Abstract
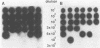
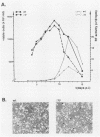
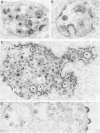
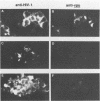
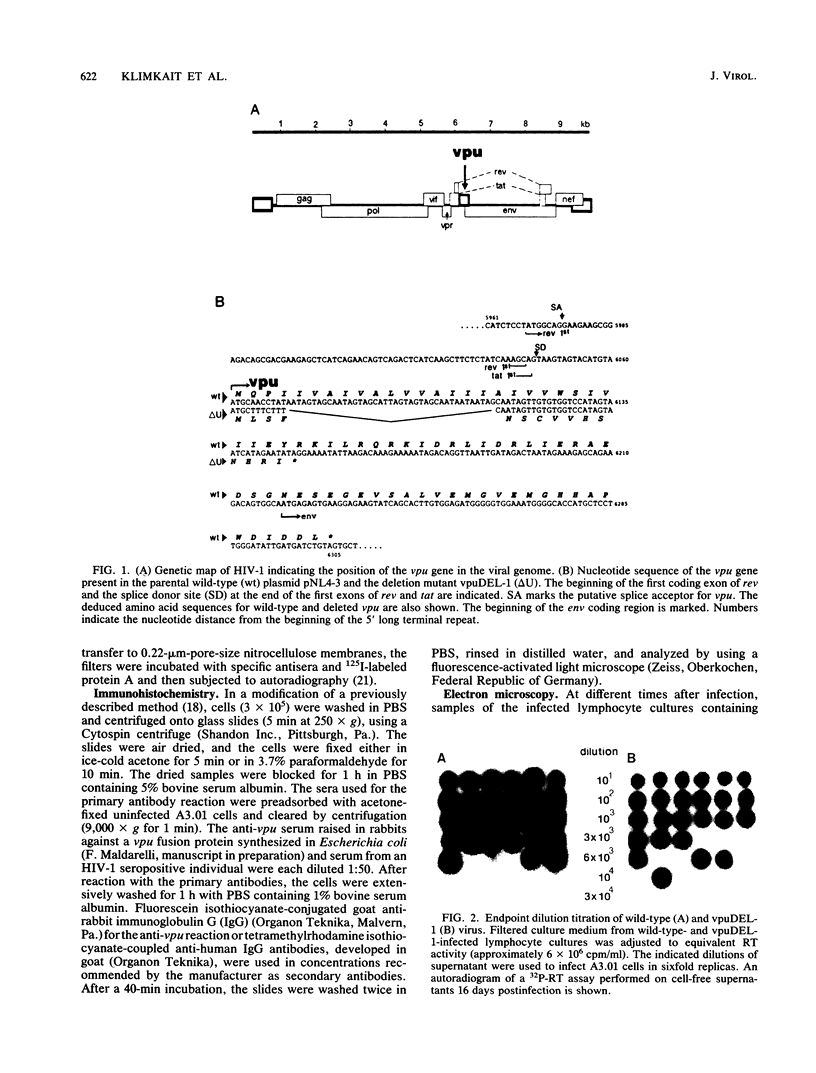
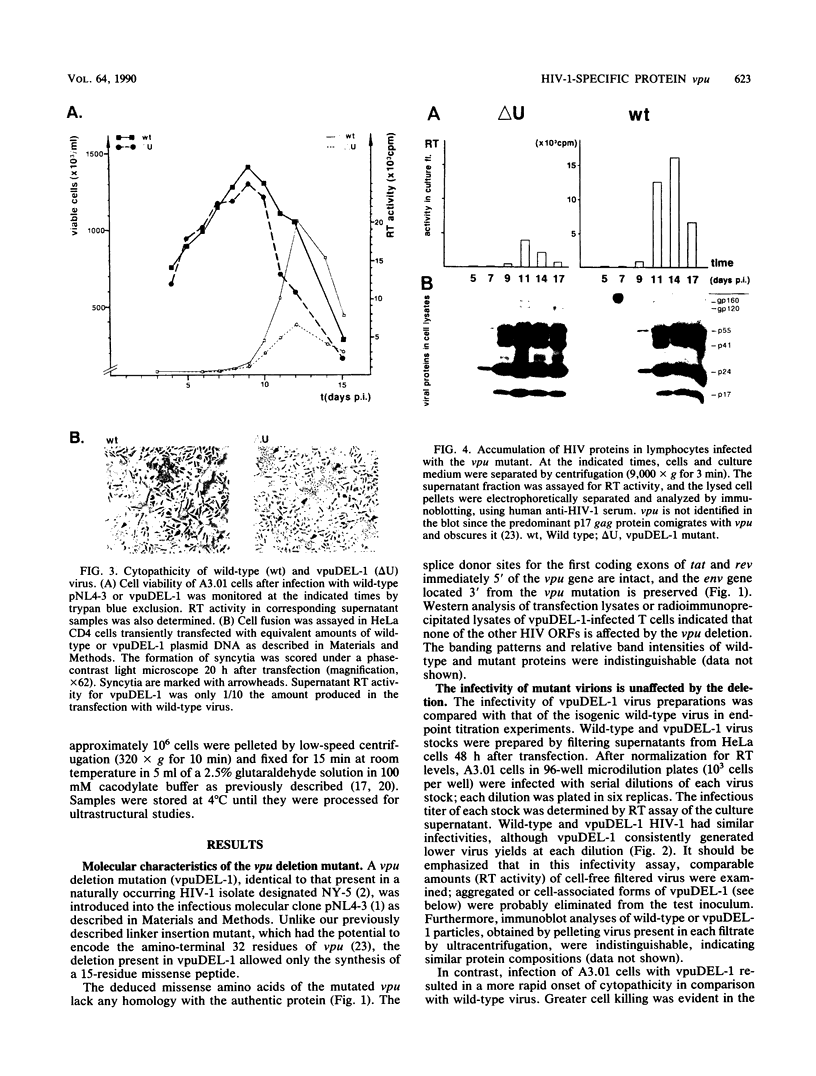
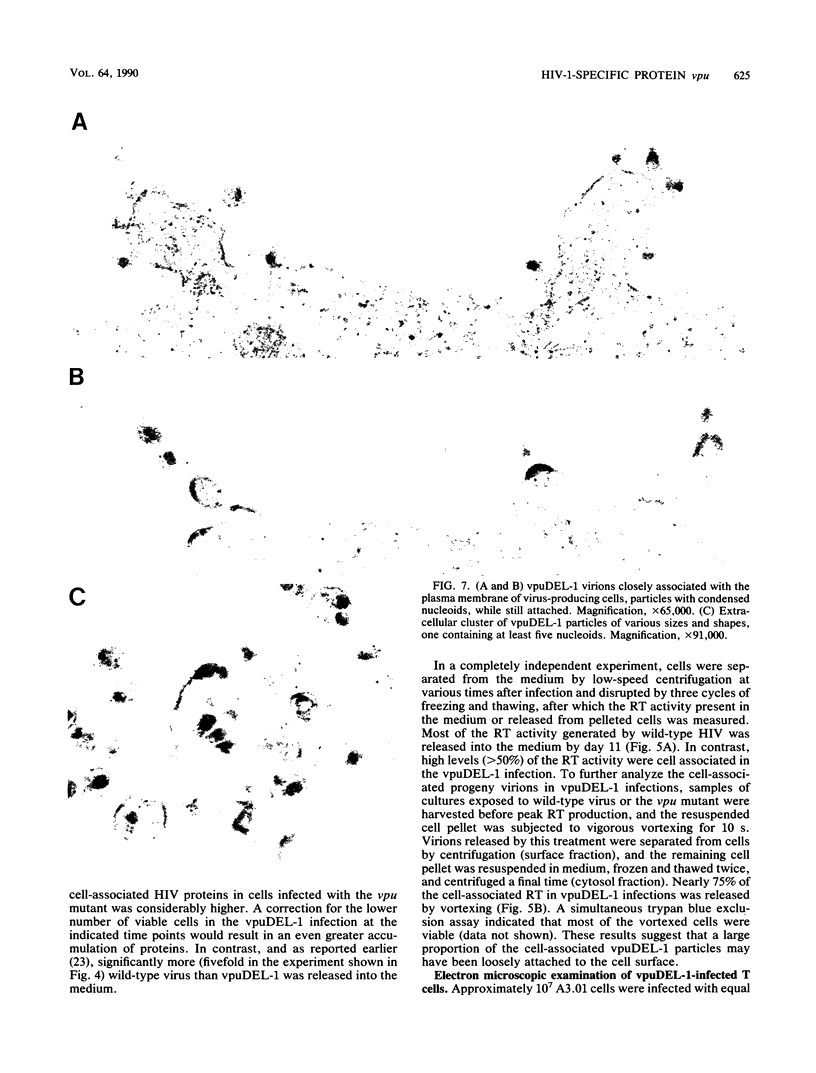
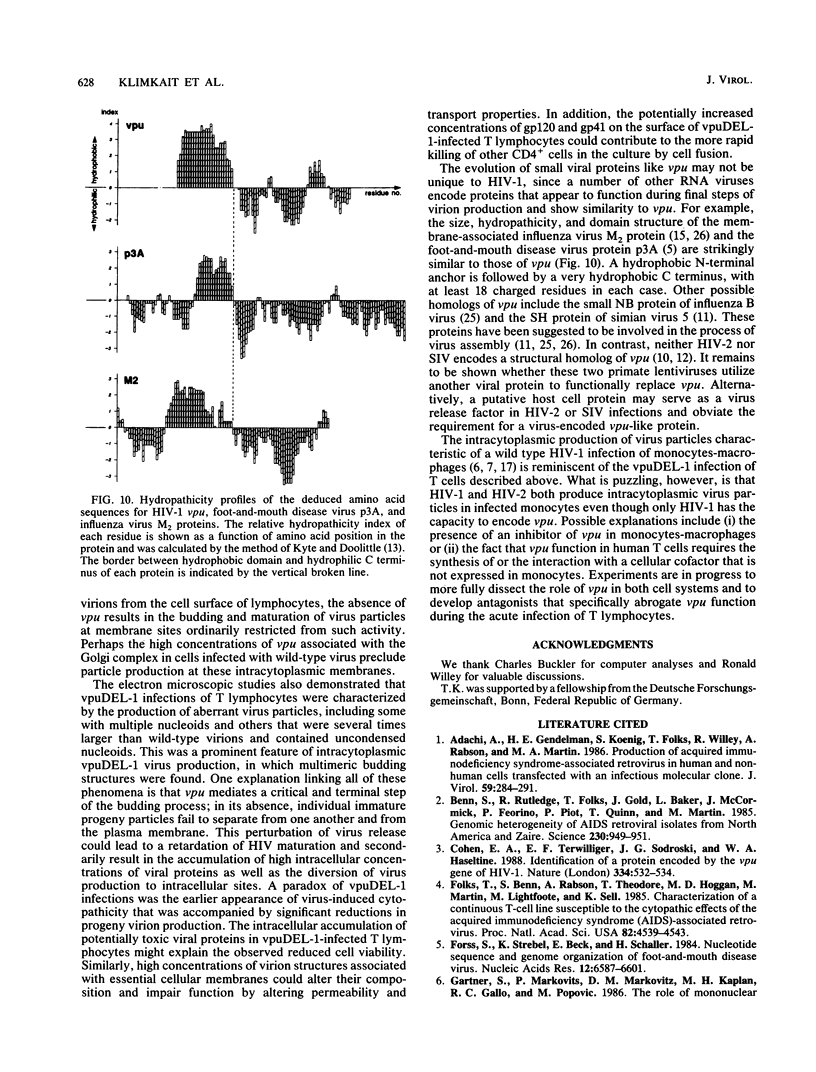
A deletion mutation affecting vpu was introduced into an infectious molecular clone of human immunodeficiency virus type 1, and the resultant phenotype was examined after infection of human T lymphocytes. The absence of vpu resulted in an accumulation of cell-associated viral proteins and impaired the release of progeny virions. Both electron microscopic and biochemical analyses indicated that a large proportion of the mutant particles was attached to the surface of infected cells. Significant variation in the size and shape of these progeny virions was observed. In addition, intracytoplasmic particles, some of which formed aberrant budding structures, were visualized in T cells infected with the vpu mutant. Indirect immunofluorescence analyses of cultures inoculated with wild-type virus with use of a vpu-specific antiserum demonstrated that vpu is mainly localized to a perinuclear region in the cytoplasm of virus-producing cells.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi A., Gendelman H. E., Koenig S., Folks T., Willey R., Rabson A., Martin M. A. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J Virol. 1986 Aug;59(2):284–291. doi: 10.1128/jvi.59.2.284-291.