Abstract
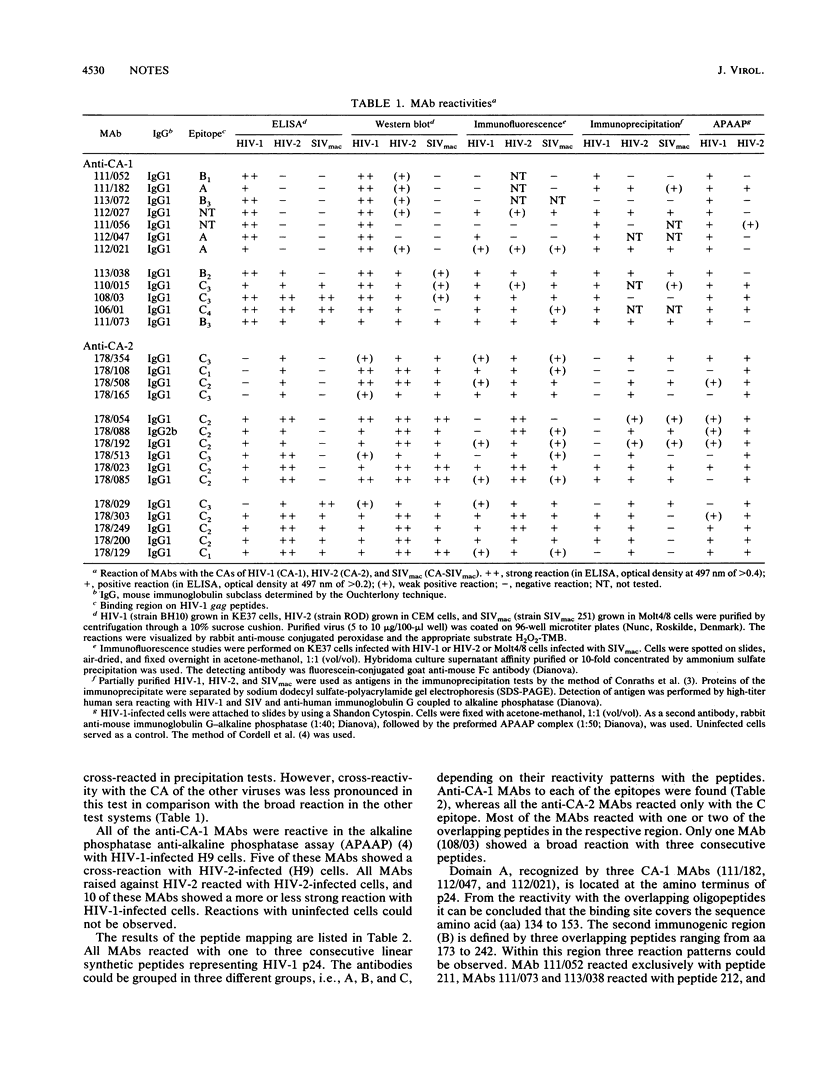
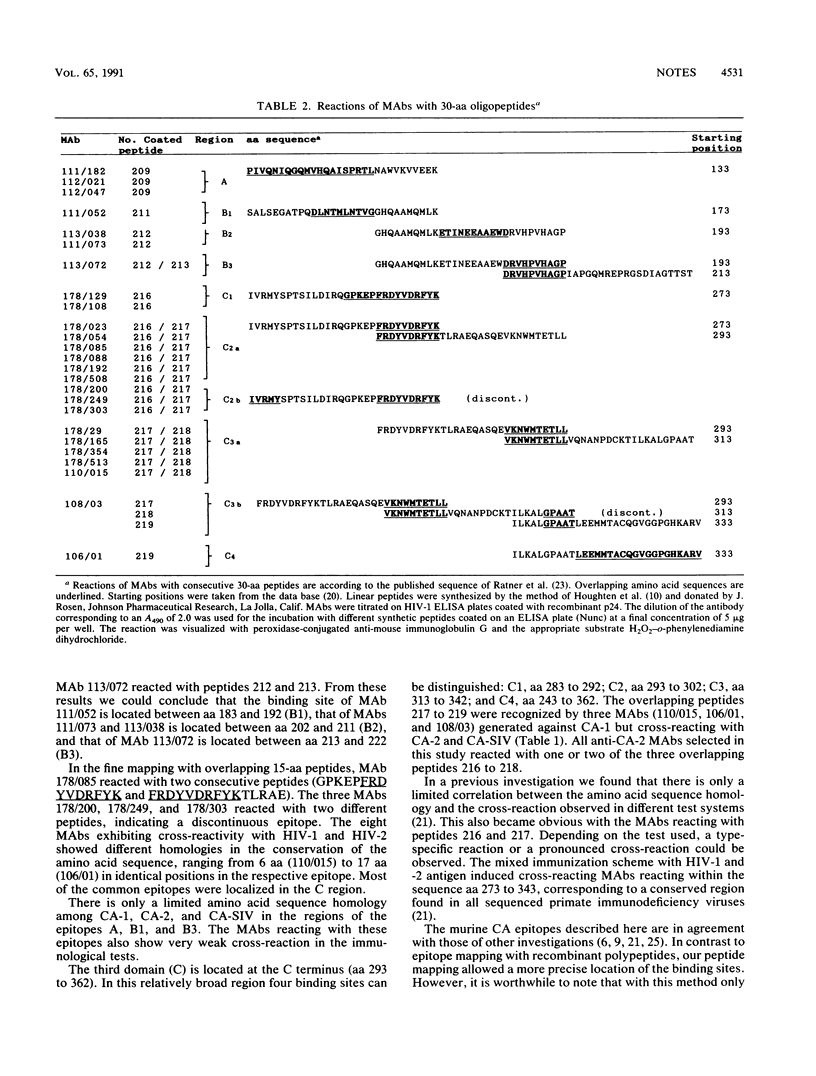
Monoclonal antibodies (MAbs) raised against the core proteins of human immunodeficiency virus type 1 (HIV-1; laboratory strain HTLV-IIIB) and HIV-2 (strain ROD) were investigated in a variety of tests, e.g., enzyme-linked immunosorbent assay (ELISA), immunostaining of Western immunoblots, immunofluorescence, immunoprecipitation, and alkaline phosphatase anti-alkaline phosphatase assay. The MAbs were grouped according to their cross-reactions. Seven HIV-1-specific MAbs reacted exclusively with HIV-1, and five showed cross-reactivity with HIV-2 and simian immunodeficiency virus of macaques in ELISA. Four of the 15 MAbs against HIV-2 reacted only with the HIV-2 protein p26. Six showed cross-reactivity with HIV-1, and five showed a broad reaction with all three viruses. Overlapping 30-amino-acid-long peptides derived from the p24 protein sequence of HIV-1 were used in an epitope-mapping system. Three different immunogenic regions (A, B, and C) could be defined. Specific regions where anti-HIV-1 and -HIV-2 MAbs cross-reacted were mapped with shorter oligopeptides.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bröker M., Jahn G., Küpper H. A. Immunoreactivity of human immunodeficiency virus (HIV-1) envelope polypeptides expressed in Escherichia coli. Behring Inst Mitt. 1988 Apr;(82):338–348. [PubMed] [Google Scholar]
- Caruso A., Terlenghi L., Ceccarelli R., Verardi R., Foresti I., Scura G., Manca N., Bonfanti C., Turano A. Liquid competition radioimmunoassay for the detection and quantitation of the HIV p24. J Virol Methods. 1987 Sep;17(3-4):199–210. doi: 10.1016/0166-0934(87)90130-3. [DOI] [PubMed] [Google Scholar]
- Conraths F. J., Pauli G., Ludwig H. Monoclonal antibodies directed against a 130K glycoprotein of bovine herpesvirus 2 cross-react with glycoprotein B of herpes simplex virus. Virus Res. 1988 Apr;10(1):53–63. doi: 10.1016/0168-1702(88)90057-3. [DOI] [PubMed] [Google Scholar]
- Cordell J. L., Falini B., Erber W. N., Ghosh A. K., Abdulaziz Z., MacDonald S., Pulford K. A., Stein H., Mason D. Y. Immunoenzymatic labeling of monoclonal antibodies using immune complexes of alkaline phosphatase and monoclonal anti-alkaline phosphatase (APAAP complexes). J Histochem Cytochem. 1984 Feb;32(2):219–229. doi: 10.1177/32.2.6198355. [DOI] [PubMed] [Google Scholar]
