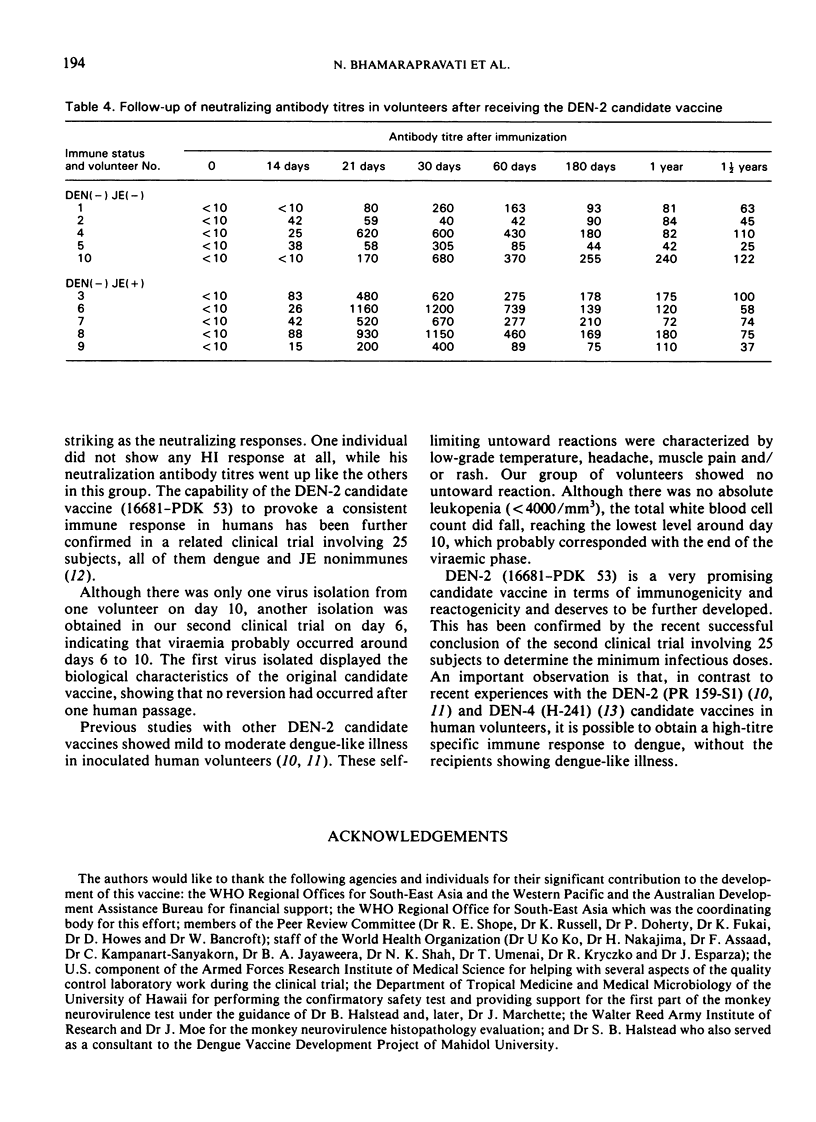
Abstract
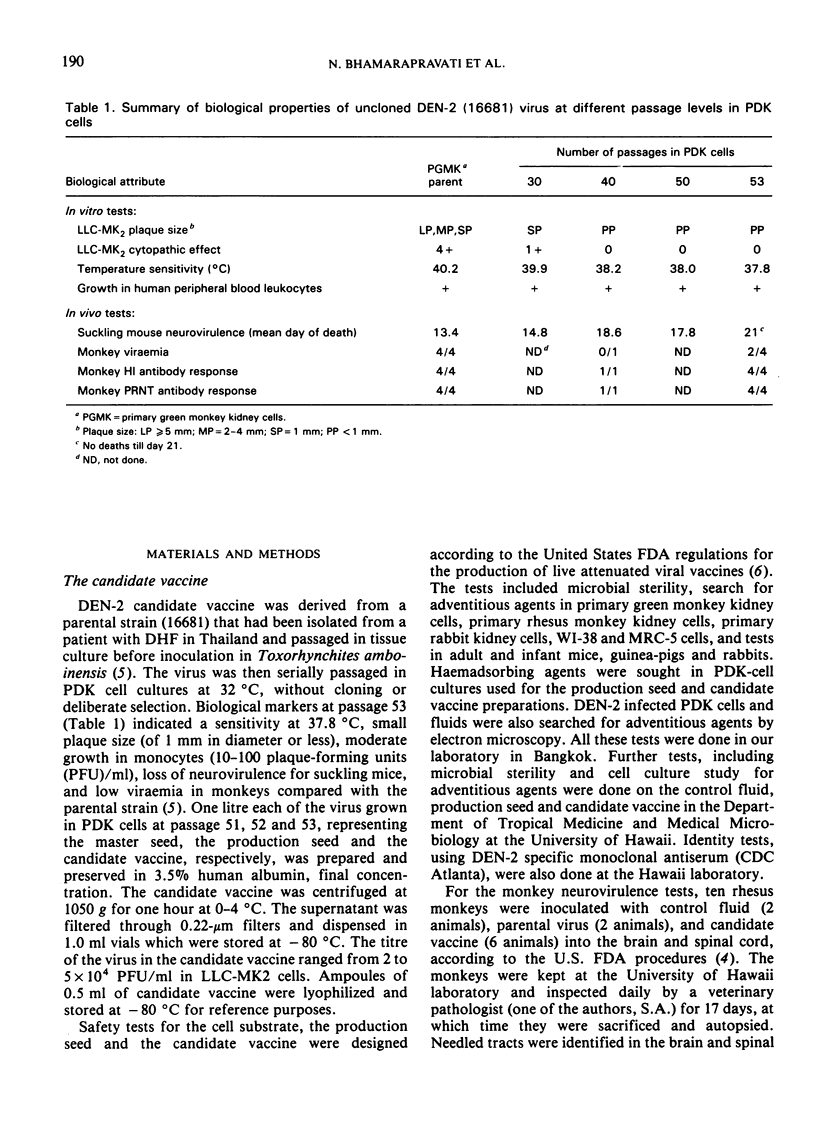
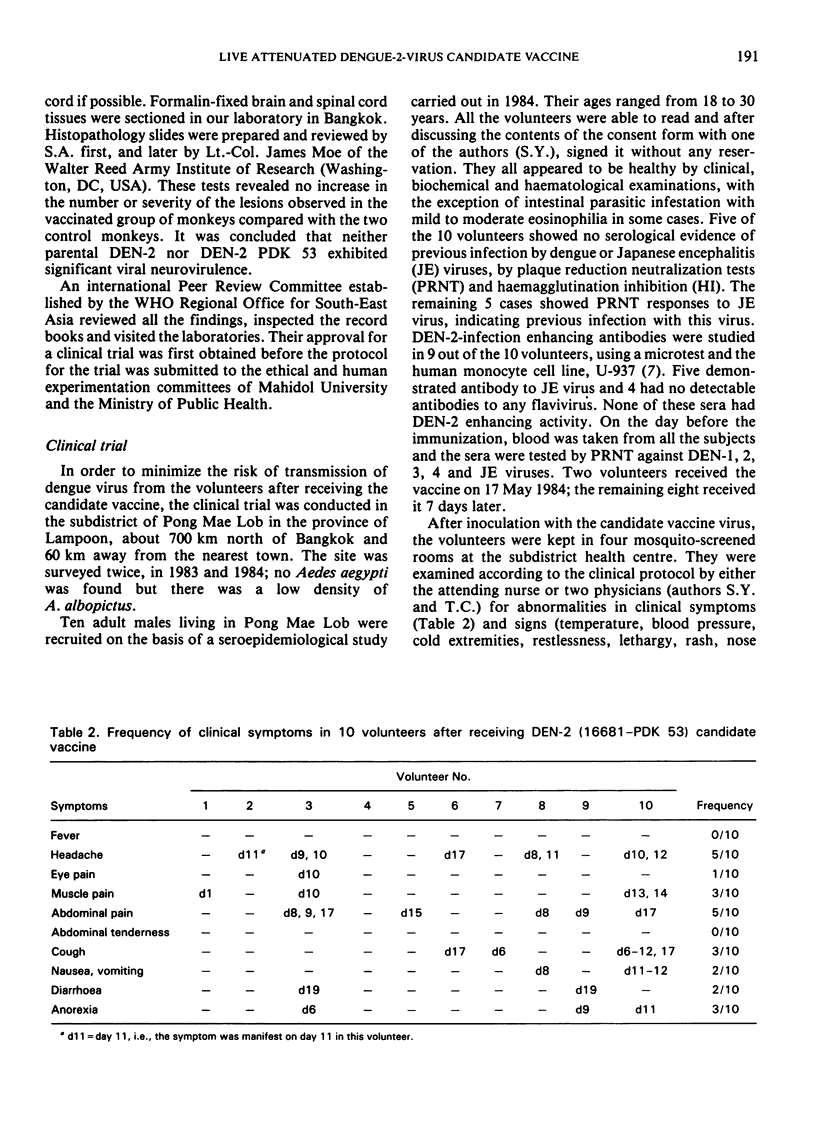
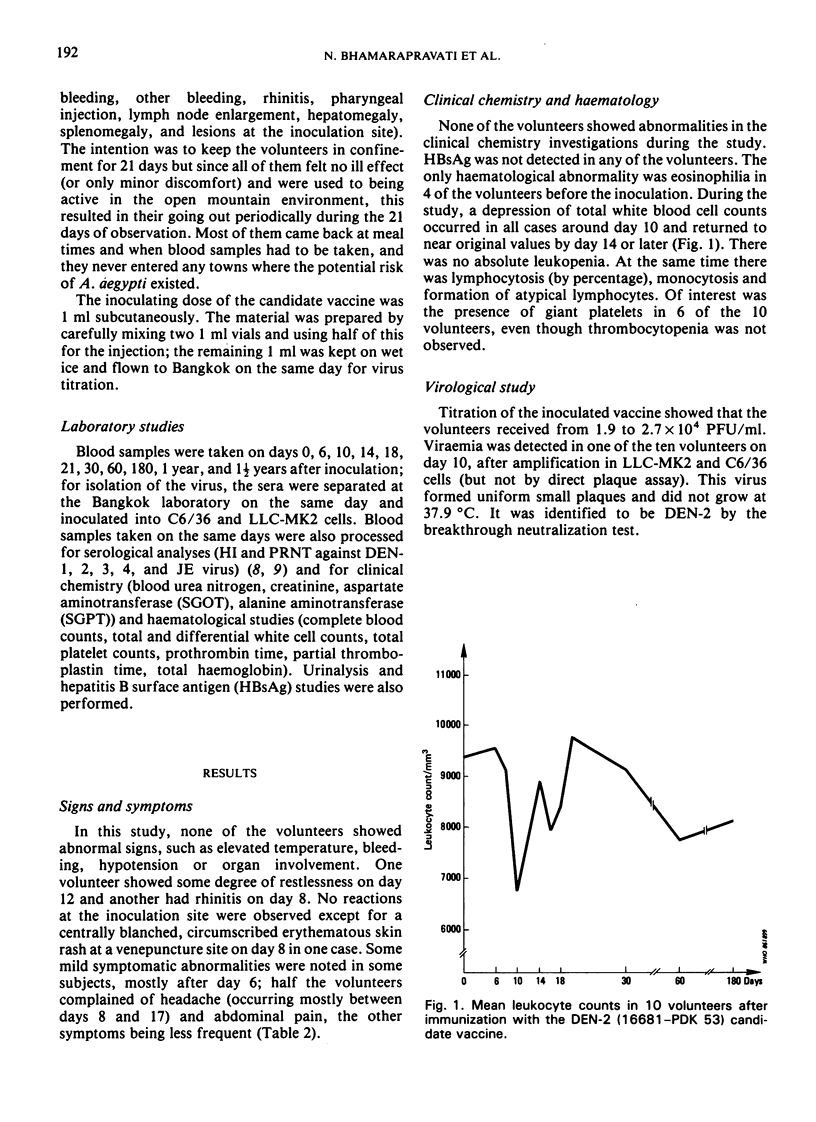
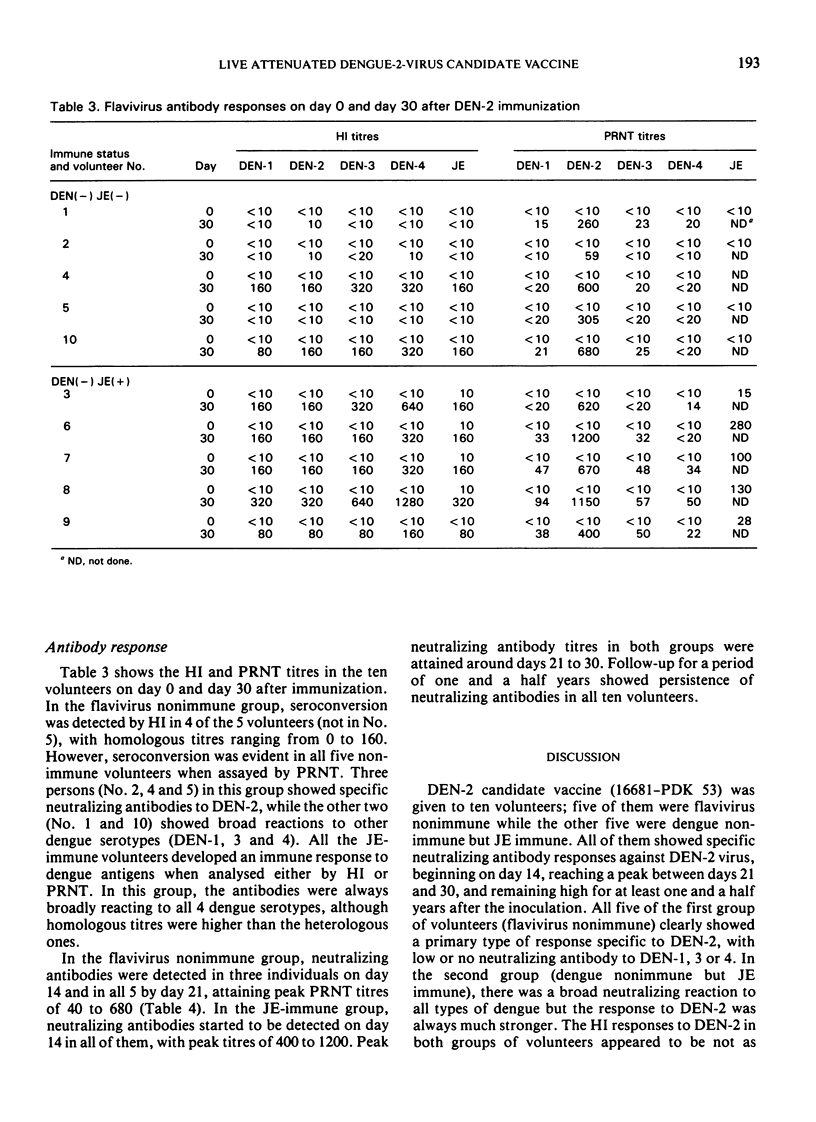
A live dengue-2 (DEN-2) candidate vaccine (strain 16681-PDK 53), attenuated by passage in primary dog kidney cells, was tested in ten adult volunteers for evaluation of the safety, infectivity and immunogenicity of a dose of 1.9-2.7 × 104 plaque-forming units. Five of the volunteers were nonimmune to either dengue or Japanese encephalitis (JE) viruses; the other five were nonimmune to dengue but immune to JE. After receiving 1.0 ml of the vaccine subcutaneously, all ten volunteers developed neutralizing