Abstract
Suppression of oxidative injury by viral-mediated transfer of the human catalase gene was tested in the optic nerves of animals with experimental allergic encephalomyelitis (EAE). EAE is an inflammatory autoimmune disorder of primary central nervous system demyelination that has been frequently used as an animal model for the human disease multiple sclerosis (MS). The optic nerve is a frequent site of involvement common to both EAE and MS. Recombinant adeno-associated virus containing the human gene for catalase was injected over the right optic nerve heads of SJL/J mice that were simultaneously sensitized for EAE. After 1 month, cell-specific catalase activity, evaluated by quantitation of catalase immunogold, was increased approximately 2-fold each in endothelia, oligodendroglia, astrocytes, and axons of the optic nerve. Effects of catalase on the histologic lesions of EAE were measured by computerized analysis of the myelin sheath area (for demyelination), optic disc area (for optic nerve head swelling), extent of the cellular infiltrate, extravasated serum albumin labeled by immunogold (for blood–brain barrier disruption), and in vivo H2O2 reaction product. Relative to control, contralateral optic nerves injected with the recombinant virus without a therapeutic gene, catalase gene inoculation reduced demyelination by 38%, optic nerve head swelling by 29%, cellular infiltration by 34%, disruption of the blood–brain barrier by 64%, and in vivo levels of H2O2 by 61%. Because the efficacy of potential treatments for MS are usually initially tested in the EAE animal model, this study suggests that catalase gene delivery by using viral vectors may be a therapeutic strategy for suppression of MS.
Keywords: free oxygen radicals/multiple sclerosis
Experimental allergic encephalomyelitis (EAE) is an inflammatory autoimmune disorder of primary central nervous system demyelination that has been frequently used as an animal model for the human disease multiple sclerosis (MS) (1, 2). The optic nerve is a frequent site of involvement in EAE and MS (3, 4, 5). In both disorders, myelin-forming oligodendroglia are the primary targets of immune-mediated injury (1, 6, 7), although other cell types are affected also. Demyelinated axons exhibit hydropic degeneration with dissolution of microtubules and neurofilaments (3). Even endothelial cells that appear ultrastructurally intact have lost their ability to maintain the integrity of the blood–brain barrier (BBB) (8–10). Consequently, treatments for EAE, and eventually MS, must protect not only oligodendroglia, but also axons and endothelial cells against structural and functional mediators of tissue injury.
Reactive oxygen species (ROS) such as superoxide and nitric oxide (NO), released by inflammatory cells, are mediators of demyelination and disruption of the BBB in EAE (11, 12). The role of ROS in altering BBB permeability and demyelination has been inferred from the beneficial effect of free radical scavengers and antioxidants on the neurologic deficits and histopathologic lesions associated with EAE (11–17). ROS scavengers include catalase and superoxide dismutase. Superoxide dismutase dismutes superoxide to hydrogen peroxide (H2O2) and catalase detoxifies the H2O2 to H2O and O2. Exogenous catalase has been previously shown to reduce disruption of the BBB and demyelination of the optic nerve in EAE (12, 13).
Limitations to the use of catalase protein are several fold. First, catalase must be administered daily, even with conjugation of polyethylene glycol, to prolong the half life of the enzyme (18–20). Second, exogenous catalase is effective only during the periods of active BBB disruption when this high molecular weight protein is able to penetrate the central nervous system (CNS). Third, optic neuritis recurs in part due to the inability of catalase protein to cross the BBB after integrity is restored by the catalase-mediated detoxification of H2O2.
Genetic augmentation of cellular defenses against ROS may help surmount these problems. Viral-mediated gene delivery offers a promising way to deliver such therapeutic genes (21, 22). In fact, 2- to 4-fold increases in catalase expression have been shown in vitro in human endothelial cells 1 day after administration of viral-catalase cDNA complexes (23). Moreover, the increased catalase levels persisted for 1 week, as opposed to exogenous administration that at best achieved a 2-fold increase with repeated daily injections. Because the optic nerve is a frequent site of demyelination in both EAE and in MS and the eye is a readily accessible site for gene transfer (24, 25), we evaluated the effects of viral-mediated gene transfer of catalase on suppression of EAE in the optic nerve.
