Abstract
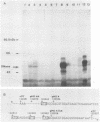
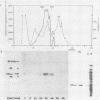
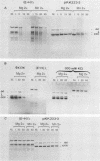
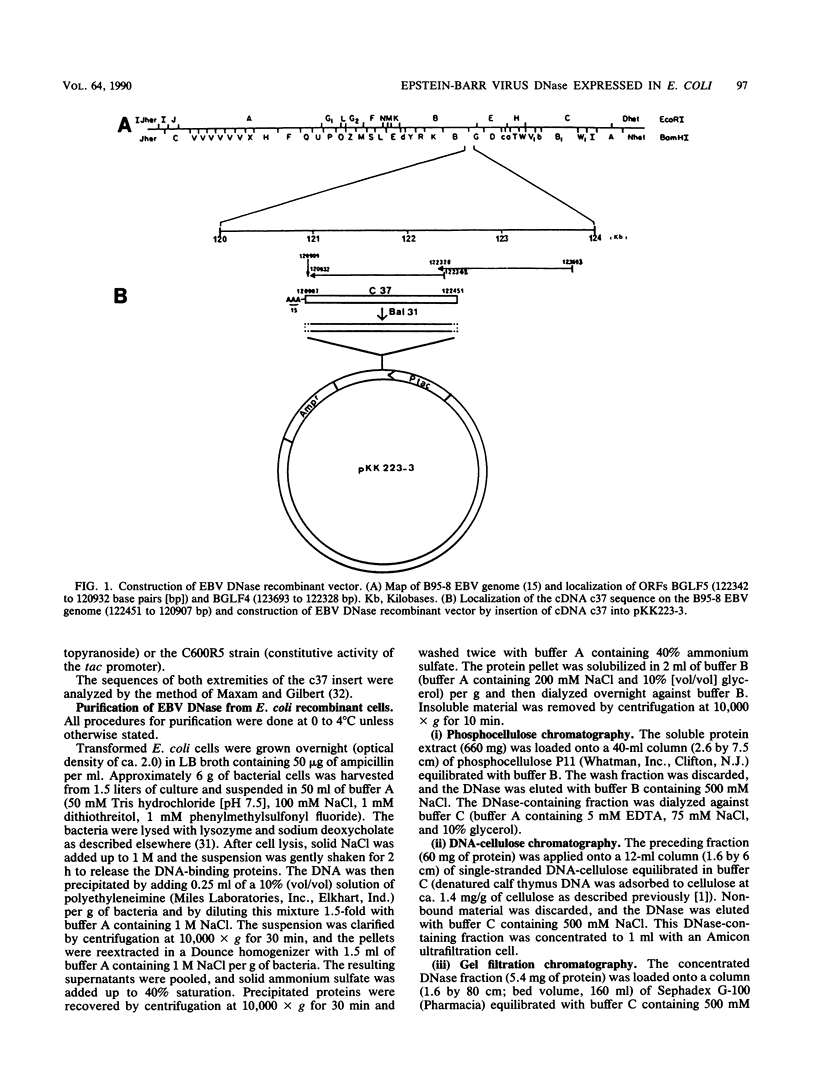
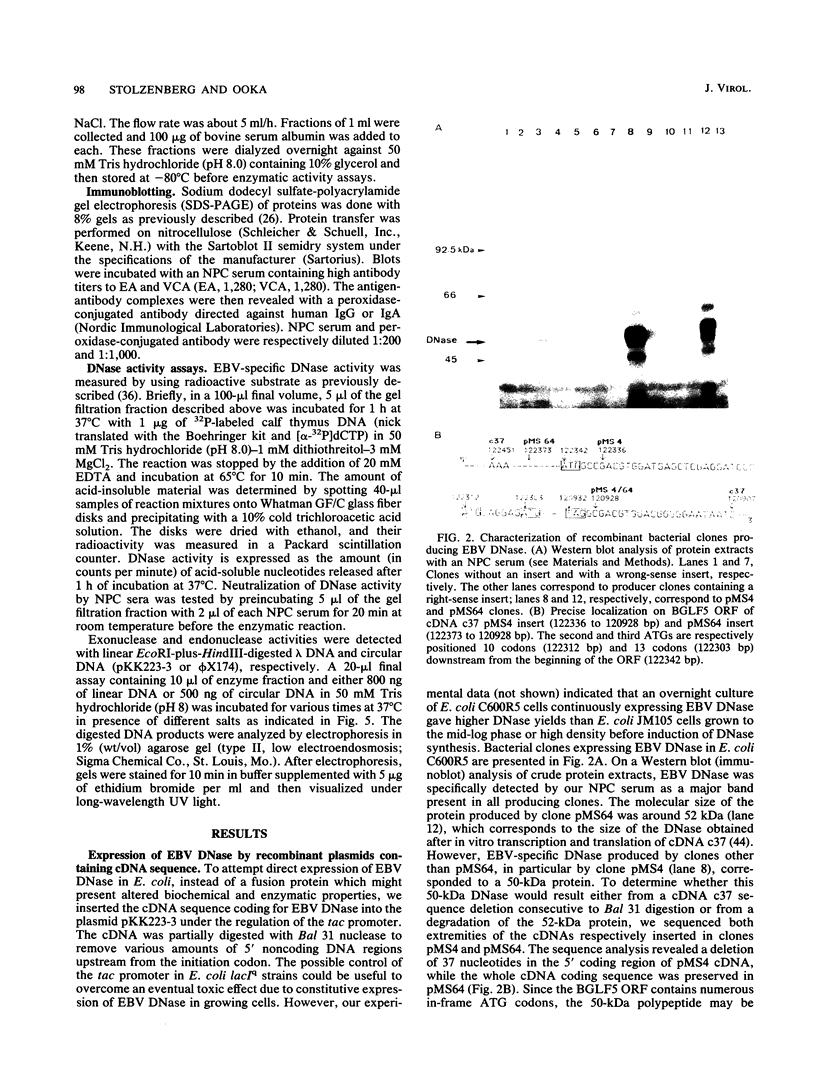
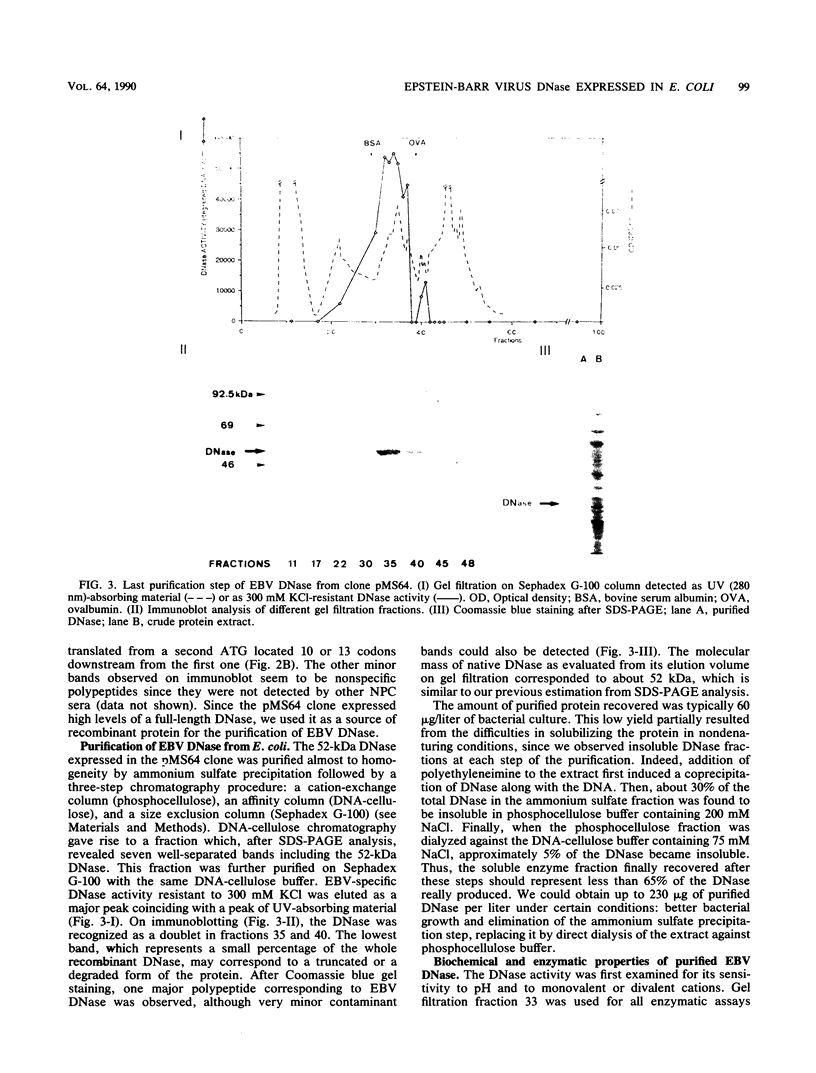
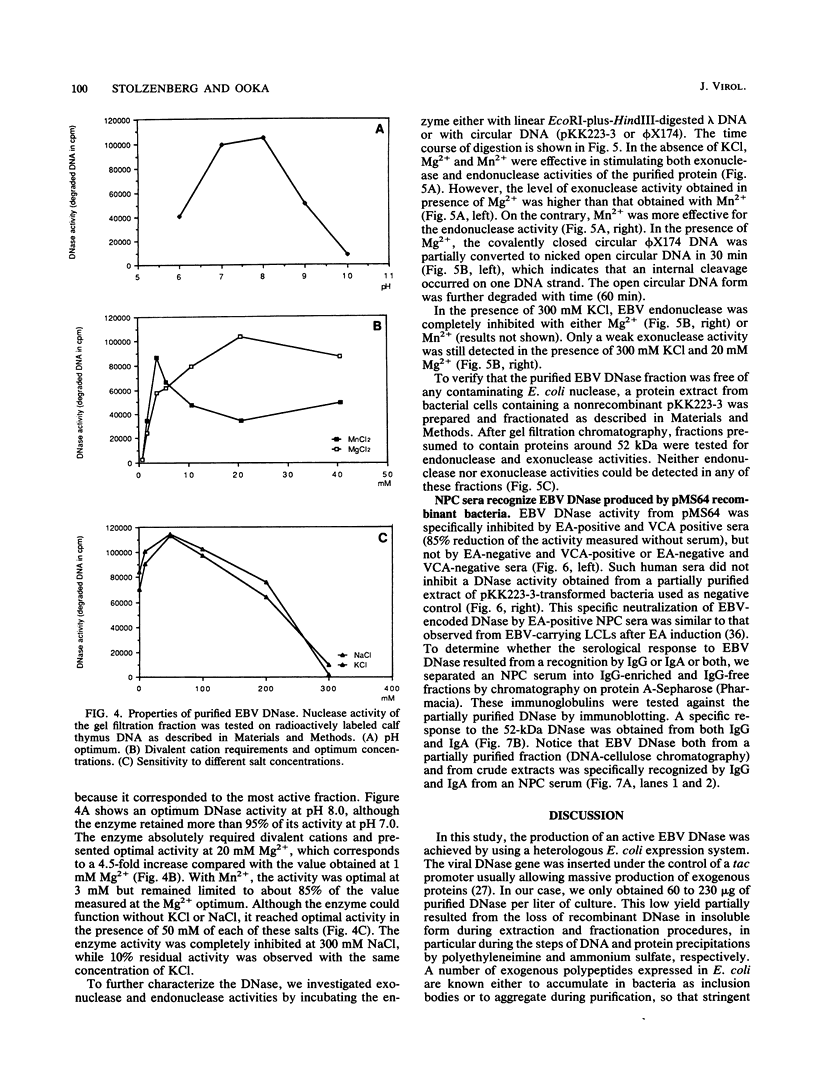
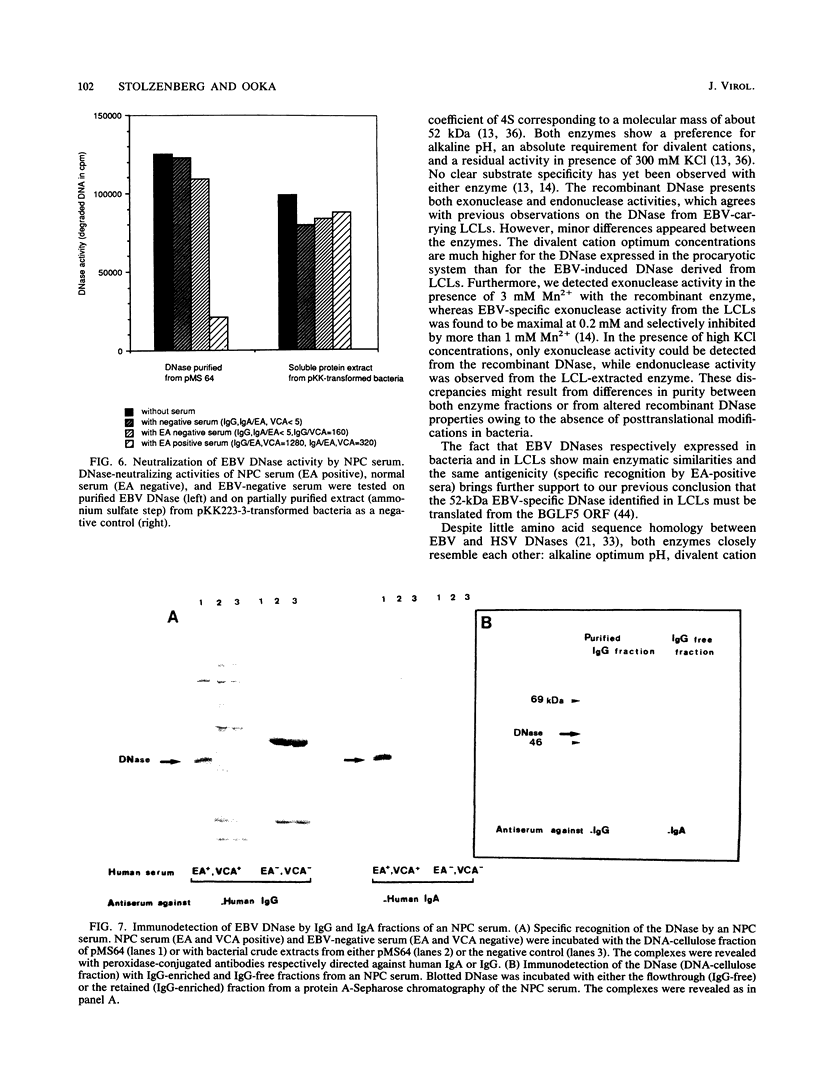
A cDNA corresponding to the BGLF5 open reading frame of the Epstein-Barr virus (EBV) genome and coding for an early DNase was inserted into the procaryotic expression vector pKK223-3. One bacterial clone producing the expected 52-kilodalton DNase was used as a source of EBV DNase. The 52-kilodalton Dnase was purified in the active form to near homogeneity by ammonium sulfate precipitation and successive chromatographies on phosphocellulose, DNA-cellulose, and gel filtration columns. The purified enzyme exhibited both exonuclease and endonuclease activities, an absolute requirement for divalent cations, an alkaline pH preference, and a typical residual activity in presence of 300 mM KCl. Moreover, the enzyme was specifically inhibited by human sera with high antibody titers to EBV early antigens. These properties are similar to those observed for EBV-induced DNase from lymphoblastoid cell extracts. In addition, the enzyme was recognized by both immunoglobulin G and A serum fractions from patients with nasopharyngeal carcinoma (NPC). From these results and previous studies which demonstrated the value of antibody titers to this viral DNase as an NPC marker, it appears that EBV-encoded DNase produced in a heterologous expression system could be used in the development of a specific and early NPC diagnosis test.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baer R., Bankier A. T., Biggin M. D., Deininger P. L., Farrell P. J., Gibson T. J., Hatfull G., Hudson G. S., Satchwell S. C., Séguin C. DNA sequence and expression of the B95-8 Epstein-Barr virus genome. Nature. 1984 Jul 19;310(5974):207–211. doi: 10.1038/310207a0. [DOI] [PubMed] [Google Scholar]
