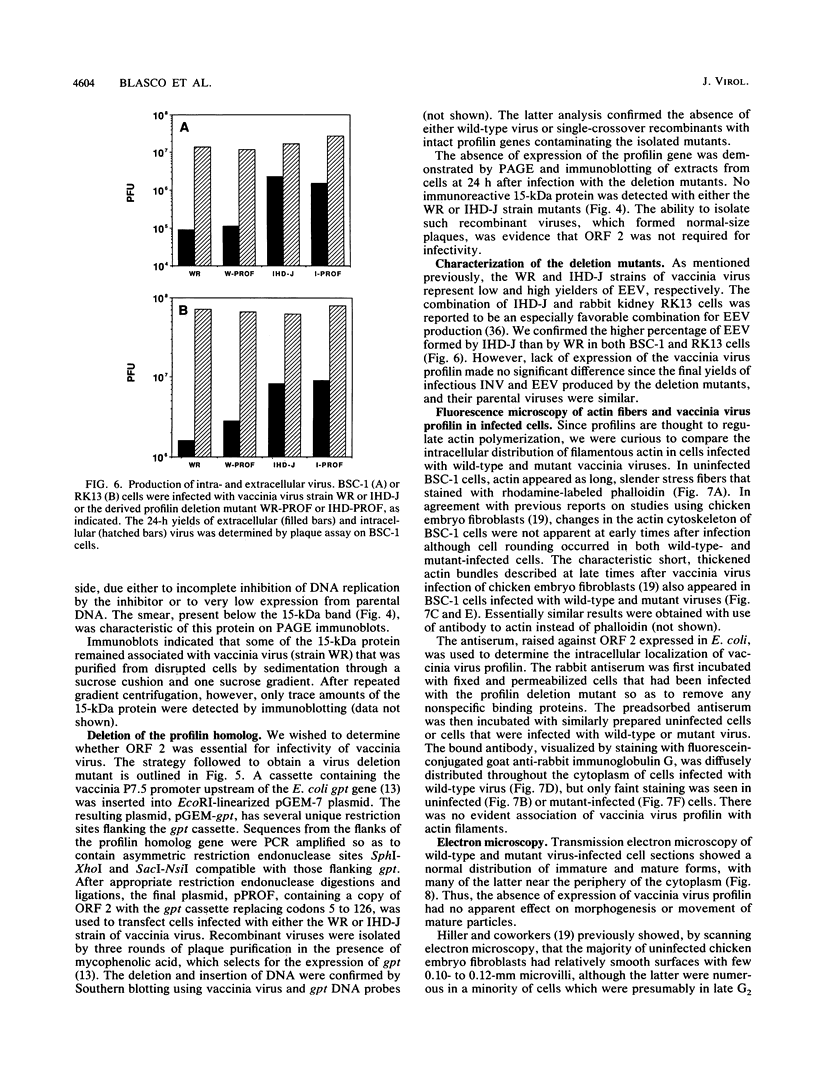
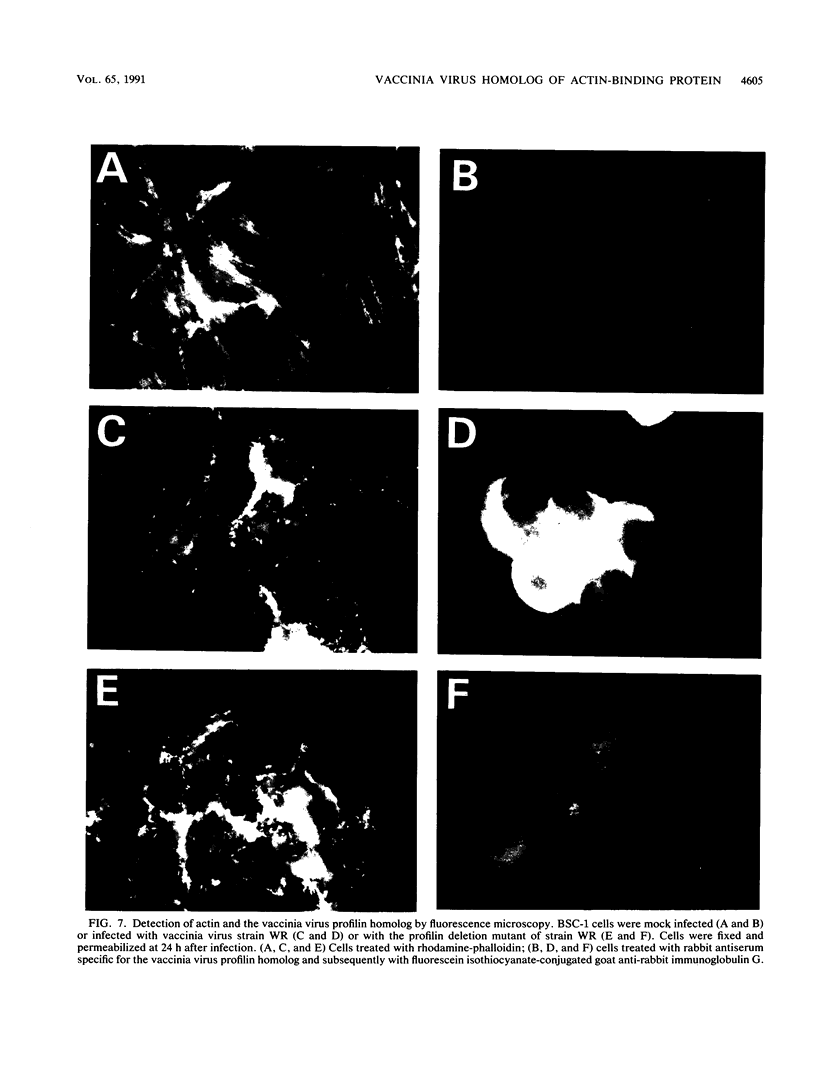
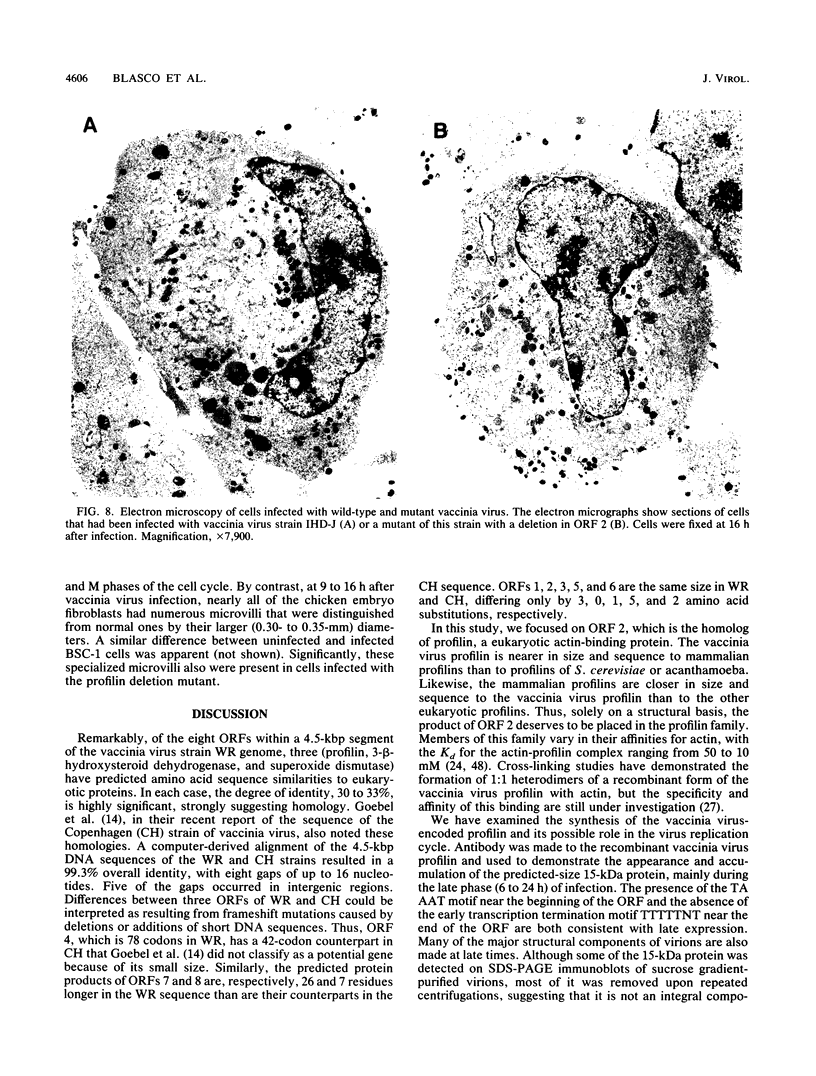
Abstract
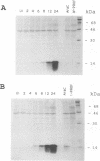
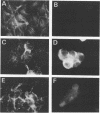
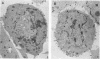
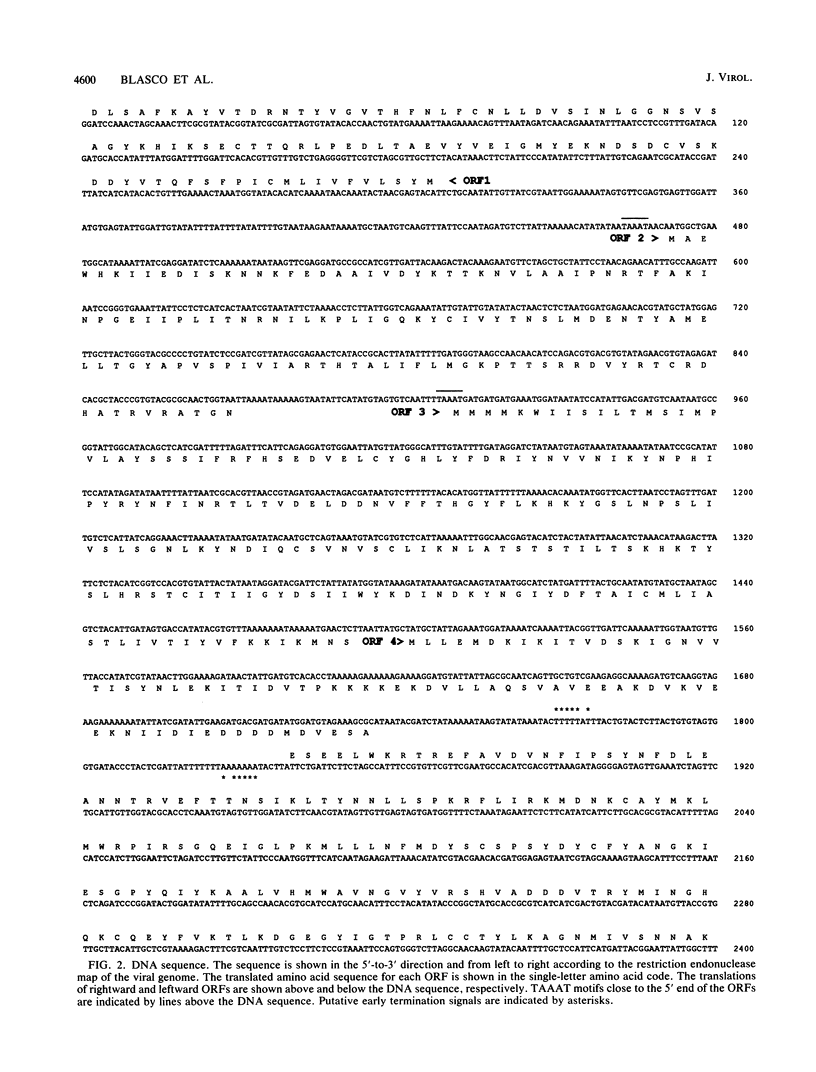
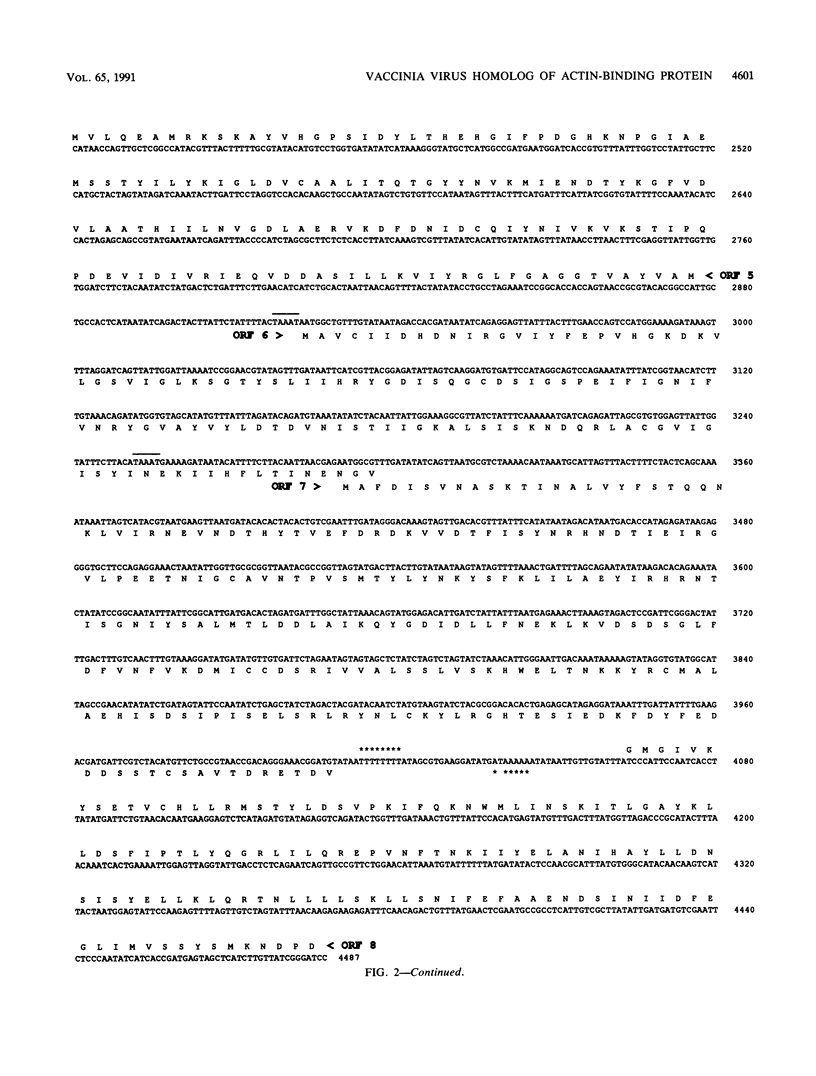
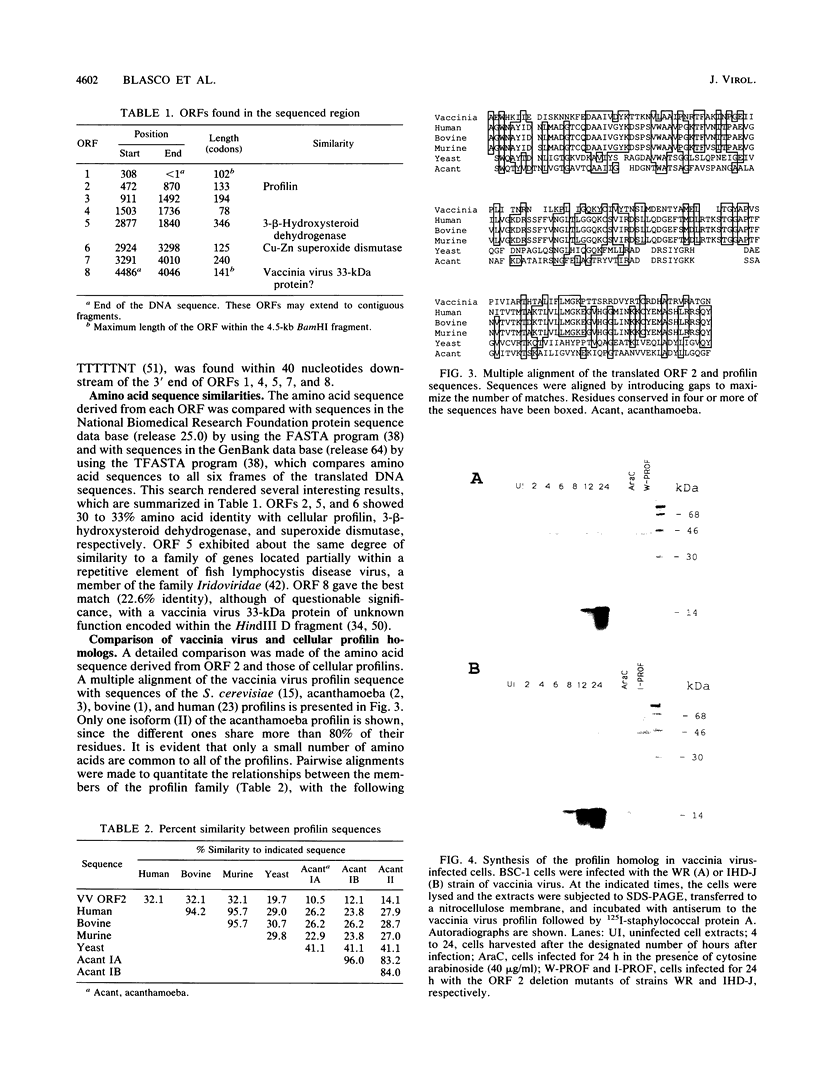
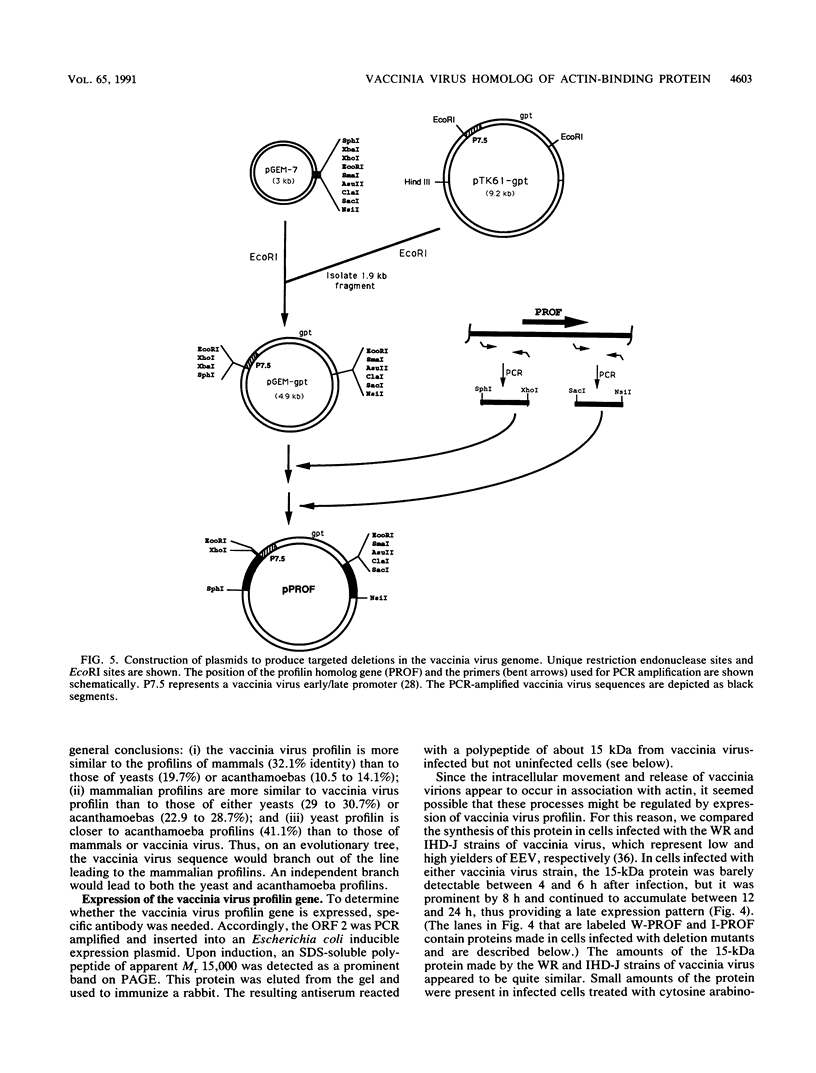
A 4,500-bp BamHI fragment, located within the HindIII A segment of the vaccinia virus genome, was found to contain eight potential coding regions for polypeptides of 78 to 346 amino acids. The open reading frames with 133, 346, and 125 codons were homologous to profilin (an actin-binding protein), 3-beta-hydroxysteroid dehydrogenase, and Cu-Zn superoxide dismutase, respectively. Sequence alignments indicated that the vaccinia virus and mammalian profilins were more closely related to each other than to known profilins of other eukaryotes. The expression and possible role of the profilin homolog in the virus replicative cycle were therefore investigated. Antibody raised to Escherichia coli expressed vaccinia virus profilin was used to demonstrate the synthesis of the 15-kDa polypeptide at late times after vaccinia virus infection of mammalian cells. The protein accumulated in the cytoplasm, but only trace amounts remained associated with highly purified virions. The isolation of vaccinia virus mutants (in strains WR and IHD-J), with nearly the entire profilin