Abstract
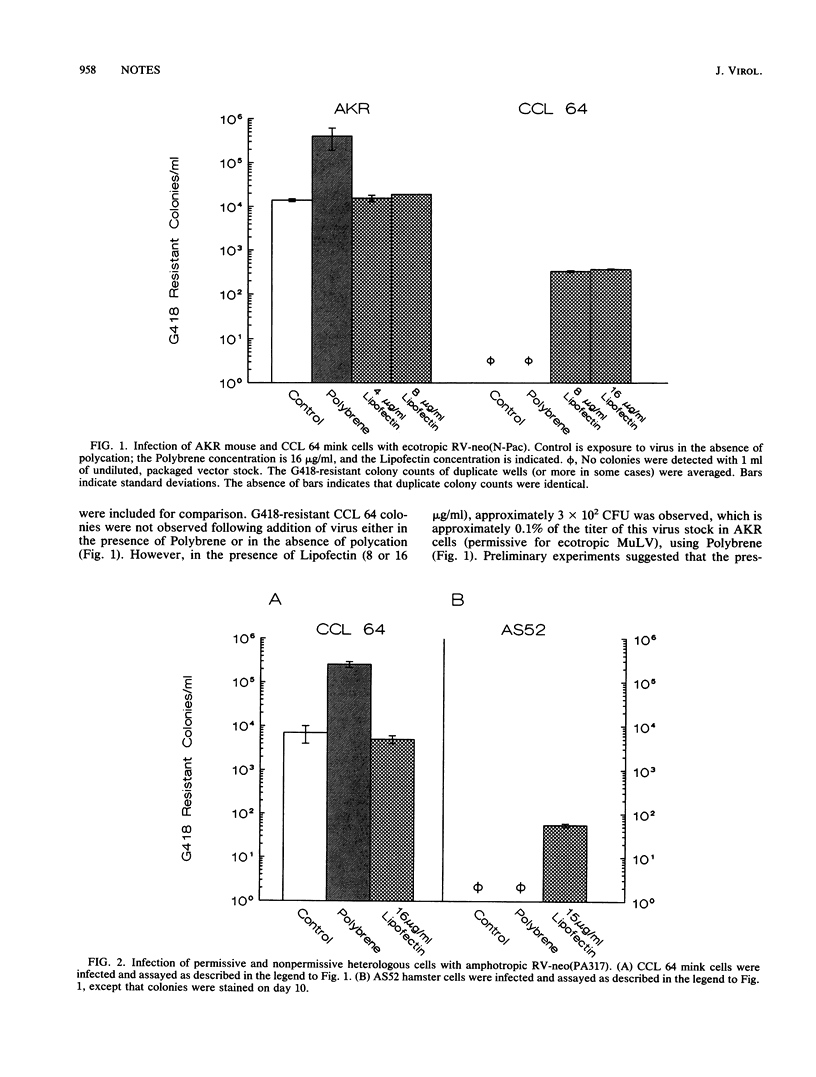
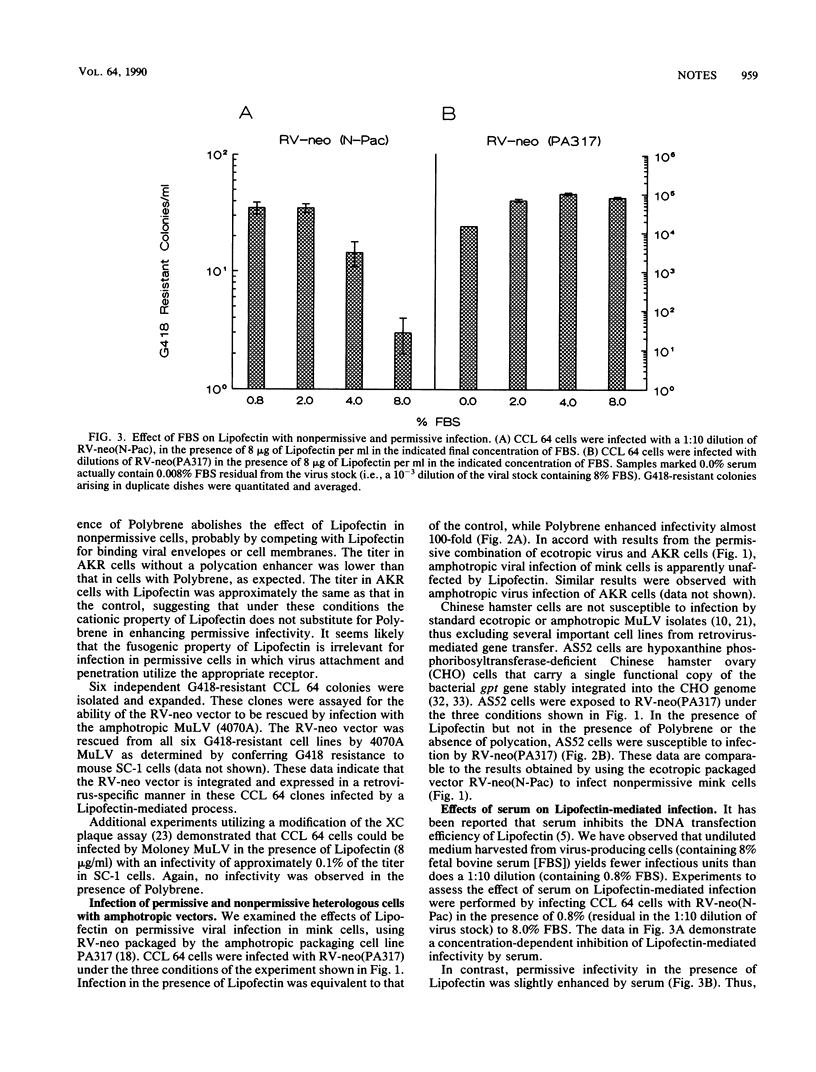
We have used cationic liposomes (Lipofectin) to facilitate retrovirus infection of cells lacking the homologous viral receptor. Ecotropic murine leukemia virus and packaged retroviral vectors were shown to infect mink cells, and amphotropic packaged retroviral vectors were shown to infect hamster cells in the presence of Lipofectin but not in the presence of Polybrene. Lipofectin-mediated infection of cells lacking the homologous receptor results in a titer approximately 0.1% of the titer in cells with the homologous receptor, using the standard Polybrene protocol. The use of Lipofectin may provide a simple means to experimentally infect a wide variety of cells with viruses not normally infectious for the species, tissue, or cell type of interest.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boone L. R., Innes C. L., Glover P. L., Linney E. Development and characterization of an Fv-1-sensitive retrovirus-packaging system: single-hit titration kinetics observed in restrictive cells. J Virol. 1989 Jun;63(6):2592–2597. doi: 10.1128/jvi.63.6.2592-2597.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimmock N. J. Review article initial stages in infection with animal viruses. J Gen Virol. 1982 Mar;59(Pt 1):1–22. doi: 10.1099/0022-1317-59-1-1. [DOI] [PubMed] [Google Scholar]
- Duc-Nguyen H. Enhancing effect of diethylaminoethyl-dextran on the focus-forming titer of a murine sarcoma virus (Harvey strain). J Virol. 1968 Jun;2(6):643–644. doi: 10.1128/jvi.2.6.643-644.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faller D. V., Baltimore D. Liposome encapsulation of retrovirus allows efficient superinfection of resistant cell lines. J Virol. 1984 Jan;49(1):269–272. doi: 10.1128/jvi.49.1.269-272.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felgner P. L., Gadek T. R., Holm M., Roman R., Chan H. W., Wenz M., Northrop J. P., Ringold G. M., Danielsen M. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7413–7417. doi: 10.1073/pnas.84.21.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felgner P. L., Ringold G. M. Cationic liposome-mediated transfection. Nature. 1989 Jan 26;337(6205):387–388. doi: 10.1038/337387a0. [DOI] [PubMed] [Google Scholar]
