Abstract
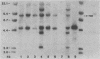
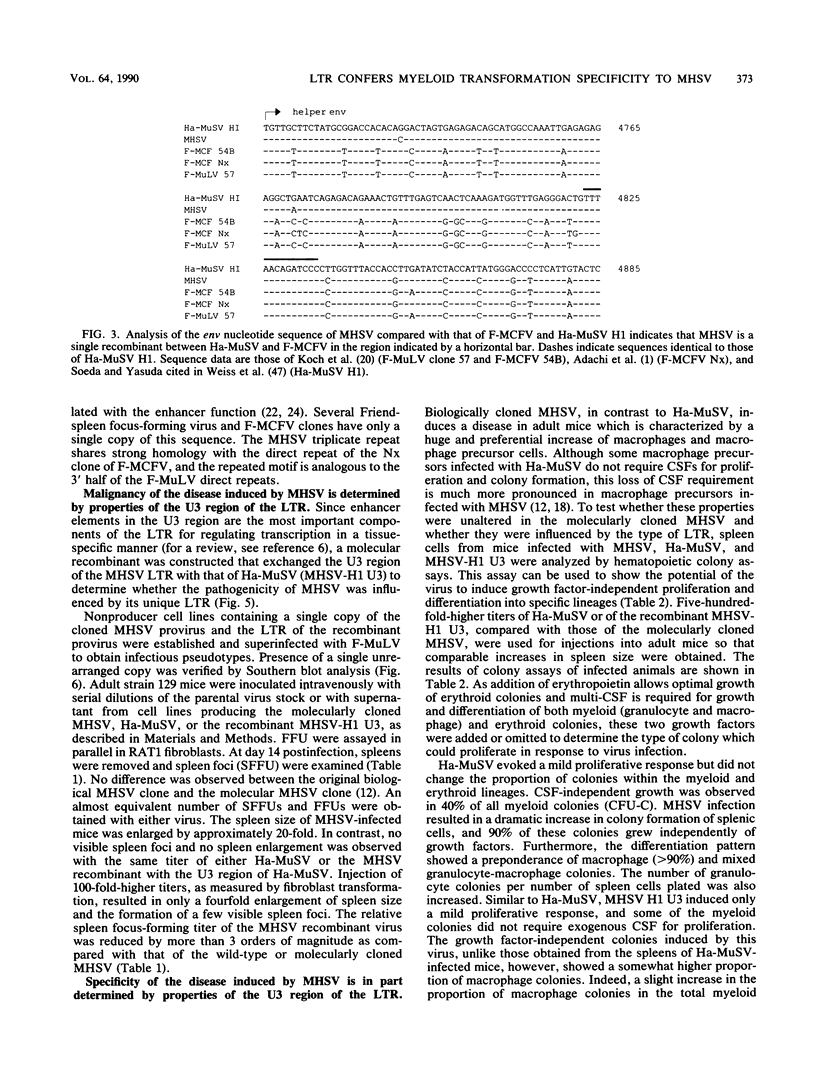
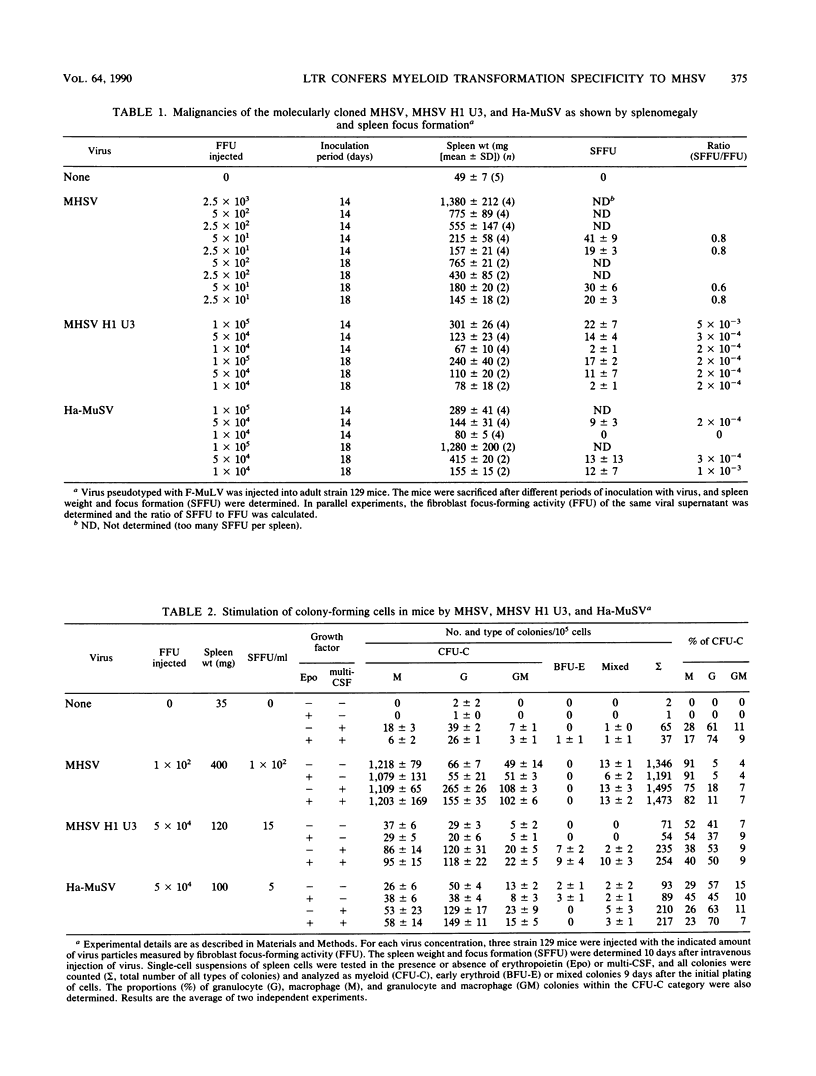
The malignant histiocytosis sarcoma virus (MHSV), in contrast to other viruses with the ras oncogene, induces acute histiocytosis in newborn and adult mice. Molecular structure and function studies were initiated to determine the basis of its unique macrophage-transforming potential. Characterization of the genomic structure showed that the virus evolved by recombination of the Harvey murine sarcoma virus (Ha-MuSV) and a virus of the Friend-mink cell focus-forming virus family. Structural analysis of MHSV showed two regions of the genome that are basically different from the Ha-MuSV: (i) the ras gene, which is altered by a point mutation in codon 181 leading to a Cys----Ser substitution of the p21 protein, and (ii) the U3 region of the long terminal repeat, which is largely derived from F-MCFV and contains a deletion of one direct repeat as well as a duplication of an altered enhancer-like region. Biological studies of Ha-MuSV, MHSV, and recombinants between the two viruses show that the U3 region of the MHSV long terminal repeat is essential for the malignancy and specificity of the disease. A contributing role of the ras point mutation in determining macrophage specificity, however, cannot be excluded.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi A., Sakai K., Kitamura N., Nakanishi S., Niwa O., Matsuyama M., Ishimoto A. Characterization of the env gene and long terminal repeat of molecularly cloned Friend mink cell focus-inducing virus DNA. J Virol. 1984 Jun;50(3):813–821. doi: 10.1128/jvi.50.3.813-821.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celander D., Haseltine W. A. Tissue-specific transcription preference as a determinant of cell tropism and leukaemogenic potential of murine retroviruses. Nature. 1984 Nov 8;312(5990):159–162. doi: 10.1038/312159a0. [DOI] [PubMed] [Google Scholar]
- Chang E. H., Maryak J. M., Wei C. M., Shih T. Y., Shober R., Cheung H. L., Ellis R. W., Hager G. L., Scolnick E. M., Lowy D. R. Functional organization of the Harvey murine sarcoma virus genome. J Virol. 1980 Jul;35(1):76–92. doi: 10.1128/jvi.35.1.76-92.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatis P. A., Holland C. A., Hartley J. W., Rowe W. P., Hopkins N. Role for the 3' end of the genome in determining disease specificity of Friend and Moloney murine leukemia viruses. