammary gland, and proliferation of mammary and uterine epithelial cells as well as target gene induction in both organs. The outcome of this study is that not all synthetic progestins exhibit the same effects. MPA demonstrated uterine activity and mitogenic activity in the mammary gland at the same doses. In contrast, drospirenone behaved similarly to the natural hormone, progesterone, and exhibited uterine activity at doses lower than those leading to considerable proliferative effects in the mammary gland. We hypothesize that the safety of combined hormone therapy in postmenopausal women may be associated with a dissociation between the uterine and mammary gland activities of the progestin component.
FOR MANY WOMEN, reaching menopause and suffering from symptoms such as hot flushes, sleep disturbance, and vaginal dryness, hormone replacement therapy has been the treatment of choice for several decades. Because estrogens lead to enhanced uterine epithelial cell proliferation and thus increase the risk for endometrial carcinoma, women with an intact uterus are frequently treated with synthetic progestins to counteract the proliferative effects of estrogen in the uterus (1). Although progestins inhibit estradiol-induced proliferation of uterine epithelial cells, they demonstrate the opposite effect in the mammary gland. During the luteal phase of the menstrual cycle, when progesterone is present at elevated levels, increased proliferation is observed in breast epithelium (2). In addition, studies analyzing normal mammary gland development in animal models (3) or the human breast (4) have demonstrated that progestins, when added to estradiol, enhance proliferation of mammary epithelial cells. Interestingly, proliferating cells in the normal human (5) or murine (6) breast do not express the progesterone receptor (PR) or estrogen receptor (ER)-α. Therefore, in the normal mammary gland, cell proliferation in response to progesterone and estradiol appears to be regulated in a paracrine manner. One suggested paracrine mechanism involves receptor activator of nuclear factor-κΒ ligand (RANKL)-mediated activation of the nuclear factor-κΒ, which leads to enhanced cyclin D1 expression in proliferating epithelial cells devoid of ER and PR (6). The plausibility of this association between PR activation and cyclin D1 expression is strengthened by the observation that cyclin D1 and progesterone receptor B-deficient mice show the same mammary gland phenotype, i.e. reduced ductal side branching (7). In contrast to normal cells, breast cancer cells are frequently positive for ER and PR and thus show a direct proliferative response after exposure to estradiol and progesterone (8).
In the Women’s Health Initiative (WHI) study, an increased risk for breast cancer was reported in women treated with conjugated equine estrogens (CEE) plus the synthetic progestin, medroxyprogesterone acetate (MPA) (9). In contrast, women treated with CEE alone did not show an increased risk for breast cancer (10). Although these results are in agreement with those of other randomized controlled studies (11), the WHI study has been the subject of much controversy. Major criticisms of the study design focused on the continuous rather than cyclic administration of MPA, the route of hormone delivery, the age and health status of the women included in the study, and the duration of estrogen deficiency before starting hormone therapy (HT) (12,13). Importantly for the clinical interpretation of the WHI study, not all synthetic progestins and estrogens used in HT appear to have similar characteristics (13). Therefore it is possible that the increased breast cancer risk that was observed with CEE plus MPA treatment in the WHI study may not be associated with other hormonal treatments.
In the United States, the most frequently prescribed treatment for menopausal HT in nonhysterectomized women remains oral CEE plus MPA. Recently a new combination containing 17β-estradiol and the novel progestin, drospirenone, was launched for the same indication. Aside from the differences between these treatments in the inclusion of the natural hormone, estradiol, or the less well-defined steroid combinations within CEE (13), the two synthetic progestins that are included, MPA and drospirenone, are also pharmacologically different. In addition to PR activation, MPA demonstrates considerable agonistic activity on the glucocorticoid receptor (GR) and has androgenic activity (14). Drospirenone, by contrast, more closely resembles the natural hormone, progesterone, in that it is devoid of glucocorticoid activity but possesses slight antiandrogenic and strong antimineralocorticoid activity (15). Variations in the profile of nuclear receptor activation may underline the differences between synthetic progestins. For example, in vitro and in vivo studies have demonstrated that MPA, but not progesterone (16) or drospirenone (17), blunts the beneficial effects of estradiol on endothelial nitric oxide synthase activity and endothelial nitric oxide synthase expression in endothelial cells. The fundamental differences between the effects of MPA and drospirenone observed in the cardiovascular system prompted us to investigate whether these progestins exhibit similar effects in two major progesterone target organs: the uterus and the mammary gland. Therefore, we developed a mouse model that was suitable for quantitative measurements of progestin activity in both organs using a range of readout paradigms. By comparing quantitative measures of activity at the mammary gland and uterus for each progestin, we were able to analyze the relative tissue specificity of each compound. The key objective in the current study of these progestins was to determine whether there was any dissociation between the desirable effects in the uterus and the less desirable effects in the mammary gland.
Materials and Methods
Animals and drugs
17β-Estradiol and 5′-bromo-2′-deoxyuridine (BrdU) were purchased from Sigma (St. Louis, MO), whereas estrone, drospirenone, and MPA were synthesized in the laboratories of Bayer Schering Pharma AG. NMRI and C57BL/6 mice (Charles River, Sulzfeld, Germany) were maintained on a 14-h light, 10-h dark cycle and provided with food and water ad libitum. All animal procedures were in accordance with German animal welfare law and were with the permission of the District Government of Berlin.