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benn S., Rutledge R., Folks T., Gold J., Baker L., McCormick J., Feorino P., Piot P., Quinn T., Martin M. Genomic heterogeneity of AIDS retroviral isolates from North America and Zaire. Science. 1985 Nov 22;230(4728):949–951. doi: 10.1126/science.2997922. [DOI] [PubMed] [Google Scholar]
- Cohen E. A., Terwilliger E. F., Sodroski J. G., Haseltine W. A. Identification of a protein encoded by the vpu gene of HIV-1. Nature. 1988 Aug 11;334(6182):532–534. doi: 10.1038/334532a0. [DOI] [PubMed] [Google Scholar]
- Folks T., Benn S., Rabson A., Theodore T., Hoggan M. D., Martin M., Lightfoote M., Sell K. Characterization of a continuous T-cell line susceptible to the cytopathic effects of the acquired immunodeficiency syndrome (AIDS)-associated retrovirus. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4539–4543. doi: 10.1073/pnas.82.13.4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forss S., Strebel K., Beck E., Schaller H. Nucleotide sequence and genome organization of foot-and-mouth disease virus. Nucleic Acids Res. 1984 Aug 24;12(16):6587–6601. doi: 10.1093/nar/12.16.6587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartner S., Markovits P., Markovitz D. M., Kaplan M. H., Gallo R. C., Popovic M. The role of mononuclear phagocytes in HTLV-III/LAV infection. Science. 1986 Jul 11;233(4760):215–219. doi: 10.1126/science.3014648. [DOI] [PubMed] [Google Scholar]