MATERIALS AND METHODS
Recombinant Adeno-Associated Virus (rAAV).
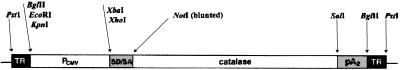
The AAV vector, pTRUF (24), was used to accept the catalase cDNA at the NotI and SalI sites. Catalase cDNA was provided by Chiron. The resulting pTR-Cat plasmid (Fig. 1, restriction map) was then amplified and purified by using cesium chloride gradient centrifugation. The resulting rAAV-Cat construct was regulated by a cytomegalovirus (CMV) immediate early promoter (24). This pTR-Cat plasmid DNA was packaged into rAAV by transfection into 293 cells using standard procedures (24, 25). The resultant rAAV-packaged pTR-Cat was assayed for rAAV by an infectious center assay (24) and gave a titer of 1 × 108 infectious units/ml. It also was tested for contaminating Adenovirus by plaque assay and wild-type AAV by infectious center assay. Both potentially contaminating viruses were found to be below detection limits, <5 orders of magnitude lower than rAAV.
Figure 1.
Restriction map of the rAAV-Cat construct. TR, terminal repeat; Pcmv, CMV immediate early promoter/enhancer; SD/SA, SV40 late viral promoter gene 16S/19S splice donor and acceptor signals; catalase, human gene for catalase; pA2, bovine growth hormone polyadenylation signal from pRc/CMV (Invitrogen).
Induction of EAE and Intraocular Injections.
Experimental allergic encephalomyelitis was induced in 20 SJL/J mice by sensitization with homologous spinal cord emulsion in complete Freunds adjuvant (Difco) that was injected subdermally into the nuchal area.
Five microliters of rAAV-Cat were injected over the right optic nerve heads of SJL/J mice. For controls, the left eyes received rAAV containing the green fluorescent protein (gfp) gene in place of the catalase gene. The mice were simultaneously sensitized to develop EAE (1, 3). Mice were maintained in veterinarian-supervised animal care facilities that are fully accredited by the American Association of Laboratory Animal Science and humanely cared for in accordance with the Association for Research in Vision and Ophthalmology Resolution on the Use of Animals in Research.
Immunohistochemistry.
One month after viral and EAE inoculations, the mice were overdosed with sodium pentobarbital. They were then perfused by cardiac puncture with fixative consisting of 4% paraformaldehyde in 0.1 M PBS buffer (pH 7.4) or, for detection of in vivo H2O2, with a mixture consisting of 2 mM cerium chloride, 10 mM 3-amino-1,2,4-triazole, 0.8 mM NADH, 0.1 M PBS buffer (pH 7.5), and 7% sucrose followed by perfusion with the fixative (20). The eyes with attached optic nerves were dissected out and further processed by either of the following procedures: (i) For H2O2 localization, tissue specimens were immersion fixed in 2.5% gluteraldehyde, postfixed in 1% osmium tetroxide, 0.1 M sodium cacodylate-HCl buffer (pH 7.4), 7% sucrose in the cold, and then dehydrated through an ethanol series to propylene oxide, infiltrated, and embedded in epoxy resin that was polymerized at 60°C overnight. (ii) For immunocytochemistry, tissue specimens were postfixed in 5.0% acrolein, 0.1 M sodium cacodylate-HCl buffer (pH 7.4) and 7% sucrose and then dehydrated through an ethanol series and embedded in LR White (Ted Pella, Redding, CA) that was polymerized at 50°C overnight. Semi-thin longitudinal sections (0.5 μm) of the optic nerve head and retrobulbar nerve were stained with toluidine blue for light microscopic examination. Ultrathin sections (90 nm) were placed on nickel grids for immunocytochemistry.