Maintenance of pregnancy assay
The establishment and maintenance of pregnancy in mammals depends strictly on progestin activity. We therefore decided to quantify the pure progestogenic activity of drospirenone and MPA in the uterus in vivo by using maintenance of pregnancy assays. The results from this assay were then planned to be used in a second step to normalize the mammary gland activities of these progestins. Adult female NMRI mice (25–30 g) were mated with fertile males. The appearance of a vaginal plug represented d 1 of pregnancy. On d 1 after conception, females were separated from males and were randomly assigned to different treatment groups (n = 8 females per group). On d 8 of pregnancy, the animals were ovariectomized and were then substituted by daily sc application of different doses of MPA (0, 0.5, 2, 8, or 16 mg/kg) or drospirenone (0, 8, 16, 32, 64, or 100 mg/kg) in combination with 0.03 μg estrone/animal, which was commenced 2 h before ovariectomy. Ovariectomy without hormonal substitution would result in immediate abortion. On d 18 after conception, the animals were killed and numbers of living embryos were counted. The number of living embryos per mouse in the sham-ovariectomized, vehicle-treated control group was set to 100%.
Mammary gland whole-mount assay
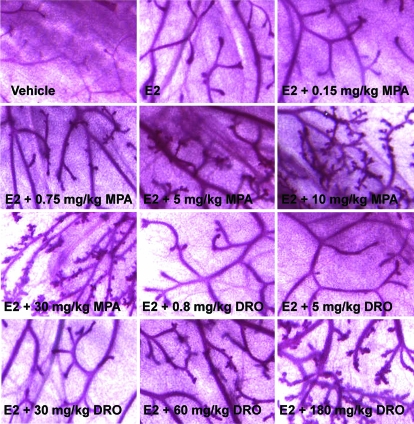
To assess the activity of MPA and drospirenone in the mammary gland, we used an experimental treatment paradigm that mimicked pregnancy-induced changes. We ovariectomized female C57BL/6 mice at the beginning of the sixth week of age. Two weeks after ovariectomy the animals were treated daily for 3 wk with sc injections of either vehicle or 100 ng 17β-estradiol plus different doses of MPA (0, 0.15, 0.75, 5, 10, or 30 mg/kg) or drospirenone (0, 0.8, 5, 30, 60, or 180 mg/kg) dissolved in ethanol/arachisoil [1 + 9 (vol/vol)]. There were eight animals in each treatment group. Two hours before the animals were killed, animals received an ip injection of BrdU dissolved in PBS (70 mg/kg body weight). Animals were killed by cervical dislocation and the left inguinal mammary gland was removed, spread on a glass slide, and fixed for 48 h at room temperature in Carnoy’s fixative (six parts 100% ethanol, three parts chloroform, one part glacial acetic acid). After staining in carmine alum (0.2% carmine alum, 0.5% aluminum potassium sulfate, 1 crystal of thymol), the mammary glands were dehydrated, cleared in xylene, and stored in Pro Taqstura (Quartett, Berlin, Germany). Photographs were taken at ×20 magnification. For each animal, the number of side branches in six different areas distal to the lymph node was determined by an investigator who was blinded to the experimental treatment the animals had received. The size of one area constituted approximately 70% of the single areas depicted in Fig. 1.
Figure 1.
Mammary gland whole-mount analysis. C57BL/6 females were ovariectomized and treated sc for 3 wk with vehicle, 100 ng estradiol (E2), or 100 ng estradiol plus different doses of MPA and drospirenone (n = 8 animals per treatment group). Representative whole-mount preparations for each treatment group are shown.
Determination of mammary and uterine epithelial cell proliferation
To assess mammary and uterine epithelial cell proliferation in response to combined estradiol/progestin treatment within the same animal, one uterine horn and the dorsal two thirds of the right inguinal mammary gland of mice treated in the mammary gland assay were removed and fixed in 4% formalin at 4 C overnight. Tissues were embedded in paraffin and cut into 5-μm slices. BrdU immunostaining using the mouse monoclonal anti-BrdU antibody (M0744; DakoCytomation, Hamburg, Germany) was performed as described previously (18). The total number of BrdU-positive cells in the ductal epithelium was determined evaluating four complete transverse mammary gland sections per animal.
To estimate the mean ID100 for uterine progestin activity in a semiquantitative manner, we calculated the mean for the minimal progestin dose required to achieve the maximal effect (i.e. progestin induced suppression of uterine epithelial cell proliferation to vehicle treated control levels) and the dose preceding this minimal dose. At the minimal dose exhibiting the maximal effect, at least seven of eight animals were required to show suppression of uterine epithelial cell proliferation to control levels.
Quantitative RT-PCR
For gene expression analysis, we used the organs of mice killed for the mammary gland assay. The ventral third of the right inguinal mammary gland (without lymph node) and one uterine horn were frozen rapidly in liquid nitrogen. RNA was isolated after homogenization of tissues in guanidinium thiocyanate (19). Five micrograms of RNA were digested with deoxyribonuclease I and reversely transcribed with random hexamers using the SuperScript III first-strand synthesis system (Invitrogen, Carlsbad, CA). Real-time TaqMan PCR analysis was performed using the ABI Prism 7700 sequence detection system according to the manufacturer’s instructions (PE Applied Biosystems, Foster City, CA). Prevalidated probes and primers for murine RANKL (catalog no. Mm00441908_m1), indoleamine-pyrrole 2,3 dioxygenase (INDO) (catalog no. Mm00492586_m1), cyclin D1 (catalog no. Mm00432358_m1), cytokeratin 18 (catalog no. Mm01601702_g1), lactotransferrin (LTF) (catalog no. Mm00434787_m1), and TATA-box-binding-protein (catalog no. Mm00446973_m1) were purchased from PE Applied Biosystems. Relative mRNA levels were calculated by the comparative ΔCt-method. In the mammary gland, expression levels of RANKL, cyclin D1, and INDO were normalized to cytokeratin 18, whereas in the uterus, the expression level of LTF was normalized to TATA-box-binding-protein.
