Abstract
Fischer rat fibroblasts, naturally resistant to killing by the fibrotropic strain of minute virus of mice [(parvovirus MVM(p)], became sensitive to MVM when transformed by polyomavirus. This sensitization did not involve an increase in the percentage of cells which synthesized viral capsid antigens or in the percentage of cells which produced infectious virus. The addition of anti-MVM antiserum to the growth medium of MVM-infected cells had only a small effect on their survival rates, indicating that the majority of the killing effect of MVM occurs in a single cycle of infection. The data indicate that cell killing by MVM is independent of infectious virus production and thus support the notion that the preferential cytolytic effect is affected by viral cytotoxic gene products which accumulate to intolerable levels in transformed cells but not in normal ones. Finally, using cells transformed with polyomavirus and genomic and subgenomic clones of polyomavirus, we showed that the extent of sensitization to killing by MVM depended on the transforming agent used.
Full text
PDF
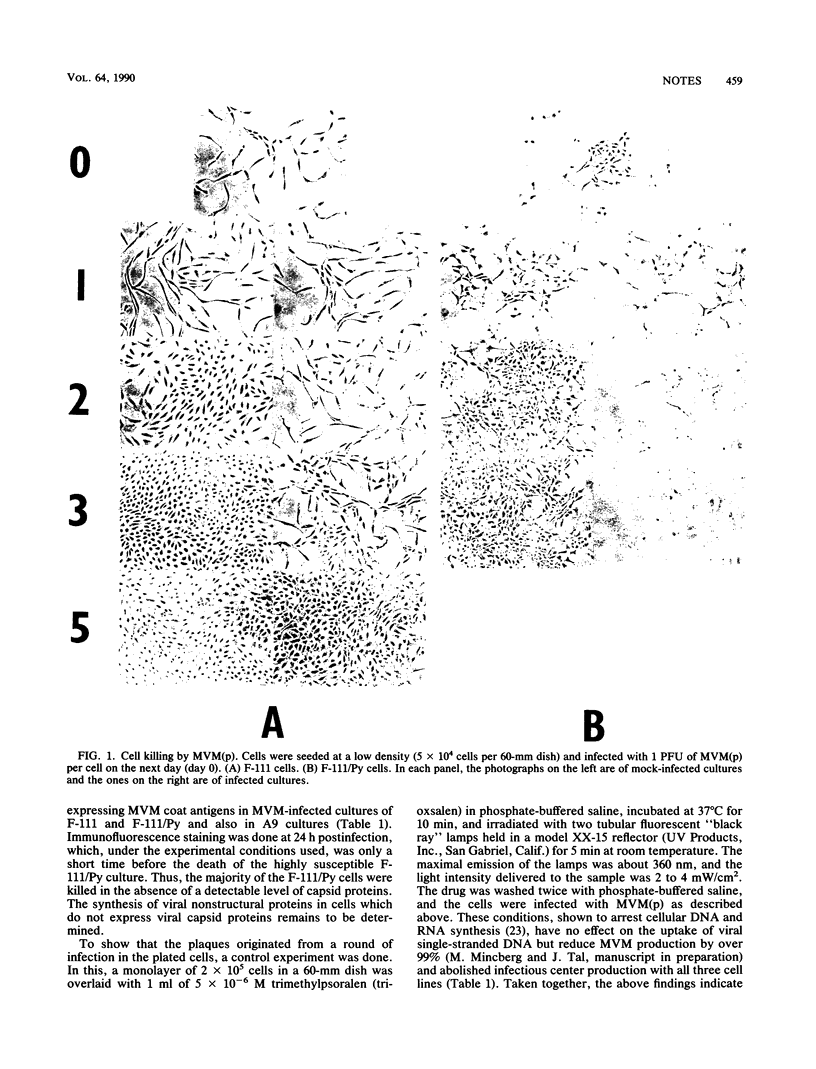


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergs V. V. Rat virus-mediated suppression of leukemia induction by Moloney virus in rats. Cancer Res. 1969 Sep;29(9):1669–1672. [PubMed] [Google Scholar]
- Chen Y. Q., de Foresta F., Hertoghs J., Avalosse B. L., Cornelis J. J., Rommelaere J. Selective killing of simian virus 40-transformed human fibroblasts by parvovirus H-1. Cancer Res. 1986 Jul;46(7):3574–3579. [PubMed] [Google Scholar]
- Cornelis J. J., Becquart P., Duponchel N., Salomé N., Avalosse B. L., Namba M., Rommelaere J. Transformation of human fibroblasts by ionizing radiation, a chemical carcinogen, or simian virus 40 correlates with an increase in susceptibility to the autonomous parvoviruses H-1 virus and minute virus of mice. J Virol. 1988 May;62(5):1679–1686. doi: 10.1128/jvi.62.5.1679-1686.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
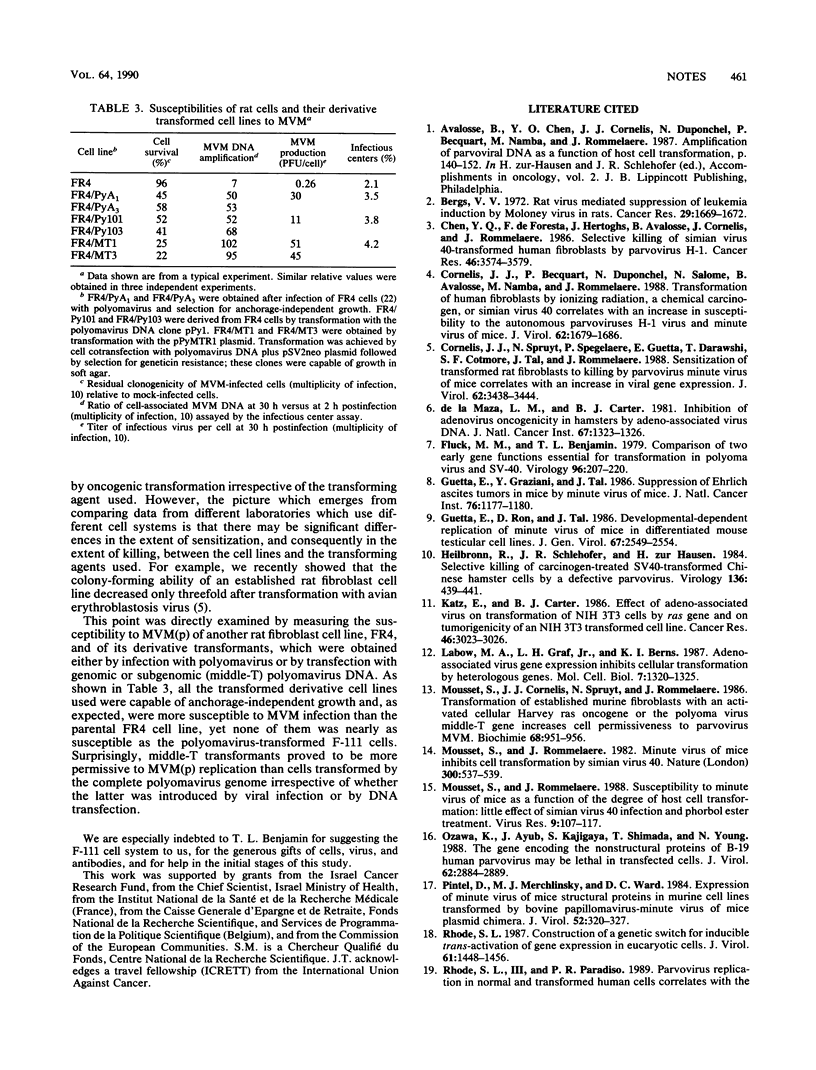
- Cornelis J. J., Spruyt N., Spegelaere P., Guetta E., Darawshi T., Cotmore S. F., Tal J., Rommelaere J. Sensitization of transformed rat fibroblasts to killing by parvovirus minute virus of mice correlates with an increase in viral gene expression. J Virol. 1988 Sep;62(9):3438–3444. doi: 10.1128/jvi.62.9.3438-3444.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guetta E., Graziani Y., Tal J. Suppression of Ehrlich ascites tumors in mice by minute virus of mice. J Natl Cancer Inst. 1986 Jun;76(6):1177–1180. [PubMed] [Google Scholar]