- HANAFUSA H., HANAFUSA T., RUBIN H. The defectiveness of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1963 Apr;49:572–580. doi: 10.1073/pnas.49.4.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanafusa T., Miyamoto T., Hanafusa H. A type of chick embryo cell that fails to support formation of infectious RSV. Virology. 1970 Jan;40(1):55–64. doi: 10.1016/0042-6822(70)90378-8. [DOI] [PubMed] [Google Scholar]
- Hartley J. W., Rowe W. P. Naturally occurring murine leukemia viruses in wild mice: characterization of a new "amphotropic" class. J Virol. 1976 Jul;19(1):19–25. doi: 10.1128/jvi.19.1.19-25.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson I. C., Lieber M. M., Todaro G. J. Mink cell line Mv 1 Lu (CCL 64). Focus formation and the generation of "nonproducer" transformed cell lines with murine and feline sarcoma viruses. Virology. 1974 Jul;60(1):282–287. doi: 10.1016/0042-6822(74)90386-9. [DOI] [PubMed] [Google Scholar]
- Kondorosi E., Duda E. Infection of cells with Sindbis virus nucleocapsids entrapped into liposomes. Biochem Biophys Res Commun. 1982 Jul 16;107(1):367–373. doi: 10.1016/0006-291x(82)91713-2. [DOI] [PubMed] [Google Scholar]
- Kozak C. A., Gromet N. J., Ikeda H., Buckler C. E. A unique sequence related to the ecotropic murine leukemia virus is associated with the Fv-4 resistance gene. Proc Natl Acad Sci U S A. 1984 Feb;81(3):834–837. doi: 10.1073/pnas.81.3.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning J. S., Hackett A. J., Darby N. B., Jr Effect of polycations on sensitivity of BALD-3T3 cells to murine leukemia and sarcoma virus infectivity. Appl Microbiol. 1971 Dec;22(6):1162–1163. doi: 10.1128/am.22.6.1162-1163.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannino R. J., Gould-Fogerite S. Liposome mediated gene transfer. Biotechniques. 1988 Jul-Aug;6(7):682–690. [PubMed] [Google Scholar]
- Martin F., MacDonald R. Liposomes can mimic virus membranes. Nature. 1974 Nov 8;252(5479):161–163. doi: 10.1038/252161a0. [DOI] [PubMed] [Google Scholar]
- McClure M. O., Marsh M., Weiss R. A. Human immunodeficiency virus infection of CD4-bearing cells occurs by a pH-independent mechanism. EMBO J. 1988 Feb;7(2):513–518. doi: 10.1002/j.1460-2075.1988.tb02839.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. D., Buttimore C. Redesign of retrovirus packaging cell lines to avoid recombination leading to helper virus production. Mol Cell Biol. 1986 Aug;6(8):2895–2902. doi: 10.1128/mcb.6.8.