Abstract
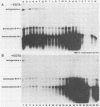
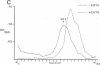
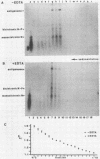
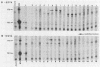
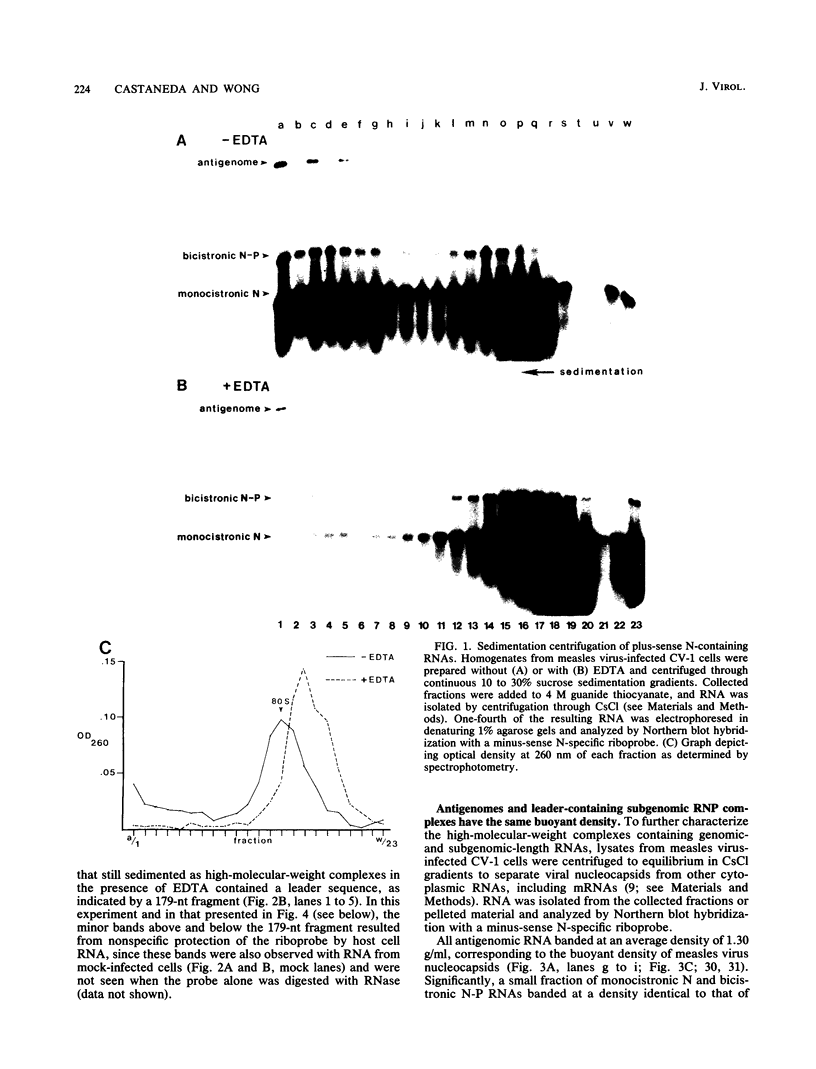
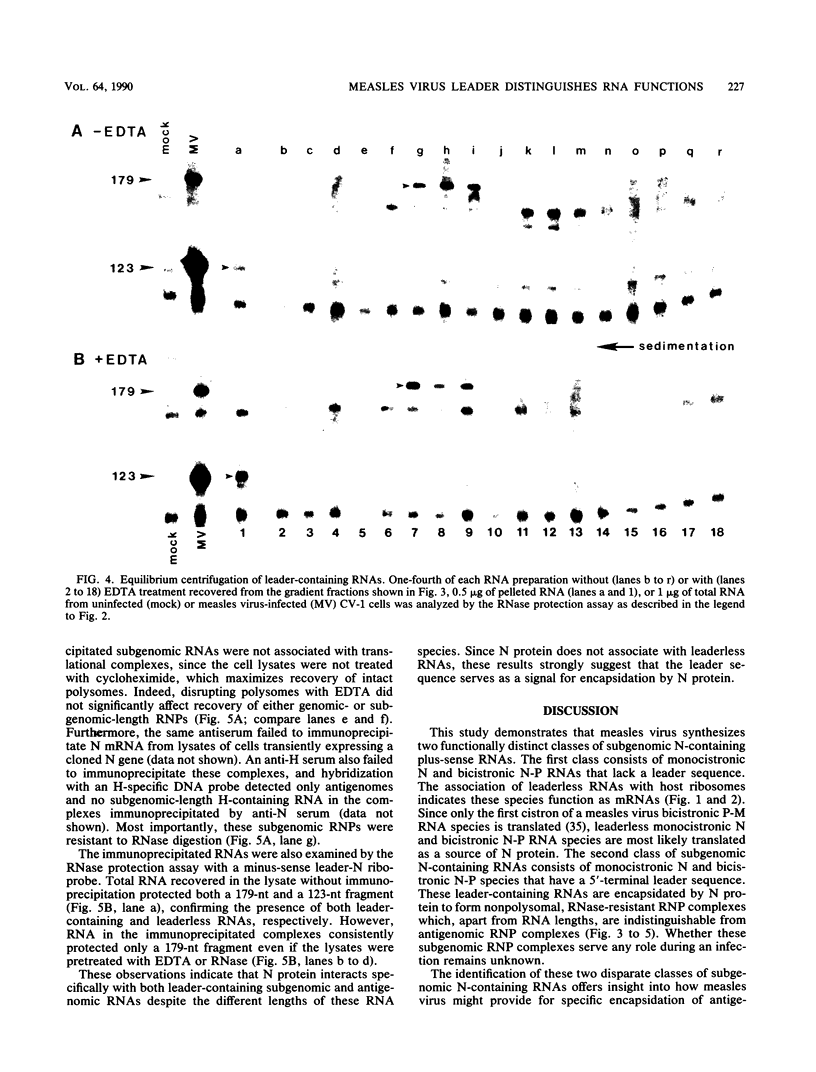
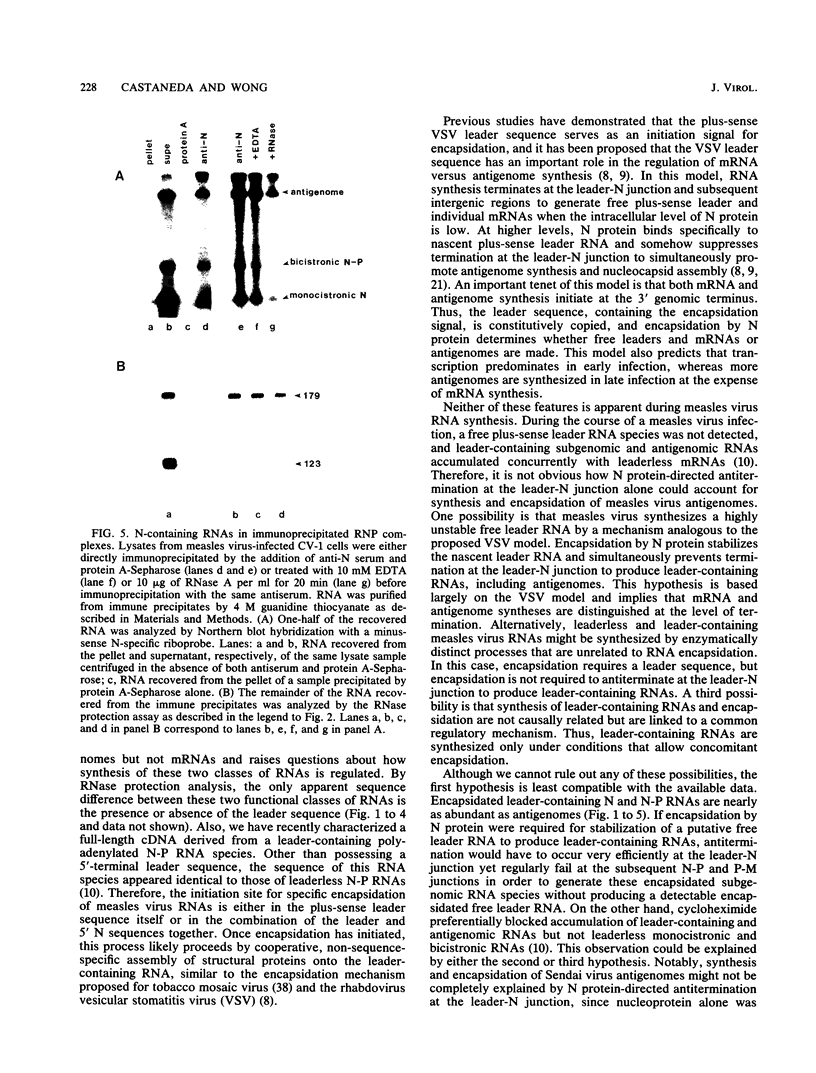
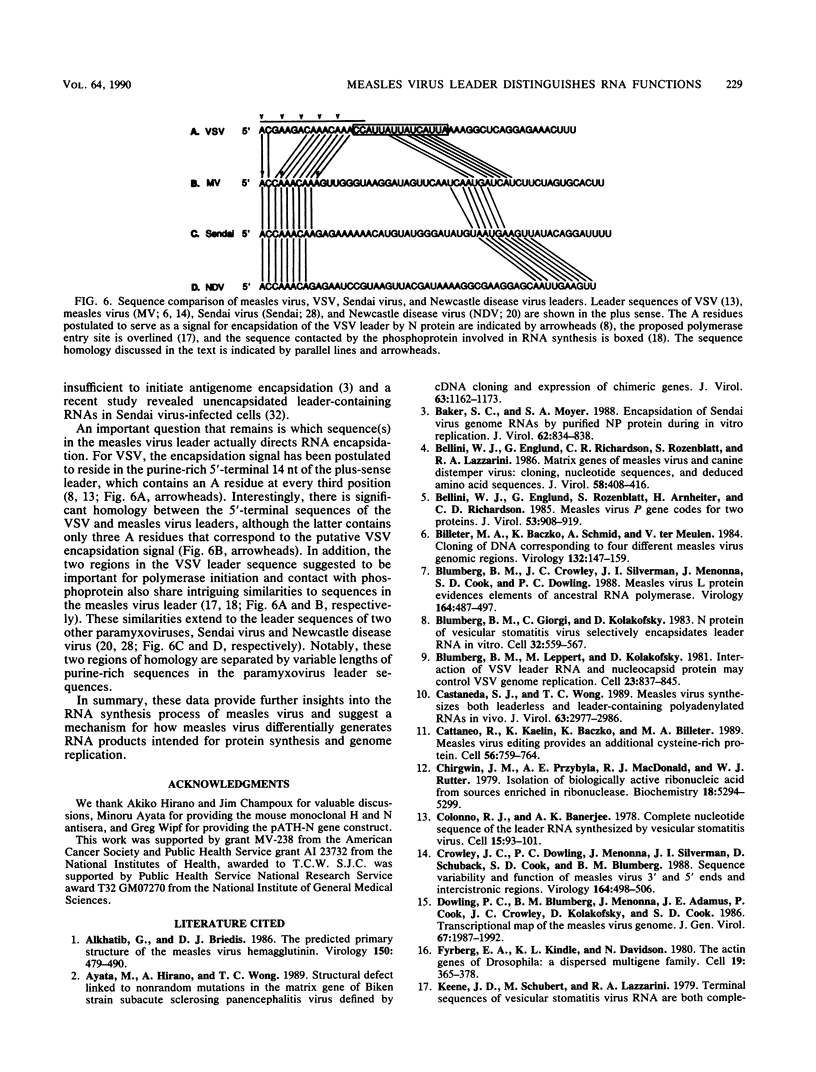
The 3'-terminal 55 nucleotides of the negative-strand measles virus RNA genome called the leader sequence is not transcribed into a detectable distinct RNA product. Most of the monocistronic N and bicistronic N-P RNAs lack the leader sequence. However, a subpopulation of the N and N-P RNAs and all of the antigenomes possess this leader. Here, we show that leader-containing subgenomic RNAs are functionally distinct from their leaderless counterparts. In measles virus-infected cells, leaderless monocistronic N and bicistronic N-P RNAs were associated with polysomes. By contrast, leader-containing N and N-P RNAs were found exclusively in nonpolysomal ribonucleoprotein complexes that were resistant to RNase and had a buoyant density of 1.30 g/ml, the same as that of antigenomic ribonucleoprotein complexes. Both antigenomic and subgenomic ribonucleoprotein complexes were specifically immunoprecipitated by antiserum against the N protein, and leaderless RNAs were not found in these complexes. These findings suggest that measles virus distinguishes RNAs destined for encapsidation or translation by the presence or absence of a leader sequence.