Abstract
A highly efficient one-pot procedure for the synthesis of indolines and their homologues based on a domino Cu-catalyzed amidation/nucleophilic substitution reaction has been developed. Substituted 2-iodophenethyl mesylates and related compounds afforded the corresponding products in excellent yields. No erosion of optical purity was observed when transforming enantiomerically pure mesylates under the reaction conditions.
The indoline moiety1 can be found in numerous biologically active alkaloid natural products2 and pharmaceuticals.3 Recently, highly efficient indoline-based organic dyes for dye-sensitized solar cells have also been developed.4
Since our earlier reports on the Pd-catalyzed intramolecular amination reactions for the formation of indolines,5 a variety of intramolecular transition metal-catalyzed amination and amidation processes have emerged for the synthesis of N-protected indolines (Scheme 1, eq. 1).6,7,8 More versatile routes toward the synthesis of the indoline core incorporate an intermolecular Pd-catalyzed amidation or amination reaction as part of a sequential or domino process (Scheme 1, eq. 2 and 3).9 Although, this strategy represents a significant improvement in the modular synthesis of indolines, several drawbacks limit the reported methods. Specifically, certain methods only allow access to 3-substituted,9a 2-substituted9c or non-substituted9d,e indolines, and the Pd-catalyzed C–C/C–N coupling of bromoalkylamines with an aryl iodide requires ortho-substituted aryl iodides and a para-nitrophenyl-protected amine.9f We felt that a one-pot procedure for the synthesis of indolines that overcomes these limitations would be highly desirable.
Scheme 1.
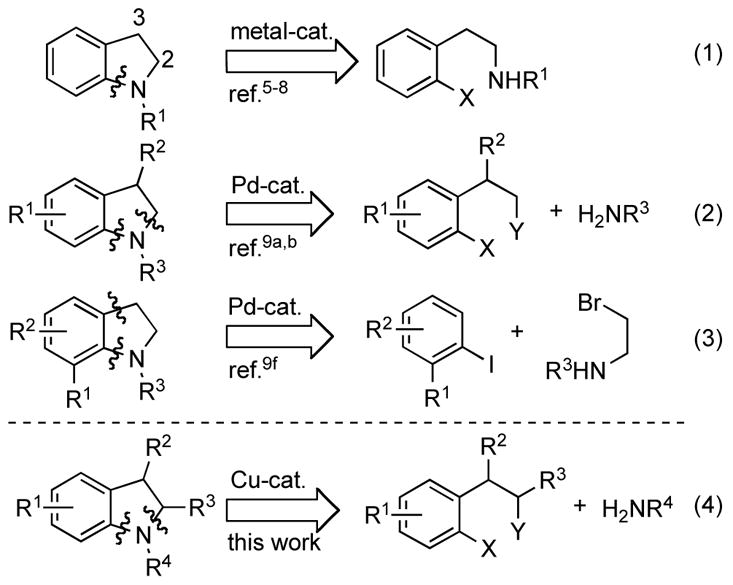
Known and Envisioned Strategies for the Synthesis of Indolines
Herein, we report the development of a general domino Cu-catalyzed amidation/nucleophilic substitution process for the synthesis of substituted indolines and their homologues (Scheme 1, eq. 4).10
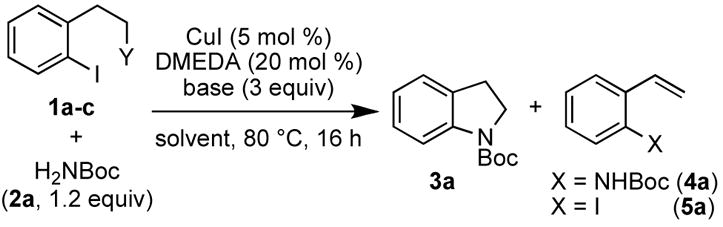
We began our investigation with 1-iodo-2-(2-iodoethyl)benzene (1a) and tert-butylcarbamate (2a) as the model substrates to examine the reaction conditions, which we previously reported for the Cu-catalyzed amidation of aryl halides (Table 1) [5 mol % CuI, 20 mol % N,N′-dimethylethylenediamine (DMEDA), Cs2CO3 in THF].7a,11 Only low conversion of 1a was observed at room temperature after 16 h. At 80 °C, however, full conversion and up to 37% of the N-Boc-protected indoline 3a were obtained, along with 23% of 2-N-Boc-styrene (4a). Systematic variation of the solvent, base, and diamine-ligand did not increase the yield of the desired product, although varying amounts of the products 4a and 5a were observed (Table 1, entries 1–7).