- Guetta E., Ron D., Tal J. Development-dependent replication of minute virus of mice in differentiated mouse testicular cell lines. J Gen Virol. 1986 Nov;67(Pt 11):2549–2554. doi: 10.1099/0022-1317-67-11-2549. [DOI] [PubMed] [Google Scholar]
- Heilbronn R., Schlehofer J. R., zur Hausen H. Selective killing of carcinogen-treated SV40-transformed Chinese hamster cells by a defective parvovirus. Virology. 1984 Jul 30;136(2):439–441. doi: 10.1016/0042-6822(84)90180-6. [DOI] [PubMed] [Google Scholar]
- Katz E., Carter B. J. Effect of adeno-associated virus on transformation of NIH 3T3 cells by ras gene and on tumorigenicity of an NIH 3T3 transformed cell line. Cancer Res. 1986 Jun;46(6):3023–3026. [PubMed] [Google Scholar]
- Labow M. A., Graf L. H., Jr, Berns K. I. Adeno-associated virus gene expression inhibits cellular transformation by heterologous genes. Mol Cell Biol. 1987 Apr;7(4):1320–1325. doi: 10.1128/mcb.7.4.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousset S., Cornelis J., Spruyt N., Rommelaere J. Transformation of established murine fibroblasts with an activated cellular Harvey-ras oncogene or the polyoma virus middle T gene increases cell permissiveness to parvovirus minute-virus-of-mice. Biochimie. 1986 Jul-Aug;68(7-8):951–955. doi: 10.1016/s0300-9084(86)81058-6. [DOI] [PubMed] [Google Scholar]
- Mousset S., Rommelaere J. Minute virus of mice inhibits cell transformation by simian virus 40. Nature. 1982 Dec 9;300(5892):537–539. doi: 10.1038/300537a0. [DOI] [PubMed] [Google Scholar]
- Mousset S., Rommelaere J. Susceptibility to parvovirus Minute virus of mice as a function of the degree of host cell transformation: little effect of simian virus 40 infection and phorbol ester treatment. Virus Res. 1988 Feb;9(2-3):107–117. doi: 10.1016/0168-1702(88)90026-3. [DOI] [PubMed] [Google Scholar]
- Ozawa K., Ayub J., Kajigaya S., Shimada T., Young N. The gene encoding the nonstructural protein of B19 (human) parvovirus may be lethal in transfected cells. J Virol. 1988 Aug;62(8):2884–2889. doi: 10.1128/jvi.62.8.2884-2889.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pintel D., Merchlinsky M. J., Ward D. C. Expression of minute virus of mice structural proteins in murine cell lines transformed by bovine papillomavirus-minute virus of mice plasmid chimera. J Virol. 1984 Nov;52(2):320–327. doi: 10.1128/jvi.52.2.320-327.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhode S. L., 3rd Construction of a genetic switch for inducible trans-activation of gene expression in eucaryotic cells. J Virol. 