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alkhatib G., Briedis D. J. The predicted primary structure of the measles virus hemagglutinin. Virology. 1986 Apr 30;150(2):479–490. doi: 10.1016/0042-6822(86)90312-0. [DOI] [PubMed] [Google Scholar]
- Ayata M., Hirano A., Wong T. C. Structural defect linked to nonrandom mutations in the matrix gene of biken strain subacute sclerosing panencephalitis virus defined by cDNA cloning and expression of chimeric genes. J Virol. 1989 Mar;63(3):1162–1173. doi: 10.1128/jvi.63.3.1162-1173.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker S. C., Moyer S. A. Encapsidation of Sendai virus genome RNAs by purified NP protein during in vitro replication. J Virol. 1988 Mar;62(3):834–838. doi: 10.1128/jvi.62.3.834-838.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellini W. J., Englund G., Richardson C. D., Rozenblatt S., Lazzarini R. A. Matrix genes of measles virus and canine distemper virus: cloning, nucleotide sequences, and deduced amino acid sequences. J Virol. 1986 May;58(2):408–416. doi: 10.1128/jvi.58.2.408-416.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellini W. J., Englund G., Rozenblatt S., Arnheiter H., Richardson C. D. Measles virus P gene codes for two proteins. J Virol. 1985 Mar;53(3):908–919. doi: 10.1128/jvi.53.3.908-919.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billeter M. A., Baczko K., Schmid A., Ter Meulen V. Cloning of DNA corresponding to four different measles virus genomic regions. Virology. 1984 Jan 15;132(1):147–159. doi: 10.1016/0042-6822(84)90099-0. [DOI] [PubMed] [Google Scholar]