- Ferns R. B., Partridge J. C., Spence R. P., Hunt N., Tedder R. S. Epitope location of 13 anti-gag HIV-1 monoclonal antibodies using oligopeptides and their cross reactivity with HIV-2. AIDS. 1989 Dec;3(12):829–834. doi: 10.1097/00002030-198912000-00008. [DOI] [PubMed] [Google Scholar]
- Ferns R. B., Tedder R. S., Donoghue J. L. Comparison of a monoclonal anti-HIV 1 gag solid phase with a polyclonal anti-HIV solid phase for detecting anti-HIV 1 in a competition ELISA. J Virol Methods. 1988 Jun;20(2):143–153. doi: 10.1016/0166-0934(88)90148-6. [DOI] [PubMed] [Google Scholar]
- Ferns R. B., Tedder R. S., Weiss R. A. Characterization of monoclonal antibodies against the human immunodeficiency virus (HIV) gag products and their use in monitoring HIV isolate variation. J Gen Virol. 1987 Jun;68(Pt 6):1543–1551. doi: 10.1099/0022-1317-68-6-1543. [DOI] [PubMed] [Google Scholar]
- Gelderblom H. R., Hausmann E. H., Ozel M., Pauli G., Koch M. A. Fine structure of human immunodeficiency virus (HIV) and immunolocalization of structural proteins. Virology. 1987 Jan;156(1):171–176. doi: 10.1016/0042-6822(87)90449-1. [DOI] [PubMed] [Google Scholar]
- Hinkula J., Rosen J., Sundqvist V. A., Stigbrand T., Wahren B. Epitope mapping of the HIV-1 gag region with monoclonal antibodies. Mol Immunol. 1990 May;27(5):395–403. doi: 10.1016/0161-5890(90)90163-t. [DOI] [PubMed] [Google Scholar]
- Houghten R. A. General method for the rapid solid-phase synthesis of large numbers of peptides: specificity of antigen-antibody interaction at the level of individual amino acids. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5131–5135. doi: 10.1073/pnas.82.15.5131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikuta K., Morita C., Miyake S., Ito T., Okabayashi M., Sano K., Nakai M., Hirai K., Kato S. Expression of human immunodeficiency virus type 1 (HIV-1) gag antigens on the surface of a cell line persistently infected with HIV-1 that highly expresses HIV-1 antigens. Virology. 1989 Jun;170(2):408–417. doi: 10.1016/0042-6822(89)90431-5. [DOI] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Lange J. M., Paul D. A., Huisman H. G., de Wolf F., van den Berg H., Coutinho R. A., Danner S. A., van der Noordaa J., Goudsmit J. Persistent HIV antigenaemia and decline of HIV core antibodies associated with transition to AIDS. Br Med J (Clin Res Ed) 1986 Dec 6;293(6560):1459–1462. doi: 10.1136/bmj.293.6560.