Nonspecific binding of antibodies was blocked by floating the grids on either (i) 5% normal goat serum in 0.01 M Tris-buffered saline, (pH 7.2) with Tween 20 (TBST) for 30 min for catalase immunostaining or (ii) 2% teleost gelatin and 2% nonfat dry milk in 0.01 M TBS (pH 7.2) with TBST for 30 min for albumin immunostaining. They were then reacted with rabbit anti-catalase antibodies or with rabbit anti-albumin antibodies, respectively, in the same buffer for 2 hr at room temperature. After washes in 0.1 M PBS, the grids were reacted with the secondary goat anti-rabbit IgG antibodies conjugated to 10 nm gold for 1 hr at room temperature. After washes in buffer, grids were rinsed in deionized water. For examination at low magnification transmission electron microscopy, the immunogold particles were enlarged by silver enhancement using a kit (Ted Pella, Redding, PA) according to the manufacturer’s specifications. To check for nonspecific binding of the secondary antibody, control grids were incubated in the buffer, followed by the gold-labeled antibody. Immunolabled and control specimens were photographed by transmission electron microscopy without poststaining.
Morphometric Analysis.
Morphometric analysis was performed in masked fashion as previously described (26). Briefly, images of toluidine blue stained sections of the optic nerve were captured with a video camera mounted on a light microscope and then the digital image was entered into computer memory. After initial calibration with a stage micrometer, the optic nerve head areas were manually traced using the nih image software and a MacIntosh Computer (Apple, Cupertino, CA). The number of glial cells and inflammatory cells in the retrobulbar optic nerve were quantitated also by thresholding of the darker staining cell nuclei. Cell-specific catalase activity and extravasated serum albumin immunogold were similarly quantitated. The immunolabeled sections were examined without poststaining by using a Hitachi H-7000 transmission electron microscope (Tokyo, Japan) operating at 75 kV. Photographs were made at a magnification of X2,500. Ten micrographs of each cell type were taken of each optic nerve. The negatives were digitized into computer memory by using a UMAX scanner (UMAX Data Systems, Fremont, CA). Silver-enhanced immunogold particles and H2O2 reaction products were enlarged to a final magnification of X7,500, thresholded, and counted with the software and computer system. Cell-specific catalase activity was quantitated by counting the number of silver-enhanced immunogold particles in endothelial cells, astroglial cells, oligodendroglial cells, axons, and microglial cells. Values were expressed as the mean ± standard error of mean for each cell type. Mean particle counts for each nerve were obtained by taking the mean value of the 10 micrographs. Each mean value was expressed as the number of particles per unit area. The extent of demyelination was quantitated by threshold measurements of the myelin sheaths that were derived from the axonal micrographs for each optic nerve. Increases in myelin sheath area (less demyelination), thereby indicated a beneficial treatment effect. Grouped t tests were used to assess differences in the myelin areas, optic nerve head areas, optic nerve cell counts, immunogold, and H2O2 particle counts between the catalase-transduced right eyes and the control left eyes.
RESULTS
Cellular Levels of Catalase.