- Blumberg B. M., Crowley J. C., Silverman J. I., Menonna J., Cook S. D., Dowling P. C. Measles virus L protein evidences elements of ancestral RNA polymerase. Virology. 1988 Jun;164(2):487–497. doi: 10.1016/0042-6822(88)90563-6. [DOI] [PubMed] [Google Scholar]
- Blumberg B. M., Giorgi C., Kolakofsky D. N protein of vesicular stomatitis virus selectively encapsidates leader RNA in vitro. Cell. 1983 Feb;32(2):559–567. doi: 10.1016/0092-8674(83)90475-0. [DOI] [PubMed] [Google Scholar]
- Blumberg B. M., Leppert M., Kolakofsky D. Interaction of VSV leader RNA and nucleocapsid protein may control VSV genome replication. Cell. 1981 Mar;23(3):837–845. doi: 10.1016/0092-8674(81)90448-7. [DOI] [PubMed] [Google Scholar]
- Castaneda S. J., Wong T. C. Measles virus synthesizes both leaderless and leader-containing polyadenylated RNAs in vivo. J Virol. 1989 Jul;63(7):2977–2986. doi: 10.1128/jvi.63.7.2977-2986.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo R., Kaelin K., Baczko K., Billeter M. A. Measles virus editing provides an additional cysteine-rich protein. Cell. 1989 Mar 10;56(5):759–764. doi: 10.1016/0092-8674(89)90679-x. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Colonno R. J., Banerjee A. K. Complete nucleotide sequence of the leader RNA synthesized in vitro by vesicular stomatitis virus. Cell. 1978 Sep;15(1):93–101. doi: 10.1016/0092-8674(78)90085-5. [DOI] [PubMed] [Google Scholar]