gene replaced by the E. coli gpt gene, indicated that the protein is not essential for infectivity. The characteristic vaccinia virus-induced changes in actin fibers, seen by fluorescence microscopy, occurred in cells infected with the mutant. Moreover, the virus-encoded profilin homolog was not required for actin-associated events, including intracellular virus movement to the periphery of the cell, formation of specialized microvilli, or release of mature virions, as shown by electron microscopy and yields of infectious intra- and extracellular virus.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ampe C., Markey F., Lindberg U., Vandekerckhove J. The primary structure of human platelet profilin: reinvestigation of the calf spleen profilin sequence. FEBS Lett. 1988 Feb 8;228(1):17–21. doi: 10.1016/0014-5793(88)80575-1. [DOI] [PubMed] [Google Scholar]
- Ampe C., Sato M., Pollard T. D., Vandekerckhove J. The primary structure of the basic isoform of Acanthamoeba profilin. Eur J Biochem. 1988 Jan 4;170(3):597–601. doi: 10.1111/j.1432-1033.1988.tb13739.x. [DOI] [PubMed] [Google Scholar]
- Ampe C., Vandekerckhove J., Brenner S. L., Tobacman L., Korn E. D. The amino acid sequence of Acanthamoeba profilin. J Biol Chem. 1985 Jan 25;260(2):834–840. [PubMed] [Google Scholar]
- Brugge J. S. The p35/p36 substrates of protein-tyrosine kinases as inhibitors of phospholipase A2. Cell. 1986 Jul 18;46(2):149–150. doi: 10.1016/0092-8674(86)90729-4. [DOI] [PubMed] [Google Scholar]