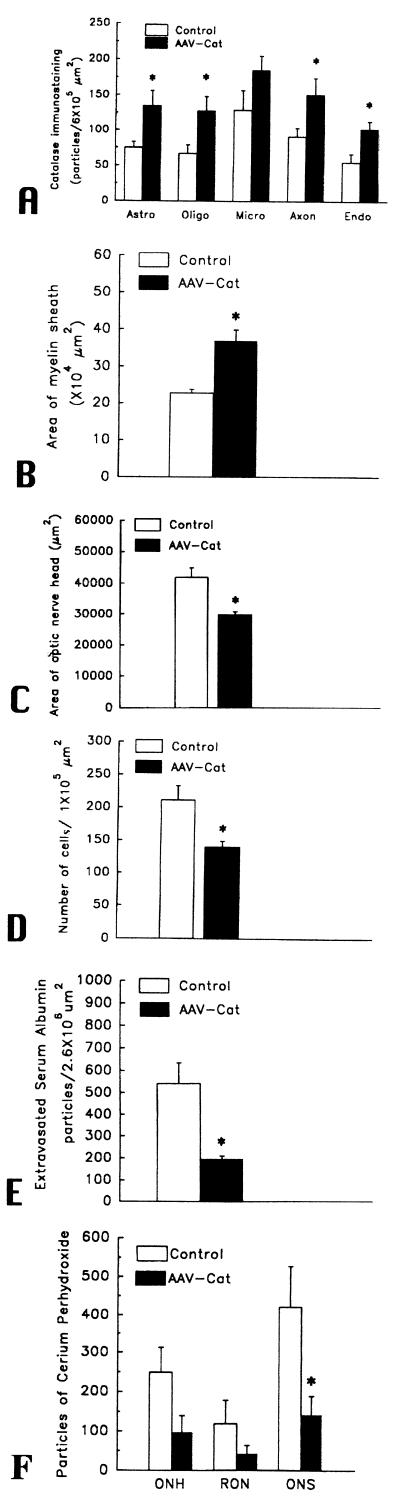
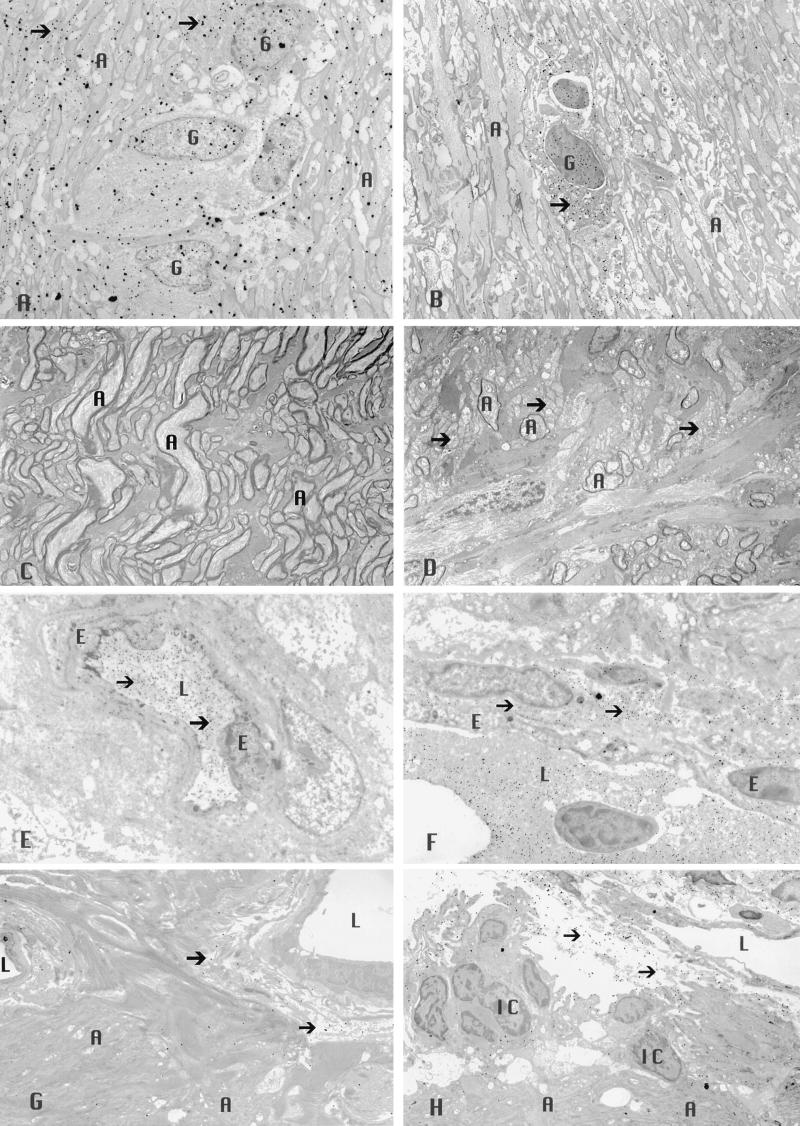
A prerequisite for demonstration of catalase-mediated suppression of EAE is the presence of increased levels of intracellular catalase in transduced tissues (23). One month after inoculation of rAAV, the levels of catalase in transduced optic nerves were increased almost 2-fold in most cell types (Fig. 2A). The highest levels of catalase transduction were seen in axons with a 2.13-fold increase (mean immunogold particles were 151 ± 23 per area of 6 × 105 μm (2) with catalase inoculation vs. 71 ± 12 for gfp inoculation, P < 0.05) and in oligodendrocytes with a 1.91-fold increase (128 ± 20 vs. 67 ± 12, P < 0.05). The levels of catalase were increased by 1.80-fold in astrocytes (135 ± 21 vs. 75 ± 8, P < 0.05) and by 1.85-fold in endothelial cells (102 ± 11 vs. 55 ± 12, P < 0.05). While the levels of catalase immunogold also were increased in microglia by 1.43-fold (185 ± 20 vs. 129 ± 28), these differences were not statistically significant. Fig. 3 shows representative transmission electron micrographs of the optic nerve inoculated with rAAV-Cat showing more catalase immunogold (Fig. 3A) than in the control nerves inoculated with rAAV-gfp (Fig. 3B). Clearly, substantially higher levels of catalase activity were achieved with rAAV-Cat.
Figure 2.
Bargraphs showing ≈2-fold increases in the mean number of catalase immunogold particles within astrocytes (astro), oligodendrocytes (oligo), microdendroglia (micro), axons, and endothelia (endo) with inoculations of rAAV-Cat (A). Bargraphs showing