Abstract
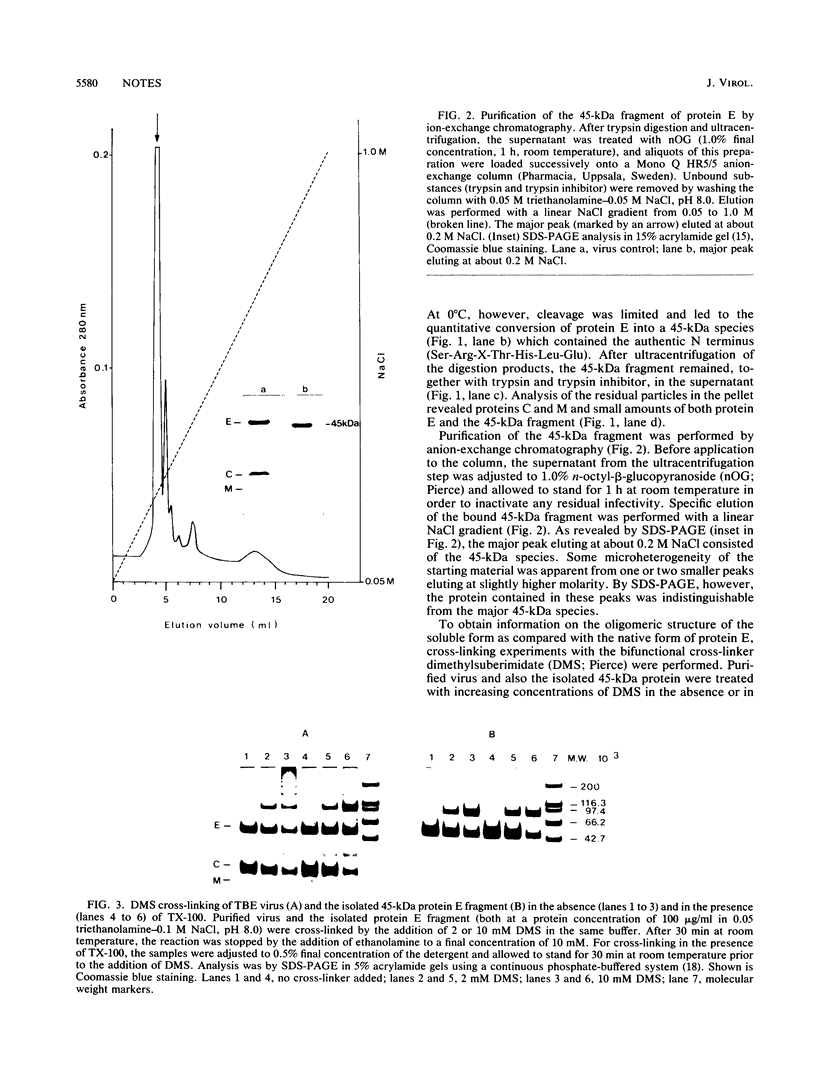
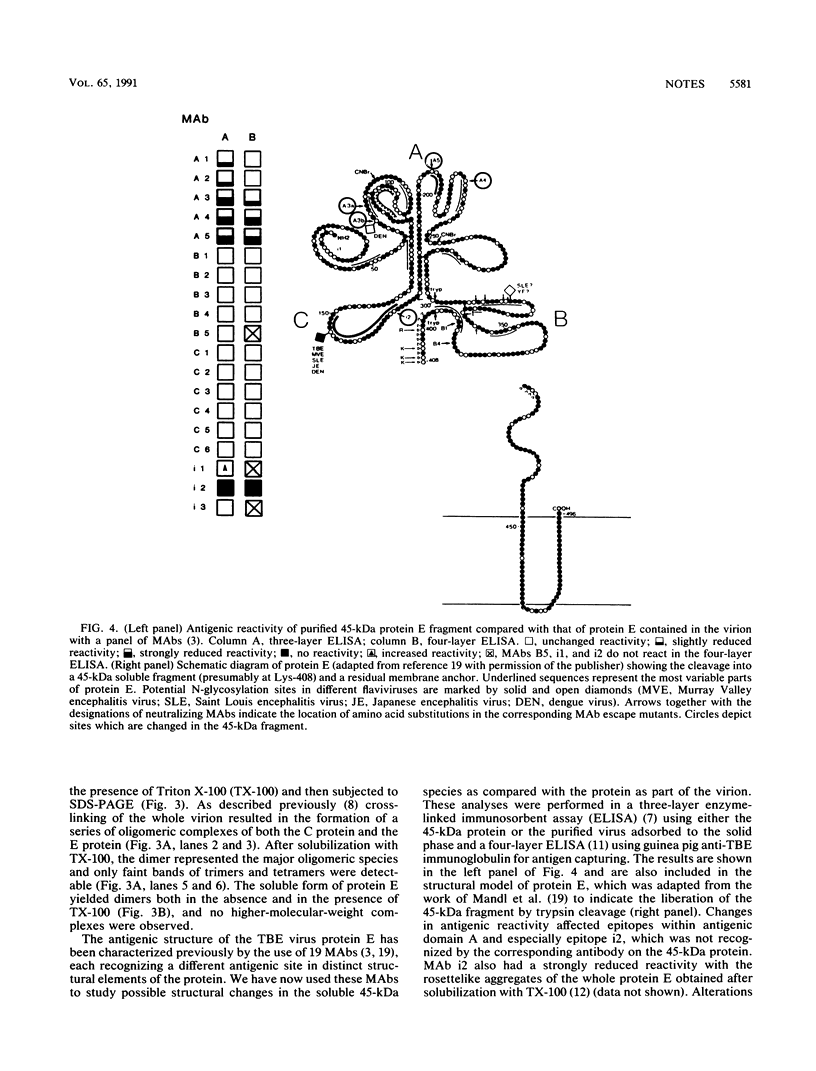
By the use of limited trypsin digestion of purified virions, we generated a membrane anchor-free and crystallizable form of the tick-borne encephalitis virus envelope glycoprotein E. It retained its reactivity with a panel of monoclonal antibodies, and only subtle structural differences from the native protein E were recognized. Treatment with the bifunctional cross-linker dimethylsuberimidate resulted in the formation of a dimer. Crystallization experiments yielded hexagonal rod-shaped crystals suitable for X-ray diffraction analysis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chambers T. J., Hahn C. S., Galler R., Rice C. M. Flavivirus genome organization, expression, and replication. Annu Rev Microbiol. 1990;44:649–688. doi: 10.1146/annurev.mi.44.100190.003245. [DOI] [PubMed] [Google Scholar]
- Gollins S. W., Porterfield J. S. pH-dependent fusion between the flavivirus West Nile and liposomal model membranes. J Gen Virol. 1986 Jan;67(Pt 1):157–166. doi: 10.1099/0022-1317-67-1-157. [DOI] [PubMed] [Google Scholar]
- Guirakhoo F., Heinz F. X., Kunz C. Epitope model of tick-borne encephalitis virus envelope glycoprotein E: analysis of structural properties, role of carbohydrate side chain, and conformational changes occurring at acidic pH. Virology. 1989 Mar;169(1):90–99. doi: 10.1016/0042-6822(89)90044-5. [DOI] [PubMed] [Google Scholar]
- Hahn C. S., Dalrymple J. M., Strauss J. H., Rice C. M. Comparison of the virulent Asibi strain of yellow fever virus with the 17D vaccine strain derived from it. Proc Natl Acad Sci U S A. 1987 Apr;84(7):2019–2023. doi: 10.1073/pnas.84.7.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn Y. S., Galler R., Hunkapiller T., Dalrymple J. M., Strauss J. H., Strauss E. G. Nucleotide sequence of dengue 2 RNA and comparison of the encoded proteins with those of other flaviviruses. Virology. 1988 Jan;162(1):167–180. doi: 10.1016/0042-6822(88)90406-0. [DOI] [PubMed] [Google Scholar]
- Heinz F. X., Berger R., Tuma W., Kunz C. A topological and functional model of epitopes on the structural glycoprotein of tick-borne encephalitis virus defined by monoclonal antibodies. Virology. 1983 Apr 30;126(2):525–537. doi: 10.1016/s0042-6822(83)80010-5. [DOI] [PubMed] [Google Scholar]
- Heinz F. X., Kunz C. Chemical crosslinking of tick-borne encephalitis virus and its subunits. J Gen Virol. 1980 Feb;46(2):301–309. doi: 10.1099/0022-1317-46-2-301. [DOI] [PubMed] [Google Scholar]
- Heinz F. X., Kunz C. Homogeneity of the structural glycoprotein from European isolates of tick-borne encephalitis virus: comparison with other flaviviruses. J Gen Virol. 1981 Dec;57(Pt 2):263–274. doi: 10.1099/0022-1317-57-2-263. [DOI] [PubMed] [Google Scholar]