- Crowley J. C., Dowling P. C., Menonna J., Silverman J. I., Schuback D., Cook S. D., Blumberg B. M. Sequence variability and function of measles virus 3' and 5' ends and intercistronic regions. Virology. 1988 Jun;164(2):498–506. doi: 10.1016/0042-6822(88)90564-8. [DOI] [PubMed] [Google Scholar]
- Dowling P. C., Blumberg B. M., Menonna J., Adamus J. E., Cook P., Crowley J. C., Kolakofsky D., Cook S. D. Transcriptional map of the measles virus genome. J Gen Virol. 1986 Sep;67(Pt 9):1987–1992. doi: 10.1099/0022-1317-67-9-1987. [DOI] [PubMed] [Google Scholar]
- Fyrberg E. A., Kindle K. L., Davidson N., Kindle K. L. The actin genes of Drosophila: a dispersed multigene family. Cell. 1980 Feb;19(2):365–378. doi: 10.1016/0092-8674(80)90511-5. [DOI] [PubMed] [Google Scholar]
- Keene J. D., Schubert M., Lazzarini R. A. Terminal sequences of vesicular stomatitis virus RNA are both complementary and conserved. J Virol. 1979 Oct;32(1):167–174. doi: 10.1128/jvi.32.1.167-174.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keene J. D., Thornton B. J., Emerson S. U. Sequence-specific contacts between the RNA polymerase of vesicular stomatitis virus and the leader RNA gene. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6191–6195. doi: 10.1073/pnas.78.10.6191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurilla M. G., Stone H. O., Keene J. D. RNA sequence and transcriptional properties of the 3' end of the Newcastle disease virus genome. Virology. 1985 Sep;145(2):203–212. doi: 10.1016/0042-6822(85)90154-0. [DOI] [PubMed] [Google Scholar]