- CAIRNS J. The initiation of vaccinia infection. Virology. 1960 Jul;11:603–623. doi: 10.1016/0042-6822(60)90103-3. [DOI] [PubMed] [Google Scholar]
- Carlin C. R., Tollefson A. E., Brady H. A., Hoffman B. L., Wold W. S. Epidermal growth factor receptor is down-regulated by a 10,400 MW protein encoded by the E3 region of adenovirus. Cell. 1989 Apr 7;57(1):135–144. doi: 10.1016/0092-8674(89)90179-7. [DOI] [PubMed] [Google Scholar]
- DALES S. The uptake and development of vaccinia virus in strain L cells followed with labeled viral deoxyribonucleic acid. J Cell Biol. 1963 Jul;18:51–72. doi: 10.1083/jcb.18.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison A. J., Moss B. Structure of vaccinia virus early promoters. J Mol Biol. 1989 Dec 20;210(4):749–769. doi: 10.1016/0022-2836(89)90107-1. [DOI] [PubMed] [Google Scholar]
- Davison A. J., Moss B. Structure of vaccinia virus late promoters. J Mol Biol. 1989 Dec 20;210(4):771–784. doi: 10.1016/0022-2836(89)90108-3. [DOI] [PubMed] [Google Scholar]
- DeFilippes F. M. Restriction enzyme mapping of vaccinia virus DNA. J Virol. 1982 Jul;43(1):136–149. doi: 10.1128/jvi.43.1.136-149.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkner F. G., Moss B. Escherichia coli gpt gene provides dominant selection for vaccinia virus open reading frame expression vectors. J Virol. 1988 Jun;62(6):1849–1854. doi: 10.1128/jvi.62.6.1849-1854.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebel S. J., Johnson G. P., Perkus M. E., Davis S. W., Winslow J. P., Paoletti E. The complete DNA sequence of vaccinia virus. Virology. 1990 Nov;179(1):247-66, 517-63. doi: 10.1016/0042-6822(90)90294-2. [DOI] [PubMed] [Google Scholar]