Table 1.
Optimization of the Domino Cu-Catalyzed Amidation/Cyclization Reaction
 | ||||||
|---|---|---|---|---|---|---|
| entry | Y | base | solvent | yield 3aa | yield 4a | yield 5a |
| 1b | I (1a) | Cs2CO3 | THF | 6% | - | 14% |
| 2 | 1a | Cs2CO3 | THF | 37% | 23% | - |
| 3 | 1a | Cs2CO3 | 1,4-dioxane | 35% | 11% | 6% |
| 4 | 1a | Cs2CO3 | toluene | 3% | - | 11% |
| 5c | 1a | Cs2CO3 | THF | 40% | 31% | - |
| 6 | 1a | K3PO4 | THF | 9% | 23% | 14% |
| 7 | 1a | K2CO3 | THF | - | - | 33% |
| 8 | Cl (1b) | Cs2CO3 | THF | 87% | - | |
| 9 | OMs (1c) | Cs2CO3 | THF | 89%d | - | - |
GC yield with dodecane as an internal standard; 99% conversion of 1, unless indicated otherwise.
Experiment performed at rt; 41% conversion of 1a.
Racemic trans-1,2-N,N′-dimethylcyclohexanediamine was used as a ligand.
Isolated yield.
Variation of the nucleofuge proved to be crucial. Switching from the phenethyl iodide 1a to the phenethyl chloride 1b or the phenethyl mesylate 1c resulted in exclusive formation of the desired product 3a in high yields (87% and 89%, respectively).
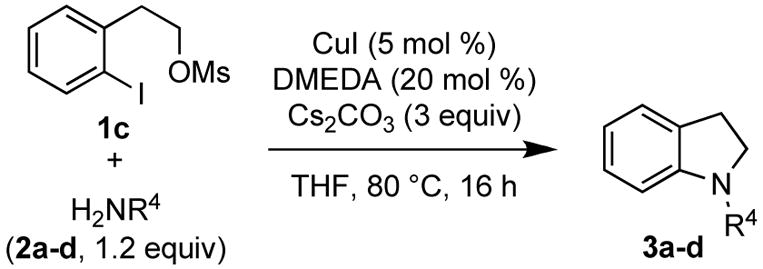
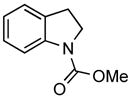
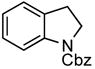
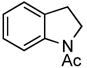
Under the optimized reaction conditions 2-iodophenethyl mesylate (1c) reacted equally efficiently with other commonly used carbamates 2b–c and amides 2d and yielded the corresponding N-protected indolines 3b–d in comparably high yields without formation of any side products (Table 2).
Table 2.
Synthesis of N-protected Indolines via Domino Cu-Catalyzed Amidation/Cyclization Reaction
 | |||
|---|---|---|---|
| entry | R4 | product | yielda |
| 1 | Boc (2a) |
 3a 3a
|
89% |
| 2 | C(O)OMe (2b) |
 3b 3b
|
90% |
| 3 | Cbz (2c) |
 3c 3c
|
90% |
| 4 | Ac (2d) |
 3d 3d
|
87% |
Yields of the isolated products are an average of two runs and the products are estimated to be over 95% pure by 1H NMR spectroscopic and GC analysis.