- Leppert M., Rittenhouse L., Perrault J., Summers D. F., Kolakofsky D. Plus and minus strand leader RNAs in negative strand virus-infected cells. Cell. 1979 Nov;18(3):735–747. doi: 10.1016/0092-8674(79)90127-2. [DOI] [PubMed] [Google Scholar]
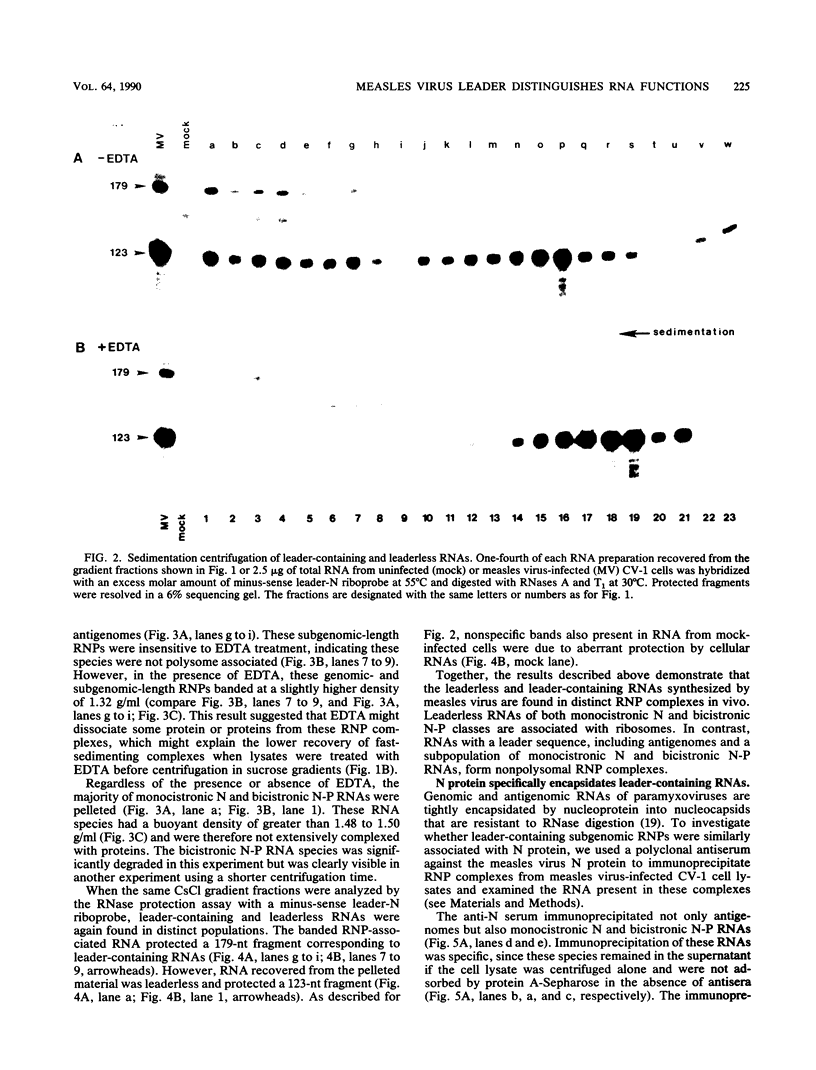
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pape L. K., Koerner T. J., Tzagoloff A. Characterization of a yeast nuclear gene (MST1) coding for the mitochondrial threonyl-tRNA1 synthetase. J Biol Chem. 1985 Dec 5;260(28):15362–15370. [PubMed] [Google Scholar]
- Richardson C., Hull D., Greer P., Hasel K., Berkovich A., Englund G., Bellini W., Rima B., Lazzarini R. The nucleotide sequence of the mRNA encoding the fusion protein of measles virus (Edmonston strain): a comparison of fusion proteins from several different paramyxoviruses. Virology. 1986 Dec;155(2):508–523. doi: 10.1016/0042-6822(86)90212-6. [DOI] [PubMed] [Google Scholar]
- Rima B. K., Baczko K., Clarke D. K., Curran M. D., Martin S. J., Billeter M. A., ter Meulen V. Characterization of clones for the sixth (L) gene and a transcriptional map for morbilliviruses. J Gen Virol. 1986 Sep;67(Pt 9):1971–1978. doi: 10.1099/0022-1317-67-9-1971. [DOI] [PubMed] [Google Scholar]