- Goldschmidt-Clermont P. J., Machesky L. M., Baldassare J. J., Pollard T. D. The actin-binding protein profilin binds to PIP2 and inhibits its hydrolysis by phospholipase C. Science. 1990 Mar 30;247(4950):1575–1578. doi: 10.1126/science.2157283. [DOI] [PubMed] [Google Scholar]
- Haarer B. K., Lillie S. H., Adams A. E., Magdolen V., Bandlow W., Brown S. S. Purification of profilin from Saccharomyces cerevisiae and analysis of profilin-deficient cells. J Cell Biol. 1990 Jan;110(1):105–114. doi: 10.1083/jcb.110.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Hiller G., Jungwirth C., Weber K. Fluorescence microscopical analysis of the life cycle of vaccinia virus in chick embryo fibroblasts. Virus-cytoskeleton interactions. Exp Cell Res. 1981 Mar;132(1):81–87. doi: 10.1016/0014-4827(81)90085-9. [DOI] [PubMed] [Google Scholar]
- Hiller G., Weber K., Schneider L., Parajsz C., Jungwirth C. Interaction of assembled progeny pox viruses with the cellular cytoskeleton. Virology. 1979 Oct 15;98(1):142–153. doi: 10.1016/0042-6822(79)90533-6. [DOI] [PubMed] [Google Scholar]
- Huang K. S., Wallner B. P., Mattaliano R. J., Tizard R., Burne C., Frey A., Hession C., McGray P., Sinclair L. K., Chow E. P. Two human 35 kd inhibitors of phospholipase A2 are related to substrates of pp60v-src and of the epidermal growth factor receptor/kinase. Cell. 1986 Jul 18;46(2):191–199. doi: 10.1016/0092-8674(86)90736-1. [DOI] [PubMed] [Google Scholar]