- Banks L., Purifoy D. J., Hurst P. F., Killington R. A., Powell K. L. Herpes simplex virus non-structural proteins. IV. Purification of the virus-induced deoxyribonuclease and characterization of the enzyme using monoclonal antibodies. J Gen Virol. 1983 Oct;64(Pt 10):2249–2260. doi: 10.1099/0022-1317-64-10-2249. [DOI] [PubMed] [Google Scholar]
- Birx D. L., Redfield R. R., Tosato G. Defective regulation of Epstein-Barr virus infection in patients with acquired immunodeficiency syndrome (AIDS) or AIDS-related disorders. N Engl J Med. 1986 Apr 3;314(14):874–879. doi: 10.1056/NEJM198604033141403. [DOI] [PubMed] [Google Scholar]
- Chen J. Y., Chen C. J., Liu M. Y., Cho S. M., Hsu M. M., Lynn T. C., Shieh T., Tu S. M., Lee H. H., Kuo S. L. Antibodies to Epstein-Barr virus-specific DNase in patients with nasopharyngeal carcinoma and control groups. J Med Virol. 1987 Sep;23(1):11–21. doi: 10.1002/jmv.1890230103. [DOI] [PubMed] [Google Scholar]
- Chen J. Y., Hwang L. Y., Beasley R. P., Chien C. S., Yang C. S. Antibody response to Epstein-Barr-virus-specific DNase in 13 patients with nasopharyngeal carcinoma in Taiwan: a retrospective study. J Med Virol. 1985 Jun;16(2):99–105. doi: 10.1002/jmv.1890160202. [DOI] [PubMed] [Google Scholar]
- Chen J. Y., Liu M. Y., Chen C. J., Hsu M. M., Tu S. M., Lee H. H., Kuo S. L., Yang C. S. Antibody to Epstein-Barr virus-specific DNase as a marker for the early detection of nasopharyngeal carcinoma. J Med Virol. 1985 Sep;17(1):47–49. doi: 10.1002/jmv.1890170107. [DOI] [PubMed] [Google Scholar]