Determination of dissociation of uterine and mammary gland effects and statistical analyses
Calculations of the parameters for the dose-response curves for progestin activity in different readout paradigms were performed using PROC NLIN in SAS (SAS Institute, Cary, NC), using the original data. The ED50 and ID50 values were obtained from the respective dose-response curves. The 95% confidence intervals were computed using the bootstrap method (20,21) for maintenance of pregnancy, inhibition of E2-induced LTF and INDO expression, ductal sidebranching, and BrdU incorporation in mammary epithelial cells. The graphical display of the dose-response curves was performed using Sigma Plot (Systat Software, San Jose, CA).
To assess the degree of dissociation between the uterine and mammary gland effects of MPA and drospirenone, we calculated dissociation factors (DFs) by dividing ED50 values for mammary gland readouts by ED50 or ID50 values for uterine readouts. By definition, a DF of 1 indicates that a progestin possesses equivalent activity in the uterus and the mammary gland at the same dose. A DF lower than 1 implies that the progestin is active in the mammary gland at doses lower than those required for activity in the uterus, whereas a DF greater than 1 indicates that the progestin exerts uterine effects at doses lower than those required for activity in the mammary gland. For hormone therapy, the latter profile (i.e. DF > 1) would be highly desirable. Whenever possible, the following rules were applied for the calculation of absolute DFs: 1) only readouts of the same type were compared, i.e. functional readouts were compared only with other functional readouts, and molecular readouts were compared only with other molecular readouts; 2) ED50 values were divided by ED50 values and ID50 values were divided by ID50 values. The DFs and the corresponding 95% confidence intervals were calculated using the bootstrap approach, which incorporates the difference in dose-response curves between drospirenone and MPA and the difference between uterine and mammary gland effects. It should be noted that, for the absolute DF, a confidence interval with a lower limit above 1 indicates a statistically significant, favorable dissociation of uterine and mammary gland effects. To estimate the extent of the differences between drospirenone and MPA, relative DFs were calculated by division of the absolute DFs for drospirenone by the absolute DFs for MPA. For the relative DF, a confidence interval above 1 indicates a significantly higher dissociation for drospirenone, compared with MPA. The confidence intervals were not adjusted to account for multiple comparisons. For RANKL and cyclin D1 expression analysis, the first progestin dose that demonstrated an effect significantly different from estradiol-only treatment was obtained using a step-down bootstrap approach that incorporated correlation structures between contrasts. All statistical analyses were performed in SAS (version 9.1).
Results
To analyze the dissociation of uterine and mammary gland effects for the synthetic progestins, MPA and drospirenone, we used morphological, functional, and molecular readouts. Maintenance of pregnancy assays were performed to obtain a quantitative measure of pure progestin action within the uterus. In a second set of experiments, mammary gland assays were performed. In these experiments we analyzed, within the same animal ductal side branching in the mammary gland, BrdU incorporation in mammary and uterine epithelial cells as well as target gene induction in both organs.
Maintenance of pregnancy assays for quantification of uterine progestin activity
ED50 values obtained from the maintenance of pregnancy assays were 3.6 mg/kg for MPA and 25.8 mg/kg for drospirenone (Table 1). These data reflect that MPA is approximately 7 times more potent than drospirenone in vivo in terms of progestogenic effects in the uterus.
Table 1.
Quantitative readouts for uterine and mammary gland effects of MPA and drospirenone
| Readout | MPA (mg/kg)a | Drospirenone (mg/kg)a |
|---|---|---|
| Uterus | ||
| Maintenance of pregnancy, ED50 | 3.6 (2.2, 6.0) | 25.8 (16.0, 31.6) |
| BrdU incorporation in epithelial cells, mean ID100 | 2.9 | 17.5 |
| Inhibition of E2-induced LTF expression, ID50 | 0.7 (0.5, 1.0) | 22.0 (3.6, 32.1) |
| Mammary gland | ||
| Formation of side branches, ED50 | 4.3 (2.0, 7.5) | 69.4 (60.3, 84.3) |
| BrdU incorporation in epithelial cells, ED50 | 1.2 (0.3, 4.2) | 50.5 (40.9, 64.0) |
| Inhibition of E2-induced INDO expression, ID50 | 0.09 (0.07, 0.13) | 14.1 (3.3, 19.5) |
| Cyclin D1 expression, first significant effect | 0.75 | 180 |
| RANKL expression, first significant effect | 0.75 | 180 |
E2, 17β-Estradiol.
The 95% confidence intervals are shown in parentheses.
Analysis of ductal side branching in the mammary gland
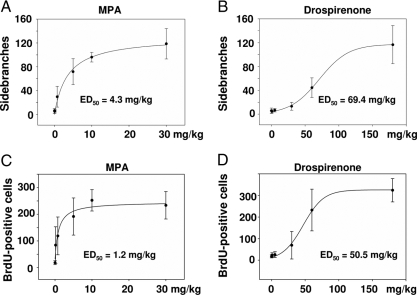
To analyze progestin effects in the mammary gland (and the uterus) under conditions that mimic early pregnancy, ovariectomized mice were treated for 3 wk, as indicated in Materials and Methods. High-power photographs of the mammary gland whole mounts are depicted in Fig. 1. After vehicle treatment, only a rudimentary duct system without side branches was visible in the fat pad of the mammary gland. Estradiol administration stimulated end-bud formation and ductal elongation as expected (22). Combined treatment with estradiol plus increasing doses of progestins clearly enhanced ductal side branching (Fig. 1). Dose-response curves were generated as shown in Fig. 2. Whereas MPA had an ED50 of 4.3 mg/kg for the stimulation of ductal side branching (Fig. 2A), the corresponding ED50 value for drospirenone was 69.4 mg/kg (Fig. 2B). These values, as well as all other consecutive measures of progestin activity, are shown in Table 1.
