Abstract
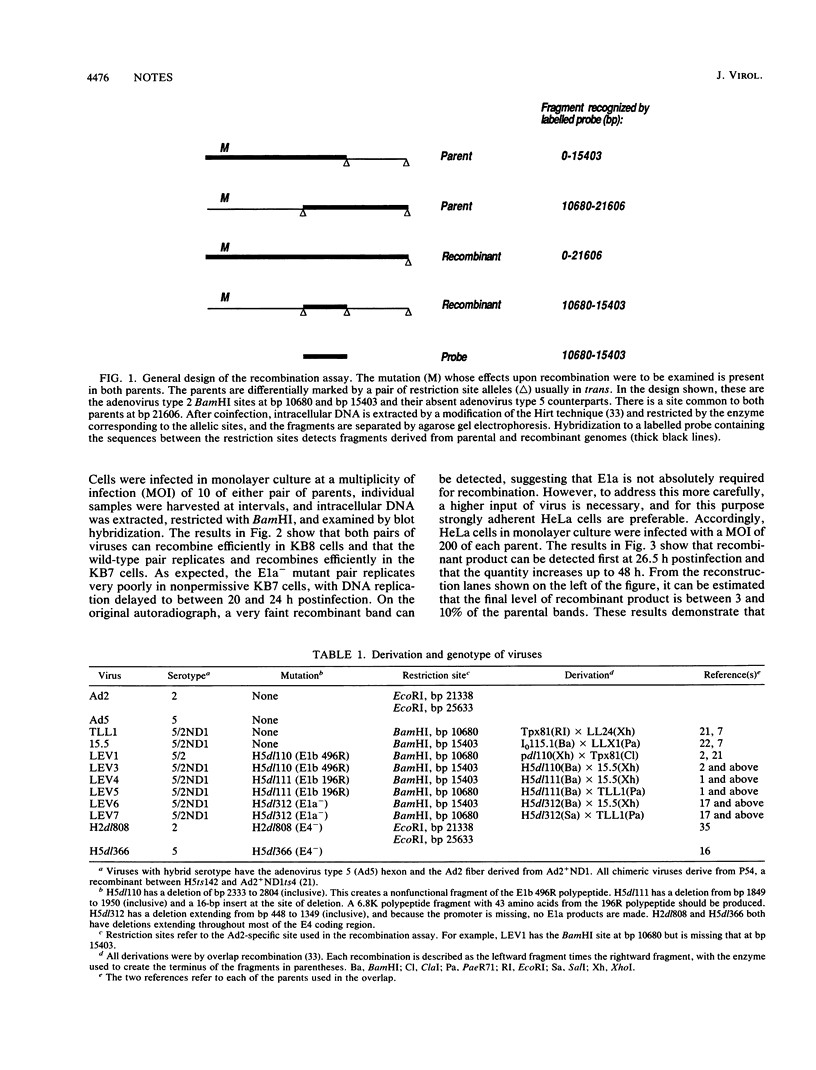
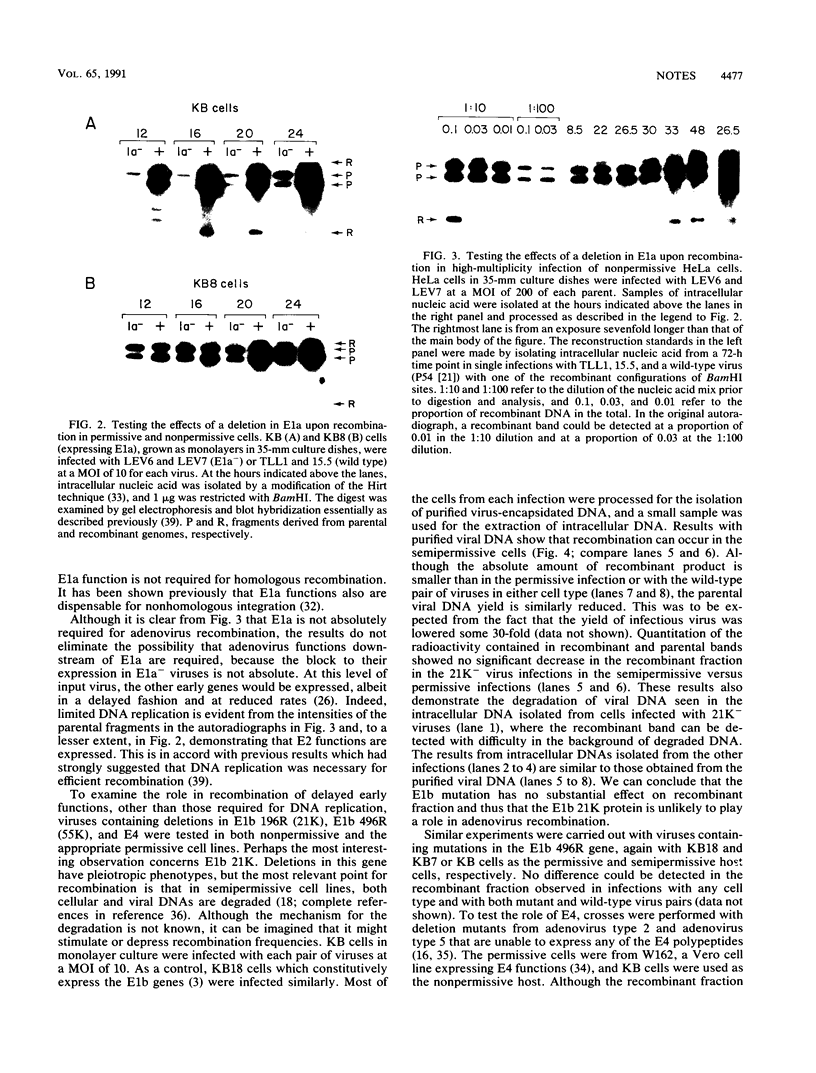
To investigate whether early genes other than those involved directly in DNA replication are required for efficient adenovirus recombination, pairs of viruses with deletions in E1a, E1b 496R, E1b 196R, or E4 and containing differing restriction site markers were used to infect both permissive and non- or semipermissive cells. Recombination was assayed among intracellular and extracellular genomes by restriction digestion and blot hybridization. Recombination was delayed in infections of nonpermissive cells with E1a- viruses until a time consistent with the late onset of DNA replication characteristic of the cell type. This shows that E1a expression is not absolutely required for adenovirus recombination. Similar tests with deletion mutations in E1b and E4 also show that these genes are not required for efficient recombination. Taken together with earlier results showing that recombination depends on DNA replication, it is likely that adenovirus recombination is a consequence of cellular repair functions acting on the substrates produced by replication.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babiss L. E., Fisher P. B., Ginsberg H. S. Effect on transformation of mutations in the early region 1b-encoded 21- and 55-kilodalton proteins of adenovirus 5. J Virol. 1984 Nov;52(2):389–395. doi: 10.1128/jvi.52.2.389-395.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babiss L. E., Ginsberg H. S. Adenovirus type 5 early region 1b gene product is required for efficient shutoff of host protein synthesis. J Virol. 1984 Apr;50(1):202–212. doi: 10.1128/jvi.50.1.202-212.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babiss L. E., Young C. S., Fisher P. B., Ginsberg H. S. Expression of adenovirus E1a and E1b gene products and the Escherichia coli XGPRT gene in KB cells. J Virol. 1983 May;46(2):454–465. doi: 10.1128/jvi.46.2.454-465.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball L. A. High-frequency homologous recombination in vaccinia virus DNA. J Virol. 1987 Jun;61(6):1788–1795. doi: 10.1128/jvi.61.6.1788-1795.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk A. J. Adenovirus promoters and E1A transactivation. Annu Rev Genet. 1986;20:45–79. doi: 10.1146/annurev.ge.20.120186.000401. [DOI] [PubMed] [Google Scholar]
- Blackwell T. K., Alt F. W. Mechanism and developmental program of immunoglobulin gene rearrangement in mammals. Annu Rev Genet. 1989;23:605–636. doi: 10.1146/annurev.ge.23.120189.003133. [DOI] [PubMed] [Google Scholar]