- Cheng Y. C., Chen J. Y., Glaser R., Henle W. Frequency and levels of antibodies to Epstein-Barr virus-specific DNase are elevated in patients with nasopharyngeal carcinoma. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6162–6165. doi: 10.1073/pnas.77.10.6162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y. C., Chen J. Y., Hoffmann P. J., Glaser R. Studies on the activity of DNase associated with the replication of the Epstein-Barr virus. Virology. 1980 Jan 30;100(2):334–338. doi: 10.1016/0042-6822(80)90524-3. [DOI] [PubMed] [Google Scholar]
- Chou J., Roizman B. Characterization of DNA sequence-common and sequence-specific proteins binding to cis-acting sites for cleavage of the terminal a sequence of the herpes simplex virus 1 genome. J Virol. 1989 Mar;63(3):1059–1068. doi: 10.1128/jvi.63.3.1059-1068.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough W. An endonuclease isolated from Epstein-Barr virus-producing human lymphoblastoid cells. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6194–6198. doi: 10.1073/pnas.77.10.6194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough W. Deoxyribonuclease activity found in Epstein--Barr virus producing lymphoblastoid cells. Biochemistry. 1979 Oct 16;18(21):4517–4521. doi: 10.1021/bi00588a009. [DOI] [PubMed] [Google Scholar]
- Dambaugh T., Beisel C., Hummel M., King W., Fennewald S., Cheung A., Heller M., Raab-Traub N., Kieff E. Epstein-Barr virus (B95-8) DNA VII: molecular cloning and detailed mapping. Proc Natl Acad Sci U S A. 1980 May;77(5):2999–3003. doi: 10.1073/pnas.77.5.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draper K. G., Devi-Rao G., Costa R. H., Blair E. D., Thompson R. L., Wagner E. K. Characterization of the genes encoding herpes simplex virus type 1 and type 2 alkaline exonucleases and overlapping proteins. J Virol. 