Figure 2.
Ductal side branching and epithelial cell proliferation in mammary glands after combined estrogen plus progestin treatment. Ovariectomized C57BL/6 females were treated for 3 wk with different doses of MPA (A and C) or drospirenone (B and D) plus 100 ng estradiol. The formation of ductal side branches within the mammary gland (A and B) as well as the proliferation of mammary epithelial cells (C and D) was analyzed. ED50 values for both progestins in the respective readout paradigms are depicted.
Mammary epithelial cell proliferation in response to progestin treatment
As a second readout of mammary gland activity, we chose stimulation of estradiol-induced epithelial cell proliferation by progestins (2,3). Dose-response curves are shown in Fig. 2. Whereas MPA stimulated mammary epithelial cell proliferation with an ED50 of 1.2 mg/kg (Fig. 2C and Table 1), drospirenone had an ED50 of 50.5 mg/kg (Fig. 2D and Table 1). Comparison of the ED50 values for stimulation of side branching and cellular proliferation revealed for drospirenone that the ED50 values were almost in the same range (compare Fig. 2, B and D, and Table 1). MPA demonstrated a nonsignificant, 3-fold greater potency for stimulation of mammary epithelial cell proliferation, compared with ductal sidebranching (compare ED50 values in Fig. 2, A and B).
Inhibition of uterine epithelial cell proliferation
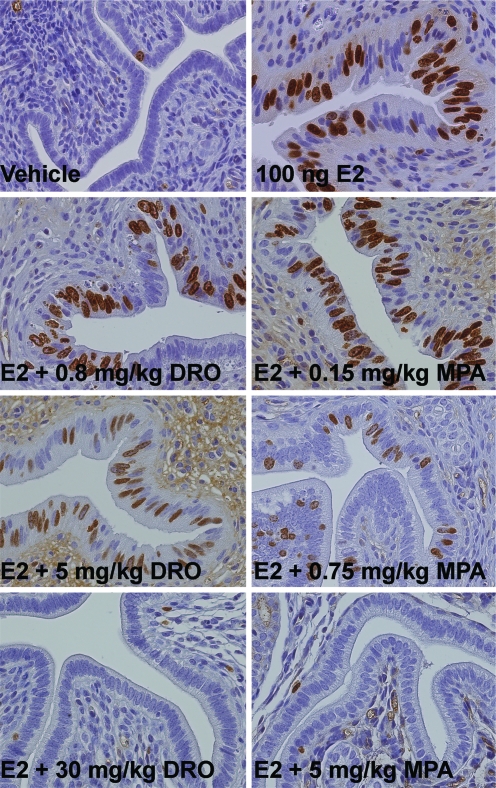
From each of the experimental animals used for the mammary gland assays, one uterine horn was processed for BrdU immunostaining. In contrast to their activity in the mammary gland, progestins inhibit estradiol-stimulated epithelial cell proliferation within the uterus (1). As depicted in Fig. 3, no or only solitary uterine epithelial cells proliferated after vehicle treatment. With estradiol treatment, there was a clear increase in epithelial cell proliferation that was reversed by coadministration of increasing progestin doses. In the case of drospirenone, vehicle levels of cellular proliferation were attained at doses between 5 and 30 mg/kg, yielding a mean ID100 of 17.5 mg/kg (Table 1). In comparison, a dose of MPA between 0.75 and 5 mg/kg was required to achieve the same effect (mean ID100 = 2.9 mg/kg, Table 1). These semiquantitative values are in agreement with the quantitative data obtained from the maintenance of pregnancy assays performed in NMRI mice (see Table 1 for respective results).
Figure 3.
Uterine epithelial cell proliferation after combined estrogen plus progestin treatment. Ovariectomized C57BL/6 mice were treated for 3 wk with vehicle, estradiol, or estradiol plus different doses of MPA and drospirenone (DRO; n = 8 animals per treatment group). Whereas there was almost no proliferation within the uterine epithelium after vehicle treatment, estradiol (E2) led to a clear increase in BrdU incorporation. Complete inhibition of estradiol-induced uterine epithelial cell proliferation was achieved by adding increasing doses of MPA or drospirenone.
Progestin-induced changes in gene expression
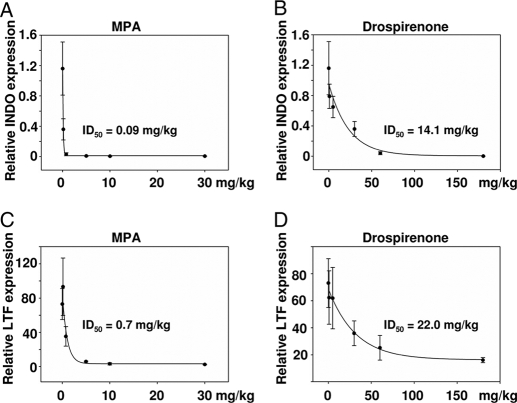
The third readout for the dissociation of uterine and mammary gland effects was progestin-induced inhibition of estradiol-stimulated gene expression in both organs. In the mammary gland, it has been demonstrated that progestins inhibit estradiol-induced INDO expression in a dose-dependent manner (23). Within the uterus, progestins antagonize the expression of the estradiol-induced target gene, LTF (24). In the mammary gland, the ID50 for inhibition of estradiol-induced INDO expression was 0.09 mg/kg for MPA (Fig. 4A and Table 1) and 14.1 mg/kg for drospirenone (Fig. 4B and Table 1). It is notable that the ID50 for MPA for inhibition of INDO expression in the mammary gland is in agreement with previously published data (23). In the uterus, the ID50 for inhibition of estradiol-stimulated LTF expression was 0.7 mg/kg for MPA (Fig. 4C and Table 1) and 22.0 mg/kg for drospirenone (Fig. 4D and Table 1).