- Heinz F. X., Tuma W., Guirakhoo F., Kunz C. A model study of the use of monoclonal antibodies in capture enzyme immunoassays for antigen quantification exploiting the epitope map of tick-borne encephalitis virus. J Biol Stand. 1986 Apr;14(2):133–141. doi: 10.1016/0092-1157(86)90032-6. [DOI] [PubMed] [Google Scholar]
- Heinz F. X., Tuma W., Kunz C. Antigenic and immunogenic properties of defined physical forms of tick-borne encephalitis virus structural proteins. Infect Immun. 1981 Jul;33(1):250–257. doi: 10.1128/iai.33.1.250-257.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzmann H., Heinz F. X., Mandl C. W., Guirakhoo F., Kunz C. A single amino acid substitution in envelope protein E of tick-borne encephalitis virus leads to attenuation in the mouse model. J Virol. 1990 Oct;64(10):5156–5159. doi: 10.1128/jvi.64.10.5156-5159.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K., Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973 Nov 15;80(4):575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- Laver W. G., Air G. M., Webster R. G., Smith-Gill S. J. Epitopes on protein antigens: misconceptions and realities. Cell. 1990 May 18;61(4):553–556. doi: 10.1016/0092-8674(90)90464-p. [DOI] [PubMed] [Google Scholar]
- Lobigs M., Usha R., Nestorowicz A., Marshall I. D., Weir R. C., Dalgarno L. Host cell selection of Murray Valley encephalitis virus variants altered at an RGD sequence in the envelope protein and in mouse virulence. Virology. 1990 Jun;176(2):587–595. doi: 10.1016/0042-6822(90)90029-q. [DOI] [PubMed] [Google Scholar]
- Mandl C. W., Guirakhoo F., Holzmann H., Heinz F. X., Kunz C. Antigenic structure of the flavivirus envelope protein E at the molecular level, using tick-borne encephalitis virus as a model. J Virol. 1989 Feb;63(2):564–571. doi: 10.1128/jvi.63.2.564-571.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandl C. W., Heinz F. X., Kunz C. Sequence of the structural proteins of tick-borne encephalitis virus (western subtype) and comparative analysis with other flaviviruses. Virology. 1988 Sep;166(1):197–205. doi: 10.1016/0042-6822(88)90161-4. [DOI] [PubMed] [Google Scholar]
- Nowak T., Wengler G. Analysis of disulfides present in the membrane proteins of the West Nile flavivirus. Virology. 1987 Jan;156(1):127–137. doi: 10.1016/0042-6822(87)90443-0. [DOI] [PubMed] [Google Scholar]
- Randolph V. B., Stollar V. Low pH-induced cell fusion in flavivirus-infected Aedes albopictus cell cultures. J Gen Virol. 1990 Aug;71(Pt 8):1845–1850. doi: 10.1099/0022-1317-71-8-1845. [DOI] [PubMed] [Google Scholar]