1986 Mar;57(3):1023–1036. doi: 10.1128/jvi.57.3.1023-1036.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feighny R. J., Henry B. E., 2nd, Pagano J. S. Epstein-Barr virus-induced deoxynuclease and the reutilization of host-cell DNA degradation products in viral DNA replication. Virology. 1981 Dec;115(2):395–400. doi: 10.1016/0042-6822(81)90121-5. [DOI] [PubMed] [Google Scholar]
- Hoffmann P. J., Cheng Y. C. DNase induced after infection of KB cells by herpes simplex virus type 1 or type 2. II. Characterization of an associated endonuclease activity. J Virol. 1979 Nov;32(2):449–457. doi: 10.1128/jvi.32.2.449-457.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann P. J., Cheng Y. C. The deoxyribonuclease induced after infection of KB cells by herpes simplex virus type 1 or type 2. I. Purification and characterization of the enzyme. J Biol Chem. 1978 May 25;253(10):3557–3562. [PubMed] [Google Scholar]
- Hoffmann P. J. Mechanism of degradation of duplex DNA by the DNase induced by herpes simplex virus. J Virol. 1981 Jun;38(3):1005–1014. doi: 10.1128/jvi.38.3.1005-1014.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Larder B., Purifoy D., Powell K., Darby G. AIDS virus reverse transcriptase defined by high level expression in Escherichia coli. EMBO J. 1987 Oct;6(10):3133–3137. doi: 10.1002/j.1460-2075.1987.tb02623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littler E., Zeuthen J., McBride A. A., Trøst Sørensen E., Powell K. L., Walsh-Arrand J. E., Arrand J. R. Identification of an Epstein-Barr virus-coded thymidine kinase. EMBO J. 1986 Aug;5(8):1959–1966. doi: 10.1002/j.1460-2075.1986.tb04450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McGeoch D. J., Dolan A., Frame M. C. DNA sequence of the region in the genome of herpes simplex virus type 1 containing the exonuclease gene and neighbouring genes. Nucleic Acids Res. 1986 Apr 25;14(8):3435–3448. doi: 10.1093/nar/14.8.3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss H. The herpes simplex virus type 2 alkaline DNase activity is essential for replication and growth. J Gen Virol. 