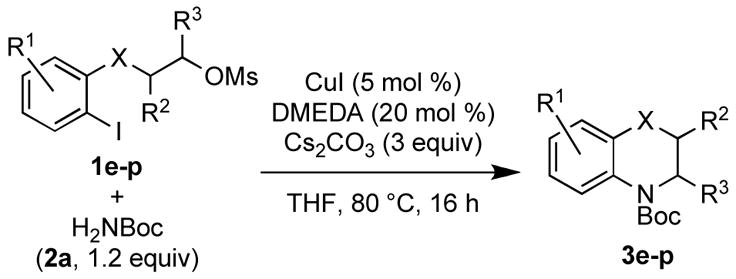
Encouraged by these results, we investigated the substrate scope of this reaction sequence. Various 2-iodophenethyl mesylates were subjected to the domino amidation sequence (Table 3).
Table 3.
Substrate Scope of the Cu-Catalyzed Domino Amidation Reaction
 | ||
|---|---|---|
| entry | product | yielda |
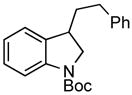
| 1 |
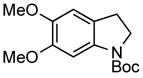 3e 3e
|
75% |
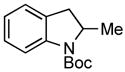
| 2 |
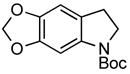 3f 3f
|
82% |
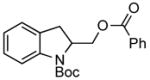
| 3 |
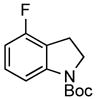 3g 3g
|
95% |
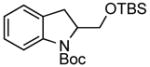
| 4 |
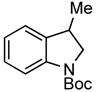 3h 3h
|
95% |
| 5 |
 3i 3i
|
92% |
| 6 |
 3k 3k
|
96% |
| 7 |
 3l 3l
|
88% |
| 8 |
 3m 3m
|
91% |
| 9 |
 3n 3n
|
74% |
| 10 |
 3o 3o
|
76% |
| 11 |
 3p 3p
|
57% |
Yields of the isolated products are an average of two runs and the products are estimated to be over 95% pure by 1H NMR spectroscopic and GC analysis.
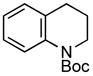
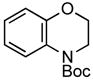
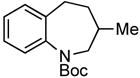
A wide variety of functional groups, such as ethers, acetals, halogens, esters, and siloxy or alkyl groups were tolerated on the aryl ring (entries 1–3) and in positions R2 and R3 (entries 4–8). In all cases, the reaction proceeded smoothly and the corresponding substituted indolines were obtained in excellent yield. This method was further applied to the synthesis of indoline homologues, which are difficult to access using the previously reported domino processes.8 The corresponding mesylates gave access to N-Boc-tetrahydroquinoline (3n), -benzoxazine (3o) and -3-methyl-2,3,4,5-tetrahydro-1H-1-benzazepine (3p) in yields up to 76% (entries 9–11).
Three distinct mechanistic pathways for this domino process can be envisioned for the formation of the indoline structure (Scheme 2): 1) base-promoted formation of 2-iodo-styrene followed by intermolecular Cu-catalyzed C–N coupling and intramolecular hydroamidation of styrene I (pathway A);12 2) intermolecular Cu-catalyzed or uncatalyzed substitution of the alkyl mesylate and subsequent Cu-catalyzed intramolecular C–N coupling with the aryl iodide II (pathway B); 3) initial intermolecular Cu-catalyzed amidation of the aryl iodide, followed by an intramolecular SN2 reaction of the carbamate or amide III onto the alkyl mesylate (pathway C).
Scheme 2. Possible Mechanistic Pathways.