Figure 4.
Inhibition of estradiol-stimulated gene expression by progestins in mammary gland and uterus. Ovariectomized C57BL/6 mice were treated over 3 wk with vehicle, 100 ng estradiol, or different doses of MPA or drospirenone plus 100 ng estradiol. In the mammary gland, estradiol-activated INDO expression was inhibited by increasing the dose of progestin (A and B), whereas in the uterus, estradiol-induced LTF expression (C and D) was down-regulated by both progestins. ID50 values were calculated for the inhibition of estradiol-induced gene expression by MPA (A and C) and drospirenone (B and D) in the mammary gland (A and B) and uterus (C and D).
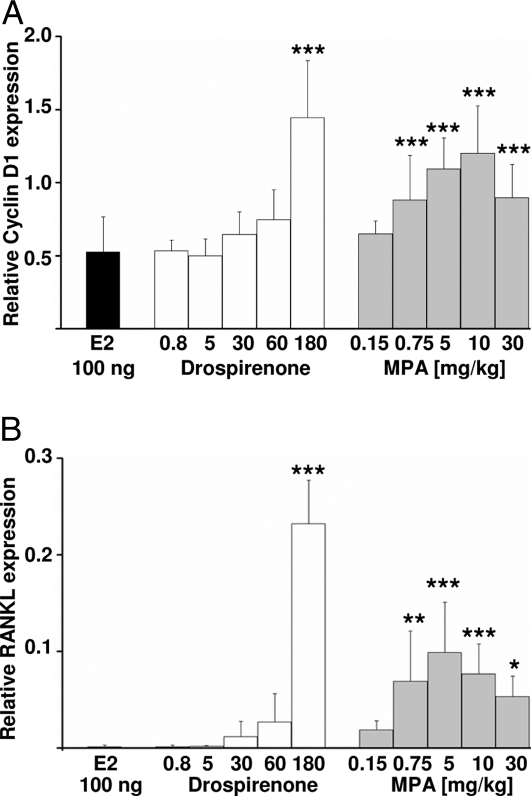
Because induction of cyclin D1 and RANKL is one paracrine mechanism for progestin-stimulated mammary epithelial cell proliferation (6), we also analyzed the induction of these two genes (Fig. 5). MPA (Fig. 5, gray bars) significantly stimulated cyclin D1 (Fig. 5A and Table 1) and RANKL expression (Fig. 5B and Table 1) at a dose of 0.75 mg/kg (Fig. 5A and Table 1). In contrast, a dose of 180 mg/kg of drospirenone (Fig. 5, white bars) was required to induce cyclin D1 (Fig. 5A and Table 1) or RANKL expression (Fig. 5B and Table 1) significantly above estradiol levels (Fig. 5A, black bar).
Figure 5.
Induction of cyclin D1 and RANKL gene expression in the mammary gland by estrogen plus progestin treatment. Mammary glands of ovariectomized C57BL/6 mice that had been treated for 3 wk with either 100 ng estradiol (E2; black bar) or 100 ng estradiol plus different doses of drospirenone (white bars) or MPA (gray bars) were analyzed for the expression of cyclin D1 (A) or RANKL (B) using quantitative RT-PCR. Note that compared with drospirenone (180 mg/kg), much lower doses of MPA (0.75 mg/kg) were required to significantly potentiate estradiol-stimulated induction of both genes. *, P < 0.05; **, P < 0.01; ***, P < 0.005 (stepdown bootstrap analysis).
Dissociation of mammary gland vs. uterine effects
To assess the dissociation of uterine and mammary gland effects for MPA and drospirenone, we determined the absolute DFs as depicted in Table 2. As a quantitative functional uterine progestin readout, we used the ED50 for maintenance of pregnancy in almost all cases. That measure, although obtained in a different cohort and strain of mice, resembled the semiquantitative data for the mean ID100 for inhibition of uterine epithelial cell proliferation obtained from C57BL/6 mice (Table 1). Maintenance of pregnancy requires progestin activity and represents an agonistic progestin readout. We therefore consider that this measure may be adopted as a valid normalization reference for the majority of the agonistic mammary gland readouts that we have analyzed.
Table 2.
Calculation of DFs for mammary gland and uterine progestin action
| Calculation of DFa | MPA absolute DF | Drospirenone absolute DF | Drospirenone relative DFb |
|---|---|---|---|
| ED50 sidebranches/ED50 pregnancy | 1.25 (0.42, 2.86) | 2.67 (2.07, 4.36) | 2.2 (0.91, 6.84) |
| ED50 BrdU Ma./ID100 BrdU ut. | 0.44 (0.10, 1.49) | 2.89 (2.32, 3.52) | 6.66 (1.85, 20.03)c |
| ED50 BrdU Ma./ED50 pregnancy | 0.33 (0.07, 1.48) | 1.97 (1.43, 3.26) | 5.91 (1.31, 29.81)c |
| ID50 INDO Ma./ID50 LTF ut. | 0.13 (0.08, 0.21) | 0.62 (0.15, 2.84) | 4.51 (1.12, 18.31)c |
| Cyclin D1 Ma./ED50 pregnancy | 0.21 (0.14, 0.34) | 6.9 (5.7, 11.22) | 33.5 (19.91, 62.61)c |
| RANKL Ma./ED50 pregnancy | 0.21 (0.14, 0.34) | 6.9 (5.7, 11.22) | 33.5 (19.91, 62.61)c |
DFs are calculated by dividing quantitative measures of mammary gland action (Ma.) by those of uterine action (ut.); absolute DF values significantly larger than 1 indicate favorable dissociation of uterine and mammary gland effects.