catalase inoculations had the following effects on EAE: reduced demyelination (increased myelin areas) (B), reduced optic nerve head edema (smaller areas) (C), decreased optic nerve cell count (D), reduced extravasated immunogold-labeled serum albumin (suppressed disruption of the BBB) (E), and reduced in vivo levels of H2O2 in the optic nerve head (ONH), retrobulbar optic nerve (RON) and the optic nerve sheath (ONS) (F). ∗, statistically significant.
Figure 3.
Transmission electron micrographs of the optic nerve showing increased levels of catalase immunogold (arrows) are evident within the cytoplasm and nuclei of glial cells and within axons after rAAV-Cat inoculation (×2,500) (A), relative to that seen with rAAV-gfp inoculation (×2,500) (B). Less demyelinated axons were evident with rAAV-Cat inoculation (×2,500) (C), whereas naked axons (arrows) and those with thin sheaths of myelin were prominent in the control nerves that received rAAV-gfp (×2,500) (D). The rAAV-Cat inoculation reduced extravasation of immunogold-labeled serum albumin (arrows) that was predominantly confined to the intravascular compartment (×3,500) (E). Comparison with control nerves (inoculated with rAAV-gfp) showing marked extravasation of immunogold-labeled serum albumin (arrows) into the perivascular space (×4,000) (F). AAV catalase gene inoculation reduced in vivo levels of H2O2 (arrows) (×2,500) (G), whereas more H2O2 reaction product particles (arrows) were evident in the control nerves (×2,500) (H). A, axon; E, endothelial cell; G, glial cell; L, lumen; IC, inflammatory cell.
Demyelination.