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4408–4411. doi: 10.1073/pnas.80.14.4408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cichutek K., Duesberg P. H. Harvey ras genes transform without mutant codons, apparently activated by truncation of a 5' exon (exon -1). Proc Natl Acad Sci U S A. 1986 Apr;83(8):2340–2344. doi: 10.1073/pnas.83.8.2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DesGroseillers L., Rassart E., Jolicoeur P. Thymotropism of murine leukemia virus is conferred by its long terminal repeat. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4203–4207. doi: 10.1073/pnas.80.14.4203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhar R., Ellis R. W., Shih T. Y., Oroszlan S., Shapiro B., Maizel J., Lowy D., Scolnick E. Nucleotide sequence of the p21 transforming protein of Harvey murine sarcoma virus. Science. 1982 Sep 3;217(4563):934–936. doi: 10.1126/science.6287572. [DOI] [PubMed] [Google Scholar]
- Dube S., Kung H. J., Bender W., Davidson N., Ostertag W. Size, subunit composition, and secondary structure of the Friend virus genome. J Virol. 1976 Oct;20(1):264–272. doi: 10.1128/jvi.20.1.264-272.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis R. W., DeFeo D., Maryak J. M., Young H. A., Shih T. Y., Chang E. H., Lowy D. R., Scolnick E. M. Dual evolutionary origin for the rat genetic sequences of Harvey murine sarcoma virus. J Virol. 1980 Nov;36(2):408–420. doi: 10.1128/jvi.36.2.408-420.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Franz T., Löhler J., Fusco A., Pragnell I., Nobis P., Padua R., Ostertag W. Transformation of mononuclear phagocytes in vivo and malignant histiocytosis caused by a novel murine spleen focus-forming virus. Nature. 1985 May 9;315(6015):149–151. doi: 10.1038/315149a0. [DOI] [PubMed] [Google Scholar]
- Fredrickson T. N., O'Neill R. R., Rutledge R. A., Theodore T. S., Martin M. A., Ruscetti S. K., Austin J. B., Hartley J. W. Biologic and molecular characterization of two newly isolated ras-containing murine leukemia viruses. J Virol. 1987 Jul;61(7):2109–2119. doi: 10.1128/jvi.61.7.2109-2119.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfarb M. P., Weinberg R. A. Generation of novel, biologically active Harvey sarcoma viruses via apparent illegitimate recombination. J Virol. 1981 Apr;38(1):136–150. doi: 10.1128/jvi.38.1.136-150.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green P. L., Kaehler D. A., McKearn J., Risser R. Substitution of the LTR of Abelson murine leukemia virus does not alter the cell type of virally induced tumors. Oncogene. 1988 Jun;2(6):585–592. [PubMed] [Google Scholar]
- Johnson G. R., Ostertag W., Nicola N. A. Proliferation in vivo and in vitro of haemopoietic progenitor cells induced by AF-1, a new ras-containing retrovirus. Haematol Blood Transfus. 1985;29:376–379. doi: 10.1007/978-3-642-70385-0_77. [DOI] [PubMed] [Google Scholar]