Relative DFs for drospirenone were calculated as absolute DF drospirenone/absolute DF MPA.
Relative DF values significantly larger than 1 indicate a favorable dissociation profile of drospirenone as compared with MPA that has relative DF values of 1.
Almost all the absolute DFs indicated that, in contrast to MPA, drospirenone was characterized by a favorable dissociation of uterine and mammary gland effects in vivo (Table 2). With the exception of the INDO to LTF ratio, all absolute DFs for drospirenone were significantly greater than 1, whereas the corresponding DFs for MPA were not significantly different from 1 or were even lower than 1. Whereas drospirenone had uterine activity at doses lower than those that were efficacious in the mammary gland, MPA was equally active in the uterus and the mammary gland at the same doses. The high proliferative activity of MPA in the mammary gland was striking, as indicated at the functional level by the DFs derived by dividing BrdU incorporation in mammary epithelial cells by maintenance of pregnancy or inhibition of uterine epithelial cell proliferation, respectively. At the molecular level, proliferative mammary gland activity was reflected by the DFs derived by dividing cyclin D1 (or RANKL) induction by maintenance of pregnancy.
In addition to absolute DFs, we also calculated relative DFs that allowed direct comparison of the tissue selectivity of MPA and drospirenone (Table 2). Comparing the relative DFs measuring proliferative activity, drospirenone showed significantly greater dissociation (6- to 33-fold) of uterine vs. proliferative mammary gland effects than MPA.
Discussion
A possible association between the use of menopausal HT and an elevated risk for breast cancer has been discussed for decades (25). Over the last few years, several randomized trials have reported an elevated risk for breast cancer related to combined estrogen-progestin use (9,11). Treatment with CEE only (10) or estrogen/micronized natural progesterone (26) appears not to elevate risk for breast cancer. Given that synthetic progestins have distinct characteristics (15), a major unanswered question is whether different estrogen/synthetic progestin combinations have similar or diverse effects in breast tissue.
The results of the present study using a mouse model indicate that MPA and drospirenone, two synthetic progestins widely used in hormone therapy, possess clear differences in activity at two major progesterone-dependent target organs. MPA demonstrated activity in the mammary gland at the same doses that were required for uterine activity. In contrast, drospirenone was characterized by a dissociation of uterine and mammary gland effects showing uterine activity at doses lower than those required for appreciable mammary gland activity. These conclusions hold true for almost all the readout paradigms analyzed. The single exception was the absolute DF that measured inhibition of INDO expression in the mammary gland vs. inhibition of LTF expression in the uterus, in which the DF for drospirenone was lower than 1. In an attempt to simplify analysis of the effects of progestins in various target organs, recent research has focused on changes in marker gene expression rather than laborious analyses of morphological or functional parameters (23). However, to focus on gene expression alone may lead to oversimplification. Morphological changes such as ductal side branching or functional changes such as cellular proliferation are complex processes that often cannot be represented by changes in RNA-based expression levels of single genes, which is the rationale for their inclusion in the current study. We selected the INDO gene for analysis because this gene is reliably inducible by estradiol in normal mammary gland tissue, and progestins were capable of inhibiting its expression (23). As demonstrated by the relative DF that measures INDO vs. LTF repression, drospirenone (with an absolute DF lower than 1) showed a significantly greater dissociation (4.5-fold) than MPA in this readout paradigm.
Another interesting observation was that ID50 values for the inhibition of estradiol-induced gene expression by both progestins were clearly lower than ED50 values for stimulatory progestin effects. The explanation for this outcome is currently unknown. One possible explanation may be that different molecular mechanisms contribute to the stimulatory and inhibitory effects of progestins. We therefore consider that it is essential, when comparing progestins, to contrast stimulatory activities in the mammary gland with stimulatory activities in the uterus and to do the same with respective inhibitory activities. This explains why we selected the maintenance of pregnancy assay as a uterine readout for progestin activity because it reflects an agonistic progestin action like the majority of mammary gland readouts that we analyzed (side branching, epithelial cell proliferation, cyclin D1, and RANKL induction). In addition, this readout closely reflects progestin activity and thus serves as an ideal normalization reference. In contrast, the inhibition of uterine epithelial cell proliferation can be influenced, for example, by the androgenic side effects of progestins (27) and thus does not necessarily reflect pure progestin activity.
In light of various studies describing an elevated risk for breast cancer after combined estrogen-progestin therapy (9,11), we think that emphasis should be placed on direct measurements of the proliferative effects of progestins in the mammary gland. In general, treatment times in clinical studies are too short to assume that the increase in breast cancer risk during HT is due to the induction of new tumors by estrogen-progestin combinations (11). Instead, it is more likely that progestins, by stimulating epithelial cell proliferation, provoke the division of premalignant cells and promote the growth of preexisting tumors (for review see Refs. 11 and 15). For these reasons, we decided to analyze the tissue selectivity of progestins based not only on morphological changes such as ductal side branching. In addition, we directly measured proliferation of mammary epithelial cells using BrdU incorporation as well as expression of marker genes such as cyclin D1 and RANKL that reflect proliferation on a molecular level. The main finding of these experiments was that MPA has high mitogenic activity in the mammary gland, as evidenced by the four absolute DFs that characterize proliferative activity on a functional and molecular level. Drospirenone showed significantly greater dissociation (6- to 33-fold) of uterine vs. proliferative mammary gland effects when compared with MPA.