1987 May;61(5):1448–1456. doi: 10.1128/jvi.61.5.1448-1456.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhode S. L., 3rd, Paradiso P. R. Parvovirus replication in normal and transformed human cells correlates with the nuclear translocation of the early protein NS1. J Virol. 1989 Jan;63(1):349–355. doi: 10.1128/jvi.63.1.349-355.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ron D., Tal J. Coevolution of cells and virus as a mechanism for the persistence of lymphotropic minute virus of mice in L-cells. J Virol. 1985 Aug;55(2):424–430. doi: 10.1128/jvi.55.2.424-430.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ron D., Tattersall P., Tal J. Formation of a host range mutant of the lymphotropic strain of minute virus of mice during persistent infection in mouse L cells. J Virol. 1984 Oct;52(1):63–69. doi: 10.1128/jvi.52.1.63-69.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seif R., Cuzin F. Temperature-sensitive growth regulation in one type of transformed rat cells induced by the tsa mutant of polyoma virus. J Virol. 1977 Dec;24(3):721–728. doi: 10.1128/jvi.24.3.721-728.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shames B., Kalma S., Heimer Y. M., Tal J. Differential inhibition of cellular RNAs by photosensitized trioxalen. Nucleic Acids Res. 1983 Sep 24;11(18):6453–6464. doi: 10.1093/nar/11.18.6453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalholz B. A., Tattersall P. Interaction of minute virus of mice with differentiated cells: strain-dependent target cell specificity is mediated by intracellular factors. J Virol. 1983 Jun;46(3):937–943. doi: 10.1128/jvi.46.3.937-943.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toolan H. W., Ledinko N. Inhibition by H-1 virus of the incidence of tumors produced by adenovirus 12 in hamsters. Virology. 1968 Jul;35(3):475–478. doi: 10.1016/0042-6822(68)90226-2. [DOI] [PubMed] [Google Scholar]
- Toolan H. W., Rhode S. L., 3rd, Gierthy J. F. Inhibition of 7,12-dimethylbenz(a)anthracene-induced tumors in Syrian hamsters by prior infection with H-1 parvovirus. Cancer Res. 1982 Jul;42(7):2552–2555. [PubMed] [Google Scholar]
- Yang T. J. Parvovirus-induced regression of canine transmissible venereal sarcoma. Am J Vet Res. 1987 May;48(5):799–800. [PubMed] [Google Scholar]
- de la Maza L. M., Carter B. J. Inhibition of adenovirus oncogenicity in hamsters by adeno-associated virus DNA. J Natl Cancer Inst. 1981 Dec;67(6):1323–1326. [PubMed] [Google Scholar]