- Brunet L. J., Babiss L. E., Young C. S., Mills D. R. Mutations in the adenovirus major late promoter: effects on viability and transcription during infection. Mol Cell Biol. 1987 Mar;7(3):1091–1100. doi: 10.1128/mcb.7.3.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challberg M. D., Kelly T. J. Animal virus DNA replication. Annu Rev Biochem. 1989;58:671–717. doi: 10.1146/annurev.bi.58.070189.003323. [DOI] [PubMed] [Google Scholar]
- Chen M., Horwitz M. S. Dissection of functional domains of adenovirus DNA polymerase by linker-insertion mutagenesis. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6116–6120. doi: 10.1073/pnas.86.16.6116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. H., Stuart D., McFadden G. High levels of genetic recombination among cotransfected plasmid DNAs in poxvirus-infected mammalian cells. J Virol. 1988 Feb;62(2):367–375. doi: 10.1128/jvi.62.2.367-375.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint S. J., Berget S. M., Sharp P. A. Characterization of single-stranded viral DNA sequences present during replication of adenovirus types 2 and 5. Cell. 1976 Dec;9(4 Pt 1):559–571. doi: 10.1016/0092-8674(76)90038-6. [DOI] [PubMed] [Google Scholar]
- Folger K. R., Thomas K., Capecchi M. R. Nonreciprocal exchanges of information between DNA duplexes coinjected into mammalian cell nuclei. Mol Cell Biol. 1985 Jan;5(1):59–69. doi: 10.1128/mcb.5.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folger K. R., Wong E. A., Wahl G., Capecchi M. R. Patterns of integration of DNA microinjected into cultured mammalian cells: evidence for homologous recombination between injected plasmid DNA molecules. Mol Cell Biol. 1982 Nov;2(11):1372–1387. doi: 10.1128/mcb.2.11.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freimuth P. I., Ginsberg H. S. Codon insertion mutants of the adenovirus terminal protein. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7816–7820. doi: 10.1073/pnas.83.20.7816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbert D. N., Cutt J. R., Shenk T. Adenovirus early region 4 encodes functions required for efficient DNA replication, late gene expression, and host cell shutoff. J Virol. 1985 Oct;56(1):250–257. doi: 10.1128/jvi.56.1.250-257.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones N., Shenk T. Isolation of adenovirus type 5 host range deletion mutants defective for transformation of rat embryo cells. Cell. 1979 Jul;17(3):683–689. doi: 10.1016/0092-8674(79)90275-7. [DOI] [PubMed] [Google Scholar]