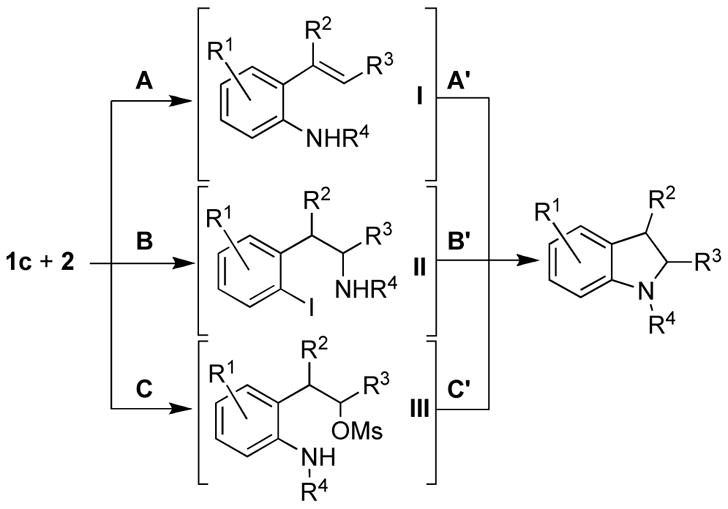
A, A′: intermolecular Cu-cat. amidation and hydroamidation.
B, B′: intermolecular Cu-cat. or uncat. amidation and intramolecular Cu-cat. amidation.
C, C′: intermolecular Cu-cat. amidation and SN2 reaction.
To elucidate the reaction mechanism, we synthesized compounds 1q, 1r, 4 and 5 (Scheme 3).
Scheme 3.
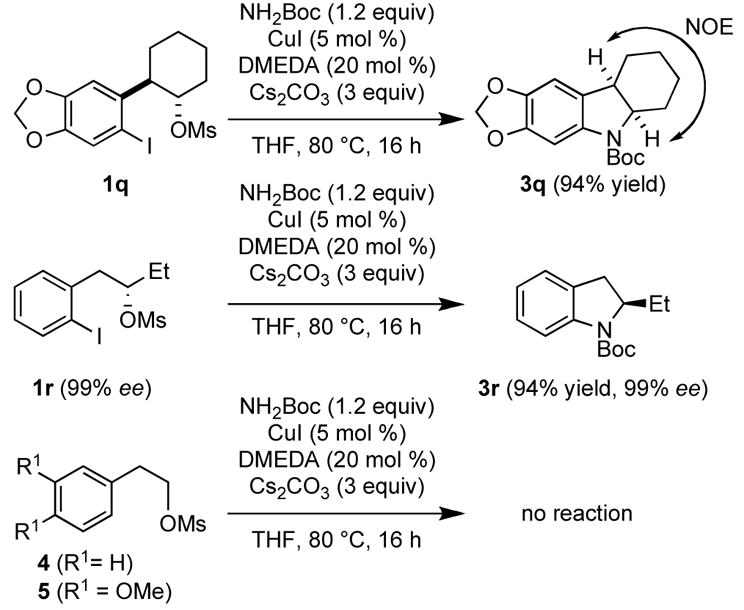
Experiments Conducted to Elucidate the Reaction Mechanism
Under the reaction conditions racemic trans mesylate 1q yielded the racemic cis-fused hexahydrocarbazole 3q - as confirmed by an NOE experiment - as a single diastereosiomer in 94%, and the enantiomerically pure mesylate 1r afforded indoline 3r in excellent yield and with 99% ee.13
Based upon these results, pathway A is unlikely to be the operative mechanism, since hydroamidation of the achiral intermediate I would lead to a mixture of cis- and trans14-products in the case of 3q and to racemization of the stereocenter in position 2 in the case of 3r. Furthermore, pathway B can be ruled out, since no substitution at the alkyl mesylate took place in model systems 4 and 5 under our reaction conditions. Finally, the fact that complete stereochemical inversion was observed in cases 1q and 1r strongly suggests a nucleophilic displacement of the mesylate group via an SN2 mechansim (pathway C in Scheme 2). Attempts to isolate reaction intermediate III were unsuccessful. Only the final product and remaining starting material could be detected by GC or NMR in various ratios over the course of the reaction.
In summary, we have developed a highly efficient domino Cu-catalyzed amidation/nucleophilic substitution reaction for the synthesis of indolines and their homologues from ortho-iodophenalkyl mesylates. The mild reaction conditions and the broad substrate scope render this method attractive and complementary to existing methods for the synthesis of indolines. Finally, this approach also allows the synthesis of enantiomerically pure indolines, since the second step proceeds with complete stereochemical inversion and therefore no erosion of optical purity.