- Rozenblatt S., Eizenberg O., Ben-Levy R., Lavie V., Bellini W. J. Sequence homology within the morbilliviruses. J Virol. 1985 Feb;53(2):684–690. doi: 10.1128/jvi.53.2.684-690.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shioda T., Hidaka Y., Kanda T., Shibuta H., Nomoto A., Iwasaki K. Sequence of 3,687 nucleotides from the 3' end of Sendai virus genome RNA and the predicted amino acid sequences of viral NP, P and C proteins. Nucleic Acids Res. 1983 Nov 11;11(21):7317–7330. doi: 10.1093/nar/11.21.7317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spindler K. R., Rosser D. S., Berk A. J. Analysis of adenovirus transforming proteins from early regions 1A and 1B with antisera to inducible fusion antigens produced in Escherichia coli. J Virol. 1984 Jan;49(1):132–141. doi: 10.1128/jvi.49.1.132-141.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stallcup K. C., Wechsler S. L., Fields B. N. Purification of measles virus and characterization of subviral components. J Virol. 1979 Apr;30(1):166–176. doi: 10.1128/jvi.30.1.166-176.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udem S. A., Cook K. A. Isolation and characterization of measles virus intracellular nucleocapsid RNA. J Virol. 1984 Jan;49(1):57–65. doi: 10.1128/jvi.49.1.57-65.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal S., Kolakofsky D. Modified model for the switch from Sendai virus transcription to replication. J Virol. 1989 May;63(5):1951–1958. doi: 10.1128/jvi.63.5.1951-1958.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