The explanation for the favorable dissociation of uterine vs. proliferative mammary gland effects seen with drospirenone, but not with MPA, is uncertain. One explanation could be that different PR ligands induce different PR conformations that have variable impacts on PR/cofactor interaction and thus gene expression in PR target tissues. If this is correct, it would not be surprising that MPA and drospirenone have diverse effects, even through they act on the same receptor. However, a second level of complexity must also be taken into account in considering these two progestins: both MPA and drospirenone bind to and activate the PR, but they also exert additional activities on other nuclear receptors. Notably, MPA has significant glucocorticoid activity (14), whereas drospirenone is inactive at the GR but behaves as strong antimineralocorticoid (15). It is tempting to speculate that the glucocorticoid activity of MPA that is lacking for drospirenone provoked the enhanced mitogenic activity of MPA in the mammary gland under our experimental conditions. Two different mouse models support this speculation and a role of GR as an activator of mammary epithelial cell proliferation. Tissue-specific knockout mice, which lose the GR protein in mammary epithelial cells in late stages of pregnancy, showed reduced cell proliferation during pregnancy-associated lobuloalveolar development (18). Knock-in mice expressing a mutant GR lacking transcriptional activity show reduced mammary epithelial cell proliferation and compromised ductal side branching during mammary gland development (28). Furthermore, mineralocorticoid receptor expression has been demonstrated in secretory epithelial cells in later stages of mammary gland development (29) (supplemental Fig. 1, published as supplemental data on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org), and it has been suggested that the mineralocorticoid receptor can compensate for GR function within the mammary gland (29). From these observations, it may be hypothesized that both glucocorticoid and mineralocorticoid activity enhance the proliferation of mammary epithelial cells. Based on this theory, the antimineralocorticoid activity of drospirenone could contribute to its reduced proliferative activity in the mammary gland when compared with MPA. Further experiments using genetically modified mice are required to test this hypothesis. Interestingly, progesterone, which activates the same nuclear receptor panel as drospirenone, behaved similarly to drospirenone in our mouse model (Otto, C., unpublished results).
Taken together, the results of our study demonstrate that not all synthetic progestins are the same. In comparison with MPA, drospirenone showed a superior in vivo profile with regard to dissociation of uterine and proliferative mammary gland effects. Drospirenone, but not MPA, appears to offer a safety window between uterine progestogenic activity and proliferative mammary gland effects. Further studies examining different estradiol/synthetic progestin regimens for hormone therapy are warranted to judge whether these preclinical findings may be applicable to humans.
Supplementary Material
Acknowledgments
The authors are grateful to A. Seltz, G. Schwarz, and S. Troelenberg (all from Bayer Schering Pharma AG) for help with ovariectomy, immunohistochemical staining, and quantitative RT-PCR. In addition, we thank Professor Orla Conneely (Baylor College, Houston, TX); Drs. H. Blode, D. Brittain, T. Faustmann, M. Schaefers, and T. Wintermantel (all from Bayer Schering Pharma AG); and Mr. B. Wolvey (Medical Marketing Service, Parexel, Uxbridge, UK) for fruitful discussions and critical reading of the manuscript.
Footnotes
Disclosure Summary: C.O., I.F., H.A., M.K., A.W., K.P., R.V., and K.-H.F. are all employees of Bayer Schering Pharma AG.
First Published Online April 17, 2008
Abbreviations: BrdU, 5′-Bromo-2′-deoxyuridine; CEE, conjugated equine estrogen; DF, dissociation factor; ER, estrogen receptor; GR, glucocorticoid receptor; HT, hormone therapy; INDO, indoleamine-pyrrole 2,3 dioxygenase; LTF, lactotransferrin; MPA, medroxyprogesterone acetate; PR, progesterone receptor; RANKL, receptor activator of nuclear factor-κΒ ligand; WHI, Women’s Health Initiative.