Supplementary Material
Supporting Information Available: Experimental procedures and characterization data for all compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
Acknowledgments
Generous financial support from the National Institutes of Health (GM 58160) is gratefully acknowledged. A. M. thanks the Alexander von Humboldt Stiftung for a Feodor Lynen postdoctoral Fellowship. We thank Alison E. Ondrus (MIT) for assistance with the NOE experiment. The Bruker Advance 400 MHz used in this work was purchased with funding from the National Institutes of Health (GM 1S10RR13886-01). The Varian NMR instruments used for this publication were supported by National Science Foundation (CHE 9808061 and DBI 9729592). We are also indebted to Merck for unrestricted support.
References
- 1.For recent examples of indoline syntheses based on non-metal-catalyzed or radical processes, see: Nicolaou KC, Roecker AJ, Pfefferkorn JA, Cao GQ. J Am Chem Soc. 2000;122:2966.Sanz Gil G, Groth UM. J Am Chem Soc. 2000;122:6789.Dunetz JR, Danheiser RL. J Am Chem Soc. 2005;127:5776. doi: 10.1021/ja051180l.Correa A, Tellitu I, Domínguez E, SanMartin R. J Org Chem. 2006;71:8316. doi: 10.1021/jo061486q.Fuwa H, Sasaki M. Org Lett. 2007;9:3347. doi: 10.1021/ol071312a.Wang Z, Wan W, Jiang H, Hao J. J Org Chem. 2007;72:9364. doi: 10.1021/jo701566v.Gilmore CD, Allan KM, Stoltz BM. J Am Chem Soc. 2008;130:1558. doi: 10.1021/ja0780582.Viswanathan R, Smith CR, Prabhakaran EN, Johnston JN. J Org Chem. 2008;73:3040. doi: 10.1021/jo702523u.Clive DLJ, Peng J, Fletcher SP, Ziffle VE, Wingert D. J Org Chem. 2008;73:2330. doi: 10.1021/jo7026307.
- 2.For selected examples, see: Boger DL, Boyce CW, Garbaccio RM, Goldberg JA. Chem Rev. 1997;97:787. doi: 10.1021/cr960095g.Sunazuka T, Hirose T, Shirahata T, Harigaya Y, Hayashi M, Komiyama K, Omura S, Smith AB., III J Am Chem Soc. 2000;122:2122.Dounay AB, Overman LE, Wrobleski AD. J Am Chem Soc. 2005;127:10186. doi: 10.1021/ja0533895.
- 3.For selected examples, see: Gruenfeld N, Stanton JL, Yuan AM, Ebetino FH, Browne LJ, Gude C, Huebner CF. J Med Chem. 1983;26:1277. doi: 10.1021/jm00363a012.Bromidge SM, Duckworth M, Forbes IT, Ham P, King FD, Thewlis KM, Blaney FE, Naylor CB, Blackburn TP, Kennett GA, Wood MD, Clarke SE. J Med Chem. 1997;40:3494. doi: 10.1021/jm970424c.Hobson LA, Nugent WA, Anderson SR, Deshmukh SS, Haley JJ, III, Liu P, Magnus NA, Sheeran P, Sherbine JP, Stone BRP, Zhu J. Org Process Res Dev. 2007;11:985.
- 4.Kuang D, Uchida S, Humphry-Baker R, Zakeeruddin SM, Grätzel M. Angew Chem Int Ed. 2008;47:1923. doi: 10.1002/anie.200705225. [DOI] [PubMed] [Google Scholar]
- 5.(a) Guram AS, Rennels RA, Buchwald SL. Angew Chem, Int Ed Engl. 1995;34:1348. [Google Scholar]; (b) Wolfe JP, Rennels RA, Buchwald SL. Tetrahedron. 1996;52:7525. [Google Scholar]
- 6.For Pd-catalyzed processes, see: Wagaw S, Rennels RA, Buchwald SL. J Am Chem Soc. 1997;119:8451.Yang BH, Buchwald SL. Org Lett. 1999;1:35. doi: 10.1021/ol9905351.Kitamura Y, Hashimoto A, Yoshikawa S, Odaira J-i, Furuta T, Kan T, Tanaka K. Synlett. 2006:115. For a Pd-catalyzed C-H activation approach, see: Watanabe T, Oishi S, Fujii N, Ohno H. Org Lett. 2008;10:1759. doi: 10.1021/ol800425z.