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinter A., Chen T. E., Lowy A., Cortez N. G., Silagi S. Ecotropic murine leukemia virus-induced fusion of murine cells. J Virol. 1986 Mar;57(3):1048–1054. doi: 10.1128/jvi.57.3.1048-1054.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portis J. L., McAtee F. J., Evans L. H. Infectious entry of murine retroviruses into mouse cells: evidence of a postadsorption step inhibited by acidic pH. J Virol. 1985 Sep;55(3):806–812. doi: 10.1128/jvi.55.3.806-812.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasheed S., Gardner M. B., Chan E. Amphotropic host range of naturally occuring wild mouse leukemia viruses. J Virol. 1976 Jul;19(1):13–18. doi: 10.1128/jvi.19.1.13-18.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohde W., Pauli G., Henning J., Friis R. R. Polyethylene glycol-mediated infection with avian sarcoma viruses. Arch Virol. 1978;58(1):55–59. doi: 10.1007/BF01315535. [DOI] [PubMed] [Google Scholar]
- Rowe W. P., Pugh W. E., Hartley J. W. Plaque assay techniques for murine leukemia viruses. Virology. 1970 Dec;42(4):1136–1139. doi: 10.1016/0042-6822(70)90362-4. [DOI] [PubMed] [Google Scholar]
- Rubin H. A VIRUS IN CHICK EMBRYOS WHICH INDUCES RESISTANCE IN VITRO TO INFECTION WITH ROUS SARCOMA VIRUS. Proc Natl Acad Sci U S A. 1960 Aug;46(8):1105–1119. doi: 10.1073/pnas.46.8.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruscetti S., Davis L., Feild J., Oliff A. Friend murine leukemia virus-induced leukemia is associated with the formation of mink cell focus-inducing viruses and is blocked in mice expressing endogenous mink cell focus-inducing xenotropic viral envelope genes. J Exp Med. 1981 Sep 1;154(3):907–920. doi: 10.1084/jem.154.3.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheele C. M., Hanafusa H. Proteins of helper-dependent RSV. Virology. 1971 Aug;45(2):401–410. doi: 10.1016/0042-6822(71)90341-2. [DOI] [PubMed] [Google Scholar]
- Steck F. T., Rubin H. The mechanism of interference between an avian leukosis virus and Rous sarcoma virus. I. Establishment of interference. Virology. 1966 Aug;29(4):628–641. doi: 10.1016/0042-6822(66)90287-x. [DOI] [PubMed] [Google Scholar]
- Steck F. T., Rubin H. The mechanism of interference between an avian leukosis virus and Rous sarcoma virus. II. Early steps of infection by RSV of cells under conditions of interference. Virology. 1966 Aug;29(4):642–653. doi: 10.1016/0042-6822(66)90288-1. [DOI] [PubMed] [Google Scholar]