- Gendelman H. E., Orenstein J. M., Martin M. A., Ferrua C., Mitra R., Phipps T., Wahl L. A., Lane H. C., Fauci A. S., Burke D. S. Efficient isolation and propagation of human immunodeficiency virus on recombinant colony-stimulating factor 1-treated monocytes. J Exp Med. 1988 Apr 1;167(4):1428–1441. doi: 10.1084/jem.167.4.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff S., Traktman P., Baltimore D. Isolation and properties of Moloney murine leukemia virus mutants: use of a rapid assay for release of virion reverse transcriptase. J Virol. 1981 Apr;38(1):239–248. doi: 10.1128/jvi.38.1.239-248.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Guyader M., Emerman M., Sonigo P., Clavel F., Montagnier L., Alizon M. Genome organization and transactivation of the human immunodeficiency virus type 2. Nature. 1987 Apr 16;326(6114):662–669. doi: 10.1038/326662a0. [DOI] [PubMed] [Google Scholar]
- Hiebert S. W., Richardson C. D., Lamb R. A. Cell surface expression and orientation in membranes of the 44-amino-acid SH protein of simian virus 5. J Virol. 1988 Jul;62(7):2347–2357. doi: 10.1128/jvi.62.7.2347-2357.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch V., Riedel N., Mullins J. I. The genome organization of STLV-3 is similar to that of the AIDS virus except for a truncated transmembrane protein. Cell. 1987 May 8;49(3):307–319. doi: 10.1016/0092-8674(87)90283-2. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lamb R. A., Zebedee S. L., Richardson C. D. Influenza virus M2 protein is an integral membrane protein expressed on the infected-cell surface. Cell. 1985 Mar;40(3):627–633. doi: 10.1016/0092-8674(85)90211-9. [DOI] [PubMed] [Google Scholar]