- 7.For Cu-catalyzed processes, see: Klapars A, Huang X, Buchwald SL. J Am Chem Soc. 2002;124:7421. doi: 10.1021/ja0260465.Yamada K, Kubo T, Tokuyama H, Fukuyama T. Synlett. 2002:231.Zhang H, Cai Q, Ma D. J Org Chem. 2005;70:5164. doi: 10.1021/jo0504464.Shafir A, Buchwald SL. J Am Chem Soc. 2006;128:8742. doi: 10.1021/ja063063b.
- 8.For a Ni-catalyzed process, see: Omar-Amrani R, Thomas A, Brenner E, Schneider R, Fort Y. Org Lett. 2003;5:2311. doi: 10.1021/ol034659w.
- 9.Aoki K, Peat AJ, Buchwald SL. J Am Chem Soc. 1998;120:3068.Deboves HJC, Hunter C, Jackson RFW. J Chem Soc Perkin Trans 1. 2002:733.Lira R, Wolfe JP. J Am Chem Soc. 2004;126:13906. doi: 10.1021/ja0460920.Ganton MD, Kerr MA. Org Lett. 2005;7:4777. doi: 10.1021/ol052086c.Ganton MD, Kerr MA. Org Lett. 2007;72:574. doi: 10.1021/jo062064j. In this case, the Pd-catalyzed amination proceeds intramolecularly: Thansandote P, Raemy M, Rudolph A, Lautens M. Org Lett. 2007;9:5255. doi: 10.1021/ol702472u.
- 10.For recent reviews on Cu-catalyzed C–N bond forming reactions, see: Kunz K, Scholz U, Ganzer D. Synlett. 2003:2428.Ley SV, Thomas AW. Angew Chem Int Ed. 2003;42:5400. doi: 10.1002/anie.200300594.Beletskaya IP, Cheprakov AV. Coord Chem Rev. 2004;248:2337.Monnier F, Taillefer M. Angew Chem Int Ed. 2008;47:3096. doi: 10.1002/anie.200703209.
- 11.(a) Klapars A, Antilla JC, Huang X, Buchwald SL. J Am Chem Soc. 2001;123:7727. doi: 10.1021/ja016226z. [DOI] [PubMed] [Google Scholar]; (b) Martín R, Rodríguez Rivero M, Buchwald SL. Angew Chem Int Ed. 2006;45:7079. doi: 10.1002/anie.200602917. [DOI] [PubMed] [Google Scholar]
- 12.For evidence of intramolecular Cu-catalyzed hydroamidation of an unsaturated moiety, see ref.10b
- 13.For recent examples of the synthesis of enantiomerically enriched indolines, see: Arp FO, Fu GC. J Am Chem Soc. 2006;128:14265. doi: 10.1021/ja0657859.Kuwano R, Kashiwabara M. Org Lett. 2006;8:2653. doi: 10.1021/ol061039x.Yamamoto H, Pandey G, Asai Y, Nakano M, Kinoshita A, Namba K, Imagawa H, Nishizawa M. Org Lett. 2007;9:4029. doi: 10.1021/ol701737x.
- 14.For examples of trans-1,2,3,4,4a,9a-hexahydrocarbazoles, see: Smolinsky G. J Am Chem Soc. 1961;83:2489.Sundberg RJ. Tetrahedron Lett. 1966;7:477.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information Available: Experimental procedures and characterization data for all compounds. This material is available free of charge via the Internet at http://pubs.acs.org.