- Stein B. S., Gowda S. D., Lifson J. D., Penhallow R. C., Bensch K. G., Engleman E. G. pH-independent HIV entry into CD4-positive T cells via virus envelope fusion to the plasma membrane. Cell. 1987 Jun 5;49(5):659–668. doi: 10.1016/0092-8674(87)90542-3. [DOI] [PubMed] [Google Scholar]
- Straubinger R. M., Papahadjopoulos D. Liposomes as carriers for intracellular delivery of nucleic acids. Methods Enzymol. 1983;101:512–527. doi: 10.1016/0076-6879(83)01035-6. [DOI] [PubMed] [Google Scholar]
- Tardieu M., Epstein R. L., Weiner H. L. Interaction of viruses with cell surface receptors. Int Rev Cytol. 1982;80:27–61. doi: 10.1016/S0074-7696(08)60366-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tindall K. R., Stankowski L. F., Jr, Machanoff R., Hsie A. W. Analyses of mutation in pSV2gpt-transformed CHO cells. Mutat Res. 1986 Apr;160(2):121–131. doi: 10.1016/0027-5107(86)90036-9. [DOI] [PubMed] [Google Scholar]
- Tindall K. R., Stankowski L. F., Jr, Machanoff R., Hsie A. W. Detection of deletion mutations in pSV2gpt-transformed cells. Mol Cell Biol. 1984 Jul;4(7):1411–1415. doi: 10.1128/mcb.4.7.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoshima K., Vogt P. K. Enhancement and inhibition of avian sarcoma viruses by polycations and polyanions. Virology. 1969 Jul;38(3):414–426. doi: 10.1016/0042-6822(69)90154-8. [DOI] [PubMed] [Google Scholar]
- Vogt P. K. DEAE-dextran: enhancement of cellular transformation induced by avian sarcoma viruses. Virology. 1967 Sep;33(1):175–177. doi: 10.1016/0042-6822(67)90109-2. [DOI] [PubMed] [Google Scholar]
- White J., Kielian M., Helenius A. Membrane fusion proteins of enveloped animal viruses. Q Rev Biophys. 1983 May;16(2):151–195. doi: 10.1017/s0033583500005072. [DOI] [PubMed] [Google Scholar]
- Wilson T., Papahadjopoulos D., Taber R. Biological properties of poliovirus encapsulated in lipid vesicles: antibody resistance and infectivity in virus-resistant cells. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3471–3475. doi: 10.1073/pnas.74.8.3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarling D. A., Keshet I. Fusion activity of virions of murine leukemia virus. Virology. 1979 May;95(1):185–196. doi: 10.1016/0042-6822(79)90413-6. [DOI] [PubMed] [Google Scholar]