- Matsuda Z., Chou M. J., Matsuda M., Huang J. H., Chen Y. M., Redfield R., Mayer K., Essex M., Lee T. H. Human immunodeficiency virus type 1 has an additional coding sequence in the central region of the genome. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6968–6972. doi: 10.1073/pnas.85.18.6968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orenstein J. M., Meltzer M. S., Phipps T., Gendelman H. E. Cytoplasmic assembly and accumulation of human immunodeficiency virus types 1 and 2 in recombinant human colony-stimulating factor-1-treated human monocytes: an ultrastructural study. J Virol. 1988 Aug;62(8):2578–2586. doi: 10.1128/jvi.62.8.2578-2586.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff M. C., Fields K. L., Hakomori S. I., Mirsky R., Pruss R. M., Winter J. Cell-type-specific markers for distinguishing and studying neurons and the major classes of glial cells in culture. Brain Res. 1979 Oct 5;174(2):283–308. doi: 10.1016/0006-8993(79)90851-5. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simionescu N., Simionescu M. Galloylglucoses of low molecular weight as mordant in electron microscopy. I. Procedure, and evidence for mordanting effect. J Cell Biol. 1976 Sep;70(3):608–621. doi: 10.1083/jcb.70.3.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strebel K., Klimkait T., Maldarelli F., Martin M. A. Molecular and biochemical analyses of human immunodeficiency virus type 1 vpu protein. J Virol. 1989 Sep;63(9):3784–3791. doi: 10.1128/jvi.63.9.3784-3791.