- Ichihashi Y., Matsumoto S., Dales S. Biogenesis of poxviruses: role of A-type inclusions and host cell membranes in virus dissemination. Virology. 1971 Dec;46(3):507–532. doi: 10.1016/0042-6822(71)90056-0. [DOI] [PubMed] [Google Scholar]
- Kwiatkowski D. J., Bruns G. A. Human profilin. Molecular cloning, sequence comparison, and chromosomal analysis. J Biol Chem. 1988 Apr 25;263(12):5910–5915. [PubMed] [Google Scholar]
- Larsson H., Lindberg U. The effect of divalent cations on the interaction between calf spleen profilin and different actins. Biochim Biophys Acta. 1988 Mar 2;953(1):95–105. doi: 10.1016/0167-4838(88)90013-1. [DOI] [PubMed] [Google Scholar]
- Lassing I., Lindberg U. Specific interaction between phosphatidylinositol 4,5-bisphosphate and profilactin. Nature. 1985 Apr 4;314(6010):472–474. doi: 10.1038/314472a0. [DOI] [PubMed] [Google Scholar]
- Luu The V., Lachance Y., Labrie C., Leblanc G., Thomas J. L., Strickler R. C., Labrie F. Full length cDNA structure and deduced amino acid sequence of human 3 beta-hydroxy-5-ene steroid dehydrogenase. Mol Endocrinol. 1989 Aug;3(8):1310–1312. doi: 10.1210/mend-3-8-1310. [DOI] [PubMed] [Google Scholar]