1986 Jun;67(Pt 6):1173–1178. doi: 10.1099/0022-1317-67-6-1173. [DOI] [PubMed] [Google Scholar]
- Ooka T., Calender A., de Turenne M., Daillie J. Effect of arabinofuranosylthymine on the replication of Epstein-Barr virus and relationship with a new induced thymidine kinase activity. J Virol. 1983 Apr;46(1):187–195. doi: 10.1128/jvi.46.1.187-195.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooka T., De Turenne M., De The G., Daillie J. Epstein-Barr virus-specific DNase activity in nonproducer Raji cells after treatment with 12-o-tetradecanoylphorbol-13-acetate and sodium butyrate. J Virol. 1984 Feb;49(2):626–628. doi: 10.1128/jvi.49.2.626-628.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooka T., Lenoir G. M., Decaussin G., Bornkamm G. W., Daillie J. Epstein-Barr virus-specific DNA polymerase in virus-nonproducer Raji cells. J Virol. 1986 May;58(2):671–675. doi: 10.1128/jvi.58.2.671-675.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooka T., Lenoir G., Daillie J. Characterization of an Epstein-Barr virus-induced DNA polymerase. J Virol. 1979 Jan;29(1):1–10. doi: 10.1128/jvi.29.1.1-10.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raab-Traub N., Flynn K., Pearson G., Huang A., Levine P., Lanier A., Pagano J. The differentiated form of nasopharyngeal carcinoma contains Epstein-Barr virus DNA. Int J Cancer. 1987 Jan 15;39(1):25–29. doi: 10.1002/ijc.2910390106. [DOI] [PubMed] [Google Scholar]