- Klingler K., Johnson G. R., Nicola N. A., Arman G., Kluge N., Ostertag W. Transformation of single myeloid precursor cells by the malignant histiocytosis sarcoma virus (MHSV): generation of growth-factor-independent myeloid colonies and permanent cell lines. J Cell Physiol. 1988 Apr;135(1):32–38. doi: 10.1002/jcp.1041350105. [DOI] [PubMed] [Google Scholar]
- Klingler K., Johnson G. R., Walker F., Nicola N. A., Decker T., Ostertag W. Macrophage cell lines transformed by the malignant histiocytosis sarcoma virus: increase of CSF receptors suggests a model for transformation. J Cell Physiol. 1987 Jul;132(1):22–32. doi: 10.1002/jcp.1041320104. [DOI] [PubMed] [Google Scholar]
- Koch W., Zimmermann W., Oliff A., Friedrich R. Molecular analysis of the envelope gene and long terminal repeat of Friend mink cell focus-inducing virus: implications for the functions of these sequences. J Virol. 1984 Mar;49(3):828–840. doi: 10.1128/jvi.49.3.828-840.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacal J. C., Anderson P. S., Aaronson S. A. Deletion mutants of Harvey ras p21 protein reveal the absolute requirement of at least two distant regions for GTP-binding and transforming activities. EMBO J. 1986 Apr;5(4):679–687. doi: 10.1002/j.1460-2075.1986.tb04267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laimins L. A., Khoury G., Gorman C., Howard B., Gruss P. Host-specific activation of transcription by tandem repeats from simian virus 40 and Moloney murine sarcoma virus. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6453–6457. doi: 10.1073/pnas.79.21.6453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laker C., Stocking C., Bergholz U., Hess N., De Lamarter J. F., Ostertag W. Autocrine stimulation after transfer of the granulocyte/macrophage colony-stimulating factor gene and autonomous growth are distinct but interdependent steps in the oncogenic pathway. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8458–8462. doi: 10.1073/pnas.84.23.8458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz J., Celander D., Crowther R. L., Patarca R., Perkins D. W., Haseltine W. A. Determination of the leukaemogenicity of a murine retrovirus by sequences within the long terminal repeat. 1984 Mar 29-Apr 4Nature. 308(5958):467–470. doi: 10.1038/308467a0. [DOI] [PubMed] [Google Scholar]
- Löhler J., Franz T., Fusco A., Pragnell I., Ostertag W. Murine retrovirus-induced malignant histiocytosis, an experimental model for the disease in humans. Leukemia. 1987 Jan;1(1):58–68. [PubMed] [Google Scholar]