- MORGAN C., ELLISON S. A., ROSE H. M., MOORE D. H. Structure and development of viruses observed in the electron microscope. II. Vaccinia and fowl pox viruses. J Exp Med. 1954 Sep 1;100(3):301–310. doi: 10.1084/jem.100.3.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackett M., Smith G. L., Moss B. General method for production and selection of infectious vaccinia virus recombinants expressing foreign genes. J Virol. 1984 Mar;49(3):857–864. doi: 10.1128/jvi.49.3.857-864.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magdolen V., Oechsner U., Müller G., Bandlow W. The intron-containing gene for yeast profilin (PFY) encodes a vital function. Mol Cell Biol. 1988 Dec;8(12):5108–5115. doi: 10.1128/mcb.8.12.5108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan C. Vaccinia virus reexamined: development and release. Virology. 1976 Aug;73(1):43–58. doi: 10.1016/0042-6822(76)90059-3. [DOI] [PubMed] [Google Scholar]
- Moss B., Ahn B. Y., Amegadzie B., Gershon P. D., Keck J. G. Cytoplasmic transcription system encoded by vaccinia virus. J Biol Chem. 1991 Jan 25;266(3):1355–1358. [PubMed] [Google Scholar]
- Niles E. G., Condit R. C., Caro P., Davidson K., Matusick L., Seto J. Nucleotide sequence and genetic map of the 16-kb vaccinia virus HindIII D fragment. Virology. 1986 Aug;153(1):96–112. doi: 10.1016/0042-6822(86)90011-5. [DOI] [PubMed] [Google Scholar]
- Payne L. G. Identification of the vaccinia hemagglutinin polypeptide from a cell system yielding large amounts of extracellular enveloped virus. J Virol. 1979 Jul;31(1):147–155. doi: 10.1128/jvi.31.1.147-155.