antibodies to DEN-2 which were maintained for at least one and a half years. None of the subjects developed abnormal signs or symptoms and the results of clinical chemistry investigations were within normal range throughout the 21 days of observation after the immunization. Virus isolated from one viraemic volunteer retained the small-plaque and temperature-sensitive growth characteristics of the vaccine virus in vitro. Further testing of this candidate vaccine in humans is indicated.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bancroft W. H., Top F. H., Jr, Eckels K. H., Anderson J. H., Jr, McCown J. M., Russell P. K. Dengue-2 vaccine: virological, immunological, and clinical responses of six yellow fever-immune recipients. Infect Immun. 1981 Feb;31(2):698–703. doi: 10.1128/iai.31.2.698-703.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckels K. H., Harrison V. R., Summers P. L., Russell P. K. Dengue-2 vaccine: preparation from a small-plaque virus clone. Infect Immun. 1980 Jan;27(1):175–180. doi: 10.1128/iai.27.1.175-180.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halstead S. B., Simasthien P. Observations related to the pathogenesis of dengue hemorrhagic fever. II. Antigenic and biologic properties of dengue viruses and their association with disease response in the host. Yale J Biol Med. 1970 Apr;42(5):276–292. [PMC free article] [PubMed] [Google Scholar]
- Kourí G., Guzmán M. G., Bravo J. Hemorrhagic dengue in Cuba: history of an epidemic. Bull Pan Am Health Organ. 1986;20(1):24–30. [PubMed] [Google Scholar]
- Putvatana R., Yoksan S., Chayayodhin T., Bhamarapravati N., Halstead S. B. Absence of dengue 2 infection enhancement in human sera containing Japanese encephalitis antibodies. Am J Trop Med Hyg. 1984 Mar;33(2):288–294. doi: 10.4269/ajtmh.1984.33.288. [DOI] [PubMed] [Google Scholar]
- Scott R. M., Eckels K. H., Bancroft W. H., Summers P. L., McCown J. M., Anderson J. H., Russell P. K. Dengue 2 vaccine: dose response in volunteers in relation to yellow fever immune status. J Infect Dis. 1983 Dec;148(6):1055–1060. doi: 10.1093/infdis/148.6.1055. [DOI] [PubMed] [Google Scholar]