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent A. G., Krust B., Rey M. A., Montagnier L., Hovanessian A. G. Cell surface expression of several species of human immunodeficiency virus type 1 major core protein. J Virol. 1989 Sep;63(9):4074–4078. doi: 10.1128/jvi.63.9.4074-4078.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leis J., Baltimore D., Bishop J. M., Coffin J., Fleissner E., Goff S. P., Oroszlan S., Robinson H., Skalka A. M., Temin H. M. Standardized and simplified nomenclature for proteins common to all retroviruses. J Virol. 1988 May;62(5):1808–1809. doi: 10.1128/jvi.62.5.1808-1809.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathiesen T., Broliden P. A., Rosen J., Wahren B. Mapping of IgG subclass and T-cell epitopes on HIV proteins by synthetic peptides. Immunology. 1989 Aug;67(4):453–459. [PMC free article] [PubMed] [Google Scholar]
- Miller M. D., Lord C. I., Stallard V., Mazzara G. P., Letvin N. L. The gag-specific cytotoxic T lymphocytes in rhesus monkeys infected with the simian immunodeficiency virus of macaques. J Immunol. 1990 Jan 1;144(1):122–128. [PubMed] [Google Scholar]
- Mills K. H., Kitchin P. A., Mahon B. P., Barnard A. L., Adams S. E., Kingsman S. M., Kingsman A. J. HIV p24-specific helper T cell clones from immunized primates recognize highly conserved regions of HIV-1. J Immunol. 1990 Mar 1;144(5):1677–1683. [PubMed] [Google Scholar]
- Minassian A. A., Kalyanaraman V. S., Gallo R. C., Popovic M. Monoclonal antibodies against human immunodeficiency virus (HIV) type 2 core proteins: cross-reactivity with HIV type 1 and simian immunodeficiency virus. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6939–6943. doi: 10.1073/pnas.85.18.6939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedrig M., Hinkula J., Weigelt W., L'age-Stehr J., Pauli G., Rosen J., Wahren B. Epitope mapping of monoclonal antibodies against human immunodeficiency virus type 1 structural proteins by using peptides. J Virol. 1989 Aug;63(8):3525–3528. doi: 10.1128/jvi.63.8.3525-3528.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedrig M., Rabanus J. P., L'Age Stehr J., Gelderblom H. R., Pauli G. Monoclonal antibodies directed against human immunodeficiency virus (HIV) gag proteins with specificity for conserved epitopes in HIV-1, HIV-2 and simian immunodeficiency virus. J Gen Virol. 1988 Aug;69(Pt 8):2109–2114. doi: 10.1099/0022-1317-69-8-2109. [DOI] [PubMed] [Google Scholar]