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne L. G., Kristenson K. Mechanism of vaccinia virus release and its specific inhibition by N1-isonicotinoyl-N2-3-methyl-4-chlorobenzoylhydrazine. J Virol. 1979 Nov;32(2):614–622. doi: 10.1128/jvi.32.2.614-622.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne L. Polypeptide composition of extracellular enveloped vaccinia virus. J Virol. 1978 Jul;27(1):28–37. doi: 10.1128/jvi.27.1.28-37.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard T. D., Cooper J. A. Actin and actin-binding proteins. A critical evaluation of mechanisms and functions. Annu Rev Biochem. 1986;55:987–1035. doi: 10.1146/annurev.bi.55.070186.005011. [DOI] [PubMed] [Google Scholar]
- Popescu L. M., Cernescu C., Moraru I. I., Constantinescu S. N., Baltà F., Manciulea M., Bràiloiu E., Buzilà L. Cell-membrane phospholipase C is involved in inducing the antiviral effect of interferon. Biosci Rep. 1989 Oct;9(5):531–539. doi: 10.1007/BF01119795. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnitzler P., Darai G. Characterization of the repetitive DNA elements in the genome of fish lymphocystis disease viruses. Virology. 1989 Sep;172(1):32–41. doi: 10.1016/0042-6822(89)90104-9. [DOI] [PubMed] [Google Scholar]
- Sherman L., Dafni N., Lieman-Hurwitz J., Groner Y. Nucleotide sequence and expression of human chromosome 21-encoded superoxide dismutase mRNA. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5465–5469. doi: 10.1073/pnas.80.18.5465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes G. V. High-voltage electron microscope study of the release of vaccinia virus from whole cells. J Virol. 