- Lai Fatt R. B., Mak S. Mapping of an adenovirus function involved in the inhibition of DNA degradation. J Virol. 1982 Jun;42(3):969–977. doi: 10.1128/jvi.42.3.969-977.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchlinsky M. Intramolecular homologous recombination in cells infected with temperature-sensitive mutants of vaccinia virus. J Virol. 1989 May;63(5):2030–2035. doi: 10.1128/jvi.63.5.2030-2035.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin N., Delsert C., Klessig D. F. Mutations that affect phosphorylation of the adenovirus DNA-binding protein alter its ability to enhance its own synthesis. J Virol. 1989 Dec;63(12):5228–5237. doi: 10.1128/jvi.63.12.5228-5237.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munz P. L., Young C. S. Polarity in adenovirus recombination. Virology. 1984 Jun;135(2):503–514. doi: 10.1016/0042-6822(84)90204-6. [DOI] [PubMed] [Google Scholar]
- Munz P. L., Young C., Young C. S. The genetic analysis of adenovirus recombination in triparental and superinfection crosses. Virology. 1983 Apr 30;126(2):576–586. doi: 10.1016/s0042-6822(83)80014-2. [DOI] [PubMed] [Google Scholar]
- Neale G. A., Kitchingman G. R. Conserved region 3 of the adenovirus type 5 DNA-binding protein is important for interaction with single-stranded DNA. J Virol. 1990 Feb;64(2):630–638. doi: 10.1128/jvi.64.2.630-638.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roovers D. J., Overman P. F., Chen X. Q., Sussenbach J. S. Linker mutation scanning of the genes encoding the adenovirus type 5 terminal protein precursor and DNA polymerase. Virology. 1991 Jan;180(1):273–284. doi: 10.1016/0042-6822(91)90032-7. [DOI] [PubMed] [Google Scholar]
- Rosenstraus M. J., Chasin L. A. Separation of linked markers in Chinese hamster cell hybrids: mitotic recombination is not involved. Genetics. 1978 Dec;90(4):735–760. doi: 10.1093/genetics/90.4.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenk T., Jones N., Colby W., Fowlkes D. Functional analysis of adenovirus-5 host-range deletion mutants defective for transformation of rat embryo cells. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):367–375. doi: 10.1101/sqb.1980.044.01.041. [DOI] [PubMed] [Google Scholar]
- Takemori N. Genetic studies with tumorigenic adenoviruses. 3. Recombination in adenovirus type 12. Virology. 1972 Jan;47(1):157–167. doi: 10.1016/0042-6822(72)90249-8. [DOI] [PubMed] [Google Scholar]
- Thomas B. J., Rothstein R. Sex, maps, and imprinting. Cell. 1991 Jan 11;64(1):1–3. doi: 10.1016/0092-8674(91)90199-9. [DOI] [PubMed] [Google Scholar]