References
- Hulka BS, Chambless LE, Kaufman DG, Fowler Jr WC, Greenberg BG 1982 Protection against endometrial carcinoma by combination-product oral contraceptives. JAMA 247:475–477 [PubMed] [Google Scholar]
- Masters JR, Drife JO, Scarisbrick JJ 1977 Cyclic variation of DNA synthesis in human breast epithelium. J Natl Cancer Inst 58:1263–1265 [DOI] [PubMed] [Google Scholar]
- Said TK, Conneely O, Medina D, O'Malley BW, Lydon JP 1997 Progesterone, in addition to estrogen, induces cyclin D1 expression in the murine mammary epithelial cell, in vivo. Endocrinology 138:3933–3939 [DOI] [PubMed] [Google Scholar]
- Hofseth LJ, Raafat AM, Osuch JR, Pathak DR, Slomski CA, Haslam SZ 1999 Hormone replacement therapy with estrogen or estrogen plus medroxyprogesterone acetate is associated with increased epithelial proliferation in the normal postmenopausal breast. J Clin Endocrinol Metab 84:4559–4565 [DOI] [PubMed] [Google Scholar]
- Clarke RB, Howell A, Potten CS, Anderson E 1997 Dissociation between steroid receptor expression and cell proliferation in the human breast. Cancer Res 57:4987–4991 [PubMed] [Google Scholar]
- Mulac-Jericevic B, Lydon JP, DeMayo FJ, Conneely OM 2003 Defective mammary gland morphogenesis in mice lacking the progesterone receptor B isoform. Proc Natl Acad Sci USA 100:9744–9749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantl V, Stamp G, Andrews A, Rosewell I, Dickson C 1995 Mice lacking cyclin D1 are small and show defects in eye and mammary gland development. Genes Dev 9:2364–2372 [DOI] [PubMed] [Google Scholar]
- Anderson E, Clarke RB 2004 Steroid receptors and cell cycle in normal mammary epithelium. J Mammary Gland Biol Neoplasia 9:3–13 [DOI] [PubMed] [Google Scholar]
- Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J 2002 Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA 288:321–333 [DOI] [PubMed] [Google Scholar]
- Writing Group for the Women’s Health Initiative Investigators 2004 Effects of conjugated equine estrogen in postmenopausal women with hysterectomy. JAMA 291:1701–1712 [DOI] [PubMed] [Google Scholar]
- Collins JA, Blake JM, Crosignani PG 2005 Breast cancer risk with postmenopausal hormonal treatment. Hum Reprod Update 11:545–560 [DOI] [PubMed] [Google Scholar]
- Klaiber EL, Vogel W, Rako S 2005 A critique of the women’s health initiative hormone therapy study. Fertil Steril 84:1589–1601 [DOI] [PubMed] [Google Scholar]
- Turgeon JL, Carr MC, Maki P, Mendelsohn ME, Wise PM 2006 Complex actions of sex steroids in adipose tissue, the cardiovascular system, and brain: insights from basic science and clinical studies. Endocr Rev 27:575–605 [DOI] [PubMed] [Google Scholar]
- Druckmann R 2003 Progestins and their effects on the breast. Maturitas 46:59–69 [DOI] [PubMed] [Google Scholar]
- Sitruk-Ware R 2004 New progestogens. A review of their effects in perimenopausal and postmenopausal women. Drugs Aging 21:865–883 [DOI] [PubMed] [Google Scholar]
- Simoncini T, Mannella P, Fornari L, Caruso A, Willis MY, Garibaldi S, Baldacci C, Genazzani AR 2004 Differential signal transduction of progesterone and medroxyprogesterone acetate in human endothelial cells. Endocrinology 145:5745–5756 [DOI] [PubMed] [Google Scholar]
- Arias-Loza PA, Hu K, Schäfer A, Bauersachs J, Quaschning T, Galle J, Jazbutyte V, Neyses L, Ertl G, Fritzemeier KH, Hegele-Hartung C, Pelzer T 2006 Medroxyprogesterone acetate but not drospirenone ablates the protective function of 17β-estradiol in aldosterone salt-treated rats. Hypertension 48:994–1001 [DOI] [PubMed] [Google Scholar]
- Wintermantel TM, Bock D, Fleig V, Greiner EF, Schütz G 2005 The epithelial glucocorticoid receptor is required for the normal timing of cell proliferation during mammary lobuloalveolar development but is dispensable for milk production. Mol Endocrinol 19:340–349 [DOI] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N 1987 Single-step method for RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162:156–159 [DOI] [PubMed] [Google Scholar]
- Efron B 1979 Bootstrap methods: another look at the jackknife. Ann Stat 7:1–26 [Google Scholar]
- Efron B, Tibshirani R 1986 Bootstrap methods for standard errors, confidence interval, and other measures of statistical accuracy. Stat Sci 1:54–75 [Google Scholar]
- Imagawa W, Yang J, Guzman R, Nandi S 1994 Control of mammary gland development. In: Knobil E, Neill JD, eds. The physiology of reproduction. New York: Raven Press; 1033–1063 [Google Scholar]
- Crabtree JS, Zhang X, Peano BJ, Zhang Z, Winneker RC, Harris HA 2006 Development of a mouse model of mammary gland versus uterus tissue selectivity using estrogen- and progesterone-regulated gene markers. J Steroid Biochem Mol Biol 101:11–21 [DOI] [PubMed] [Google Scholar]
- Kurita T, Lee K-J, Cooke PS, Lydon JP, Cunha GR 2000 Paracrine regulation of epithelial progesterone receptor and lactoferrin by progesterone in the mouse uterus. Biol Reprod 62:831–838 [DOI] [PubMed] [Google Scholar]
- Bush TL, Whiteman M, Flaws JA 2001 Hormone replacement therapy and breast cancer: a qualitative review. Obstet Gynecol 98:498–508 [DOI] [PubMed] [Google Scholar]
- Fournier A, Berrino F, Clavel-Chapelon F 2008 Unequal risks for breast cancer associated with different hormone replacement therapies: results from the E3N cohort study. Breast Cancer Res Treat 107:103–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang H, Sahlin L, Masironi B, Eriksson E, Linden Hirschberg A 2007 Effects of testosterone treatment on endometrial proliferation in postmenopausal women. J Clin Endocrinol Metab 92:2169–2175 [DOI] [PubMed] [Google Scholar]
- Reichardt HM, Horsch K, Groene H-J, Kolbus A, Beug H, Hynes N, Schuetz G 2001 Mammary gland development and lactation are controlled by different glucocorticoid receptor activities. Eur J Endocrinol 145:519–527 [DOI] [PubMed] [Google Scholar]
- Kingsley-Kallesen M, Mukhopadhyay SS, Wyszomierski SL, Schanler S, Schütz G, Rosen JM 2002 The mineralocorticoid receptor may compensate for the loss of the glucocorticoid receptor at specific stages of mammary gland development. Mol Endocrinol 16:2008–2018 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.