1976 May;18(2):636–643. doi: 10.1128/jvi.18.2.636-643.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stossel T. P., Chaponnier C., Ezzell R. M., Hartwig J. H., Janmey P. A., Kwiatkowski D. J., Lind S. E., Smith D. B., Southwick F. S., Yin H. L. Nonmuscle actin-binding proteins. Annu Rev Cell Biol. 1985;1:353–402. doi: 10.1146/annurev.cb.01.110185.002033. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Rosenberg A. H., Dunn J. J., Dubendorff J. W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- Tobacman L. S., Korn E. D. The regulation of actin polymerization and the inhibition of monomeric actin ATPase activity by Acanthamoeba profilin. J Biol Chem. 1982 Apr 25;257(8):4166–4170. [PubMed] [Google Scholar]
- Vandekerckhove J. Structural principles of actin-binding proteins. Curr Opin Cell Biol. 1989 Feb;1(1):15–22. doi: 10.1016/s0955-0674(89)80031-6. [DOI] [PubMed] [Google Scholar]
- Weinrich S. L., Hruby D. E. A tandemly-oriented late gene cluster within the vaccinia virus genome. Nucleic Acids Res. 1986 Apr 11;14(7):3003–3016. doi: 10.1093/nar/14.7.3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen L., Moss B. Oligonucleotide sequence signaling transcriptional termination of vaccinia virus early genes. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6417–6421. doi: 10.1073/pnas.84.18.6417. [DOI] [PMC free article] [PubMed] [Google Scholar]