- Tsurimoto T., Melendy T., Stillman B. Sequential initiation of lagging and leading strand synthesis by two different polymerase complexes at the SV40 DNA replication origin. Nature. 1990 Aug 9;346(6284):534–539. doi: 10.1038/346534a0. [DOI] [PubMed] [Google Scholar]
- Van Doren K., Hanahan D., Gluzman Y. Infection of eucaryotic cells by helper-independent recombinant adenoviruses: early region 1 is not obligatory for integration of viral DNA. J Virol. 1984 May;50(2):606–614. doi: 10.1128/jvi.50.2.606-614.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkert F. C., Young C. S. The genetic analysis of recombination using adenovirus overlapping terminal DNA fragments. Virology. 1983 Feb;125(1):175–193. doi: 10.1016/0042-6822(83)90072-7. [DOI] [PubMed] [Google Scholar]
- Weinberg D. H., Ketner G. A cell line that supports the growth of a defective early region 4 deletion mutant of human adenovirus type 2. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5383–5386. doi: 10.1073/pnas.80.17.5383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg D. H., Ketner G. Adenoviral early region 4 is required for efficient viral DNA replication and for late gene expression. J Virol. 1986 Mar;57(3):833–838. doi: 10.1128/jvi.57.3.833-838.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White E., Stillman B. Expression of adenovirus E1B mutant phenotypes is dependent on the host cell and on synthesis of E1A proteins. J Virol. 1987 Feb;61(2):426–435. doi: 10.1128/jvi.61.2.426-435.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. F., Ustacelebi S. Complementation and recombination with temperature-sensitive mutants of adenovirus type 5. J Gen Virol. 1971 Nov;13(2):345–348. doi: 10.1099/0022-1317-13-2-345. [DOI] [PubMed] [Google Scholar]
- Williams J., Grodzicker T., Sharp P., Sambrook J. Adenovirus recombination: physical mapping of crossover events. Cell. 1975 Feb;4(2):113–119. doi: 10.1016/0092-8674(75)90117-8. [DOI] [PubMed] [Google Scholar]
- Young C. S., Cachianes G., Munz P., Silverstein S. Replication and recombination in adenovirus-infected cells are temporally and functionally related. J Virol. 1984 Sep;51(3):571–577. doi: 10.1128/jvi.51.3.571-577.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young C. S., Silverstein S. J. The kinetics of adenovirus recombination in homotypic and heterotypic genetic crosses. Virology. 1980 Mar;101(2):503–515. doi: 10.1016/0042-6822(80)90464-x. [DOI] [PubMed] [Google Scholar]