In EAE, as well as MS, loss of the myelin sheaths that envelop axons is a hallmark of pathology at the ultrastructural level (1–3). Transmission electron microscopy of the optic nerve revealed all animals sensitized for EAE exhibited foci of demyelination, including naked axons (3). Axons enveloped by thin sheaths of myelin were frequently seen and suggested limited remyelination (1, 7). Mononuclear inflammatory cells and reactive astroglial cells comprised the optic nerve cellular infiltrate that predominantly involved the retrobulbar optic nerve. Indicative of the suppression of demyelination by catalase gene delivery with rAAV, catalase inoculated nerves exhibited a mean myelin area of 23 ± 3 × 104 per μm (2), 38% more myelin (less demyelination) than the contralateral control nerves with a mean of 37 ± 1 × 104 per μm (2) (P < 0.01) (Fig. 2B). Fig. 3 shows representative transmission electron micrographs of the optic nerve inoculated with rAAV-Cat having less demyelination (Fig. 3C) than the controls inoculated with rAAV-gfp (Fig. 3D). Therefore, rAAV gene transfer of catalase achieved therapeutic protection from EAE induced demyelination.
Optic Disc Edema.
Optic disc edema, seen in ≈50% of MS patients with acute optic neuritis (4), was evident in EAE animals in which lateral displacement of the peripapillary retina and filling of the optic cup indicated optic disc edema at the light microscopic level. Ultrastructural analysis revealed intracellular edema of unmyelinated axons contributing to the optic nerve head swelling (3). These histopathologic features were seen to some degree in both catalase-transduced nerves and contralateral control nerves. Catalase gene delivery by rAAV-Cat reduced optic disc edema, by 29%, with a mean optic nerve head area of 3.00 ± 0.10 × 104 per μm (2) vs. 4.20 ± 0.30 × 104 per μm (2) for rAAV-gfp-injected eyes (Fig. 2C). These differences were statistically significant (P < 0.01). For catalase gene-treated optic nerves, rAAV-Cat reduced EAE induced swelling of the optic nerve head.
Optic Nerve Cell Count.
For all groups, light microscopic evaluation of the myelinated segment of the optic nerve, commencing just posterior to the lamina scleralis, revealed foci of inflammatory cells and reactive astroglial cells. The rAAV-Cat gene inoculation reduced the optic nerve cell count by 34% to a mean value of 140 ± 9 cells per 105 μm (2) compared with 211 ± 22 cells per 105 μm (2) for the rAAV-gfp-injected eyes (Fig. 2D). These differences were statistically significant (P < 0.01). Thus, the rAAV-Cat construct reduced the population of cells comprising the associated cellular infiltrate in the myelinated optic nerve.
BBB Disruption.
Disruption of the BBB, a hallmark of MS (27), was seen in all animals sensitized for EAE (20). In vivo evaluation of the BBB by contrast enhanced MRI reveals enhancement of the optic nerve in most patients with acute optic neuritis and in all animals with acute EAE (28–29). A standard marker of BBB disruption is the extravasation of serum albumin (20) that is detected by immunolabeling. Transmission electron microscopy of the optic nerves revealed albumin immunogold labeling in all animals with EAE. Extravasated albumin immunogold in the perivascular compartment located the foci of BBB disruption in EAE. Albumin immunogold confined to the intravascular compartment indicated normal integrity of the BBB (20). rAAV-delivered catalase genes reduced disruption of the BBB by 64%, with a mean value of 193 ± 15 extravasated immunogold particles per 2.6 × 106 μm (2) compared with the rAAV-gfp injected nerves with a mean value of 540 ± 93 extravasated particles (Fig. 2E). These differences were statistically significant (P < 0.05). Fig. 3 shows representative transmission electron micrographs of the optic nerve inoculated with rAAV-Cat exhibiting less extravasated serum albumin (Fig. 3E) than the controls inoculated with rAAV-gfp in which a marked accumulation of extravasated albumin immunogold in the perivascular space is evident (Fig. 3F). Thus, catalase gene introduction markedly improved BBB integrity.
H2O2 Reaction Product.