- Ray W. J., Jr, Puvathingal J. M. A simple procedure for removing contaminating aldehydes and peroxides from aqueous solutions of polyethylene glycols and of nonionic detergents that are based on the polyoxyethylene linkage. Anal Biochem. 1985 May 1;146(2):307–312. doi: 10.1016/0003-2697(85)90544-5. [DOI] [PubMed] [Google Scholar]
- Roehrig J. T., Hunt A. R., Johnson A. J., Hawkes R. A. Synthetic peptides derived from the deduced amino acid sequence of the E-glycoprotein of Murray Valley encephalitis virus elicit antiviral antibody. Virology. 1989 Jul;171(1):49–60. doi: 10.1016/0042-6822(89)90509-6. [DOI] [PubMed] [Google Scholar]
- Roehrig J. T., Johnson A. J., Hunt A. R., Bolin R. A., Chu M. C. Antibodies to dengue 2 virus E-glycoprotein synthetic peptides identify antigenic conformation. Virology. 1990 Aug;177(2):668–675. doi: 10.1016/0042-6822(90)90532-v. [DOI] [PubMed] [Google Scholar]
- Summers P. L., Cohen W. H., Ruiz M. M., Hase T., Eckels K. H. Flaviviruses can mediate fusion from without in Aedes albopictus mosquito cell cultures. Virus Res. 1989 Apr;12(4):383–392. doi: 10.1016/0168-1702(89)90095-6. [DOI] [PubMed] [Google Scholar]
- Varghese J. N., Laver W. G., Colman P. M. Structure of the influenza virus glycoprotein antigen neuraminidase at 2.9 A resolution. Nature. 1983 May 5;303(5912):35–40. doi: 10.1038/303035a0. [DOI] [PubMed] [Google Scholar]
- Weis W., Brown J. H., Cusack S., Paulson J. C., Skehel J. J., Wiley D. C. Structure of the influenza virus haemagglutinin complexed with its receptor, sialic acid. Nature. 1988 Jun 2;333(6172):426–431. doi: 10.1038/333426a0. [DOI] [PubMed] [Google Scholar]
- Wengler G., Wengler G., Nowak T., Wahn K. Analysis of the influence of proteolytic cleavage on the structural organization of the surface of the West Nile flavivirus leads to the isolation of a protease-resistant E protein oligomer from the viral surface. Virology. 1987 Sep;160(1):210–219. doi: 10.1016/0042-6822(87)90062-6. [DOI] [PubMed] [Google Scholar]
- Wiley D. C., Skehel J. J. The structure and function of the hemagglutinin membrane glycoprotein of influenza virus. Annu Rev Biochem. 1987;56:365–394. doi: 10.1146/annurev.bi.56.070187.002053. [DOI] [PubMed] [Google Scholar]
- Wilson I. A., Skehel J. J., Wiley D. C. Structure of the haemagglutinin membrane glycoprotein of influenza virus at 3 A resolution. Nature. 1981 Jan 29;289(5796):366–373. doi: 10.1038/289366a0. [DOI] [PubMed] [Google Scholar]
- Winkler G., Heinz F. X., Kunz C. Characterization of a disulphide bridge-stabilized antigenic domain of tick-borne encephalitis virus structural glycoprotein. J Gen Virol. 1987 Aug;68(Pt 8):2239–2244. doi: 10.1099/0022-1317-68-8-2239. [DOI] [PubMed] [Google Scholar]