- Mathey-Prevot B., Baltimore D. Specific transforming potential of oncogenes encoding protein-tyrosine kinases. EMBO J. 1985 Jul;4(7):1769–1774. doi: 10.1002/j.1460-2075.1985.tb03849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathey-Prevot B., Nabel G., Palacios R., Baltimore D. Abelson virus abrogation of interleukin-3 dependence in a lymphoid cell line. Mol Cell Biol. 1986 Nov;6(11):4133–4135. doi: 10.1128/mcb.6.11.4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Ostertag W., Vehmeyer K., Fagg B., Pragnell I. B., Paetz W., Le Bousse M. C., Smadja-Joffe F., Klein B., Jasmin C., Eisen H. Myeloproliferative virus, a cloned murine sarcoma virus with spleen focus-forming properties in adult mice. J Virol. 1980 Feb;33(2):573–582. doi: 10.1128/jvi.33.2.573-582.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce J. H., Aaronson S. A. Myeloid cell transformation by ras-containing murine sarcoma viruses. Mol Cell Biol. 1985 Apr;5(4):667–674. doi: 10.1128/mcb.5.4.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pragnell I. B., Fusco A., Arbuthnott C., Smadja-Joffe F., Klein B., Jasmin C., Ostertag W. Analysis of the myeloproliferative sarcoma virus genome: limited changes in the prototype lead to altered target cell specificity. J Virol. 1981 Jun;38(3):952–957. doi: 10.1128/jvi.38.3.952-957.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pragnell I. B., Ostertag W., Paul J. The expression of viral and globin genes during differentiation of the Friend cell. Exp Cell Res. 1977 Sep;108(2):269–278. doi: 10.1016/s0014-4827(77)80034-7. [DOI] [PubMed] [Google Scholar]
- Privalsky M. L. Creation of a chimeric oncogene: analysis of the biochemical and biological properties of v-erbB/src fusion polypeptide. J Virol. 1987 Jun;61(6):1938–1948. doi: 10.1128/jvi.61.6.1938-1948.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy E. P., Lipman D., Andersen P. R., Tronick S. R., Aaronson S. A. Nucleotide sequence analysis of the BALB/c murine sarcoma virus transforming gene. J Virol. 1985 Mar;53(3):984–987. doi: 10.1128/jvi.53.3.984-987.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rein A., Keller J., Schultz A. M., Holmes K. L., Medicus R., Ihle J. N. Infection of immune mast cells by Harvey sarcoma virus: immortalization without loss of requirement for interleukin-3. Mol Cell Biol. 1985 Sep;5(9):2257–2264. doi: 10.1128/mcb.5.9.2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruta M., Wolford R., Dhar R., Defeo-Jones D., Ellis R. W., Scolnick E. M. Nucleotide sequence of the two rat cellular rasH genes. Mol Cell Biol. 1986 May;6(5):1706–1710. doi: 10.1128/mcb.6.5.1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stocking C., Kollek R., Bergholz U., Ostertag W. Long terminal repeat sequences impart hematopoietic transformation properties to the myeloproliferative sarcoma virus. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5746–5750. doi: 10.1073/pnas.82.17.5746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocking C., Kollek R., Bergholz U., Ostertag W. Point mutations in the U3 region of the long terminal repeat of Moloney murine leukemia virus determine disease specificity of the myeloproliferative sarcoma virus. Virology. 1986 Aug;153(1):145–149. doi: 10.1016/0042-6822(86)90015-2. [DOI] [PubMed] [Google Scholar]
- Tambourin P., Casadevall N., Choppin J., Lacombe C., Heard J. M., Fichelson S., Wendling F., Varet B. Production of erythropoietin-like activity by a murine erythroleukemia cell line. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6269–6273. doi: 10.1073/pnas.80.20.6269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taparowsky E., Suard Y., Fasano O., Shimizu K., Goldfarb M., Wigler M. Activation of the T24 bladder carcinoma transforming gene is linked to a single amino acid change. Nature. 1982 Dec 23;300(5894):762–765. doi: 10.1038/300762a0. [DOI] [PubMed] [Google Scholar]
- Topp W. C. Normal rat cell lines deficient in nuclear thymidine kinase. Virology. 1981 Aug;113(1):408–411. doi: 10.1016/0042-6822(81)90168-9. [DOI] [PubMed] [Google Scholar]
- Vande Woude G. F., Oskarsson M., Enquist L. W., Nomura S., Sullivan M., Fischinger P. J. Cloning of integrated Moloney sarcoma proviral DNA sequences in bacteriophage lambda. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4464–4468. doi: 10.1073/pnas.76.9.4464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velu T. J., Vass W. C., Lowy D. R., Tambourin P. E. Harvey murine sarcoma virus: influences of coding and noncoding sequences on cell transformation in vitro and oncogenicity in vivo. J Virol. 1989 Mar;63(3):1384–1392. doi: 10.1128/jvi.63.3.1384-1392.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willumsen B. M., Papageorge A. G., Hubbert N., Bekesi E., Kung H. F., Lowy D. R. Transforming p21 ras protein: flexibility in the major variable region linking the catalytic and membrane-anchoring domains. EMBO J. 1985 Nov;4(11):2893–2896. doi: 10.1002/j.1460-2075.1985.tb04019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ymer S., Tucker W. Q., Sanderson C. J., Hapel A. J., Campbell H. D., Young I. G. Constitutive synthesis of interleukin-3 by leukaemia cell line WEHI-3B is due to retroviral insertion near the gene. Nature. 1985 Sep 19;317(6034):255–258. doi: 10.1038/317255a0. [DOI] [PubMed] [Google Scholar]