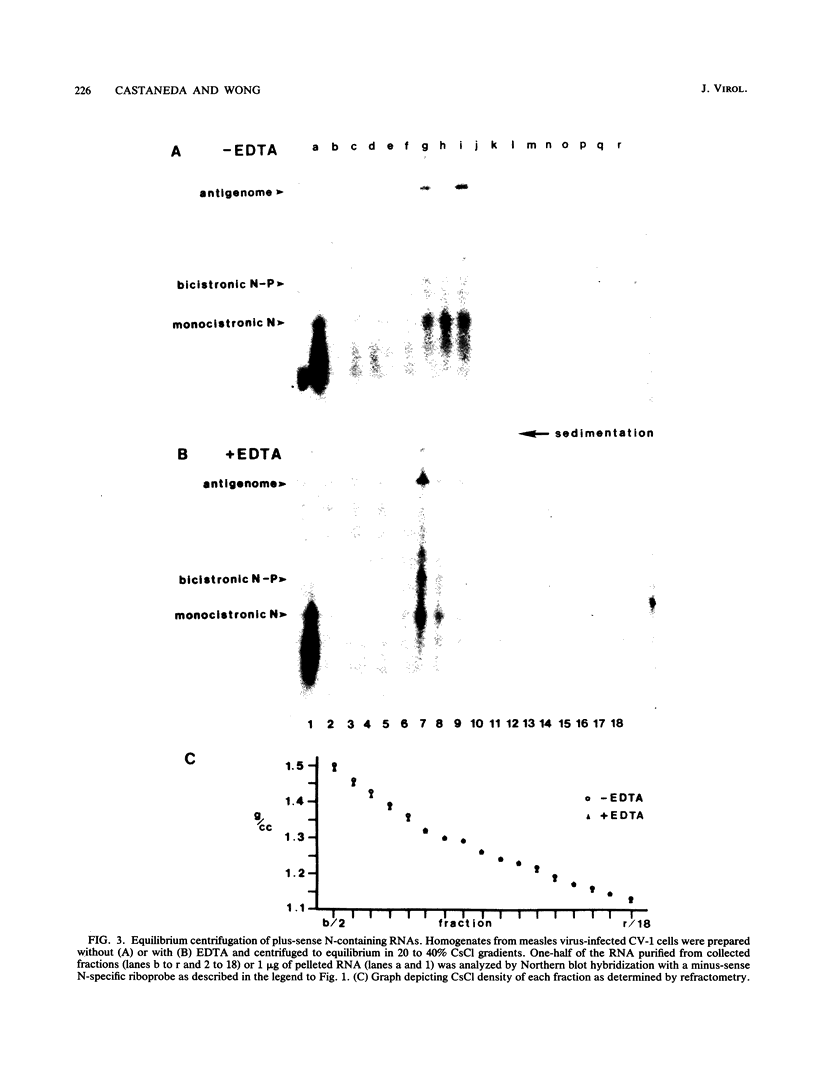
- Wilde A., Morrison T. Structural and functional characterization of Newcastle disease virus polycistronic RNA species. J Virol. 1984 Jul;51(1):71–76. doi: 10.1128/jvi.51.1.71-76.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong T. C., Hirano A. Functional cDNA library for efficient expression of measles virus-specific gene products in primate cells. J Virol. 1986 Jan;57(1):343–348. doi: 10.1128/jvi.57.1.343-348.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong T. C., Hirano A. Structure and function of bicistronic RNA encoding the phosphoprotein and matrix protein of measles virus. J Virol. 1987 Feb;61(2):584–589. doi: 10.1128/jvi.61.2.584-589.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong T. C., Kang C. Y. Isolation and characterization of a virus-specific ribonucleoprotein complex from reticuloendotheliosis virus-transformed chicken bone marrow cells. J Virol. 1978 Oct;28(1):34–44. doi: 10.1128/jvi.28.1.34-44.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa Y., Mizumoto K., Yamanouchi K. Characterization of messenger RNAs of measles virus. J Gen Virol. 1986 Dec;67(Pt 12):2807–2812. doi: 10.1099/0022-1317-67-12-2807. [DOI] [PubMed] [Google Scholar]
- Zimmern D., Butler P. J. The isolation of tobacco mosaic virus RNA fragments containing the origin for viral assembly. Cell. 1977 Jul;11(3):455–462. doi: 10.1016/0092-8674(77)90064-2. [DOI] [PubMed] [Google Scholar]