- Tan R. S., Cheng Y. C., Naegele R. F., Henle W., Glaser R., Champion J. Antibody responses to Epstein-Barr virus-specific DNase in relation to the prognosis of juvenile patients with nasopharyngeal carcinoma. Int J Cancer. 1982 Nov 15;30(5):561–565. doi: 10.1002/ijc.2910300505. [DOI] [PubMed] [Google Scholar]
- Tan R. S., Datta A. K., Cheng Y. C. Identification and characterization of a DNase induced by Epstein-Barr virus. J Virol. 1982 Dec;44(3):893–899. doi: 10.1128/jvi.44.3.893-899.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan P. J., Banks L. M., Purifoy D. J., Powell K. L. Interactions between herpes simplex virus DNA-binding proteins. J Gen Virol. 1984 Nov;65(Pt 11):2033–2041. doi: 10.1099/0022-1317-65-11-2033. [DOI] [PubMed] [Google Scholar]
- Zhang C. X., Decaussin G., de Turenne Tessier M., Daillie J., Ooka T. Identification of an Epstein-Barr virus-specific desoxyribonuclease gene using complementary DNA. Nucleic Acids Res. 1987 Mar 25;15(6):2707–2717. doi: 10.1093/nar/15.6.2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Turenne-Tessier M., Ooka T., de The G., Daillie J. Characterization of an Epstein-Barr virus-induced thymidine kinase. J Virol. 1986 Mar;57(3):1105–1112. doi: 10.1128/jvi.57.3.1105-1112.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de-Vathaire F., Sancho-Garnier H., de-Thé H., Pieddeloup C., Schwaab G., Ho J. H., Ellouz R., Micheau C., Cammoun M., Cachin Y. Prognostic value of EBV markers in the clinical management of nasopharyngeal carcinoma (NPC): a multicenter follow-up study. Int J Cancer. 1988 Aug 15;42(2):176–181. doi: 10.1002/ijc.2910420206. [DOI] [PubMed] [Google Scholar]
- zur Hausen H., Schulte-Holthausen H., Klein G., Henle W., Henle G., Clifford P., Santesson L. EBV DNA in biopsies of Burkitt tumours and anaplastic carcinomas of the nasopharynx. Nature. 1970 Dec 12;228(5276):1056–1058. doi: 10.1038/2281056a0. [DOI] [PubMed] [Google Scholar]