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strebel K., Klimkait T., Martin M. A. A novel gene of HIV-1, vpu, and its 16-kilodalton product. Science. 1988 Sep 2;241(4870):1221–1223. doi: 10.1126/science.3261888. [DOI] [PubMed] [Google Scholar]
- Symington J., Green M., Brackmann K. Immunoautoradiographic detection of proteins after electrophoretic transfer from gels to diazo-paper: analysis of adenovirus encoded proteins. Proc Natl Acad Sci U S A. 1981 Jan;78(1):177–181. doi: 10.1073/pnas.78.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willey R. L., Smith D. H., Lasky L. A., Theodore T. S., Earl P. L., Moss B., Capon D. J., Martin M. A. In vitro mutagenesis identifies a region within the envelope gene of the human immunodeficiency virus that is critical for infectivity. J Virol. 1988 Jan;62(1):139–147. doi: 10.1128/jvi.62.1.139-147.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams M. A., Lamb R. A. Determination of the orientation of an integral membrane protein and sites of glycosylation by oligonucleotide-directed mutagenesis: influenza B virus NB glycoprotein lacks a cleavable signal sequence and has an extracellular NH2-terminal region. Mol Cell Biol. 1986 Dec;6(12):4317–4328. doi: 10.1128/mcb.6.12.4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zebedee S. L., Lamb R. A. Influenza A virus M2 protein: monoclonal antibody restriction of virus growth and detection of M2 in virions. J Virol. 1988 Aug;62(8):2762–2772. doi: 10.1128/jvi.62.8.2762-2772.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]