- Schrier R. D., Gnann J. W., Jr, Landes R., Lockshin C., Richman D., McCutchan A., Kennedy C., Oldstone M. B., Nelson J. A. T cell recognition of HIV synthetic peptides in a natural infection. J Immunol. 1989 Feb 15;142(4):1166–1176. [PubMed] [Google Scholar]
- Spence R. P., Jarvill W. M., Ferns R. B., Tedder R. S., Parker D. The cloning and expression in Escherichia coli of sequences coding for p24, the core protein of human immunodeficiency virus, and the use of the recombinant protein in characterizing a panel of monoclonal antibodies against the viral p24 protein. J Gen Virol. 1989 Nov;70(Pt 11):2843–2851. doi: 10.1099/0022-1317-70-11-2843. [DOI] [PubMed] [Google Scholar]
- Sternberg M. J., Barton G. J., Zvelebil M. J., Cookson J., Coates A. R. Prediction of antigenic determinants and secondary structures of the major AIDS virus proteins. FEBS Lett. 1987 Jun 29;218(2):231–237. doi: 10.1016/0014-5793(87)81052-9. [DOI] [PubMed] [Google Scholar]
- Sutjipto S., Kodama T., Yee J., Gettie A., Jennings M., Desrosiers R. C., Marx P. A. Characterization of monoclonal antibodies that distinguish simian immunodeficiency virus isolates from each other and from human immunodeficiency virus types 1 and 2. J Gen Virol. 1990 Jan;71(Pt 1):247–249. doi: 10.1099/0022-1317-71-1-247. [DOI] [PubMed] [Google Scholar]
- Wahren B., Chiodi F., Ljunggren K., Putney S., Kurth R., Gallo R. C., Fenyö E. M. B and T cell reactivities after immunization of macaques with HIV subcomponents. AIDS Res Hum Retroviruses. 1988 Jun;4(3):199–210. doi: 10.1089/aid.1988.4.199. [DOI] [PubMed] [Google Scholar]
- Wahren B., Rosen J., Sandström E., Mathiesen T., Modrow S., Wigzell H. HIV-1 peptides induce a proliferative response in lymphocytes from infected persons. J Acquir Immune Defic Syndr. 1989;2(5):448–456. [PubMed] [Google Scholar]
- Zvelebil M. J., Sternberg M. J., Cookson J., Coates A. R. Predictions of linear T-cell and B-cell epitopes in proteins encoded by HIV-1, HIV-2 and SIVMAC and the conservation of these sites between strains. FEBS Lett. 1988 Dec 19;242(1):9–21. doi: 10.1016/0014-5793(88)80976-1. [DOI] [PubMed] [Google Scholar]