Perfusion of animals with cerium chloride results in an electron dense precipitate in the presence of endogenous H2O2 (19). Cerium perhydroxide reaction product was seen predominantly in a perivascular distribution in EAE animals. It was also seen along the apical processes of endothelial cells in normal unsensitized animals. In the interstitial optic nerve of EAE animals, the reaction product also surrounds infiltrating inflammatory cells. Decreased in vivo levels of H2O2 were seen with rAAV catalase gene inoculation. Mean particle counts in the optic nerve head were reduced by 61% in the rAAV-Cat inoculated nerves to a mean of 97 ± 43 particles per 2.6 × 106 μm (2) vs. 249 ± 64 for the rAAV-gfp injected nerves (P > 0.05) (Fig. 2F). In the retrobulbar optic nerve, reaction product counts were reduced by 66% to a value of 41 ± 23 with catalase gene inoculation vs. 119 ± 60 for control nerves (P > 0.05). In the optic nerve sheath, particle counts were reduced 75% to a mean of 142 ± 48 with catalase inoculation vs. 421 ± 107 in the control nerves (P < 0.05). Fig. 3 shows representative transmission electron micrographs of the optic nerve head inoculated with rAAV-Cat exhibiting less H2O2 derived reaction product (Fig. 3G) than the controls inoculated with rAAV-gfp in which higher levels of cerium perhydroxide particles are evident in the perivascular space (Fig. 3H).
DISCUSSION
Structural or functional injury to two cell types, endothelial cells and oligodendroglial cells, accounts for the predominant pathogenic tissue alterations leading to disruption of the BBB and demyelination, the hallmarks of both EAE and MS (1, 2, 6–10, 27). Endothelial cells comprising the BBB are the first line of defense against mediators of EAE injury to myelin, axons and oligodendroglia. Thus, restoration of BBB integrity is an important first step in limiting EAE pathology. Viral vector introduction of catalase genes into endothelial cells suppressed disruption of the BBB by 64%. A substantial effect at this focus was anticipated because previous studies have shown the foci of H2O2 generation in EAE to be predominantly perivascular (19, 20, 30). Although H2O2 is a strong oxidant that can diffuse from the sites of generation in the perivascular space and induce peroxidation of lipids in axonal membranes and myelin at remote sites in the interstitial optic nerve, restoration of BBB integrity might also have a suppressive effect on EAE by restricting other mediators of damage from access to the optic nerve. The marked reductions in perivascular cerium perhydroxide reaction product in catalase transduced nerves suggest that increased intracellular levels of catalase in endothelial cells most likely scavenged H2O2, thereby contributing to restoration of BBB integrity in EAE animals (19–20).
Oligodendroglia that form the myelin sheath are particularly vulnerable to the effects of H2O2 (31). This cell type suffers the greatest injury in both EAE and MS, culminating in the classical demyelination (1–3, 7). Accumulation of H2O2 is suppressed by antioxidant enzymes such as catalase. Although the role of catalase in suppressing demyelination had previously implicated H2O2 in the pathogenesis of CNS demyelination (12, 13, 15, 19), genetically increasing catalase levels in oligodendroglia with a single inoculation reduced demyelination as much as 38%, thus partially protecting these important cells from the adverse effects of H2O2 released into the microenvironment by the inflammatory process.
While viral promoters drive transgene expression in different cell types, promoters may be designed for cell-specific expression (25). Cell-specific promoters may have an advantage over the viral promoters by inducing a higher efficiency of transduction in targeted cells. If cell-specific promoters are to be utilized in the treatment of EAE and eventually MS, protection of endothelial cells, oligodendroglia cells and axons is desirable. This might be accomplished with the use of three viral constructs, one with a cell-specific promoter for endothelial cells, one with a promoter for oligodendroglial cells (32) and a third with a neuronal promoter for expression in axons (33). Alternatively, these promoters may be linked to therapeutic genes in a single construct, although the size constraint of AAV may limit this approach. For now, use of the CMV promoter with a broad range of expression doubled expression of catalase in all affected optic nerve cell types, with a significant impact on the suppression of EAE.
In previous protein injection studies, the integrity of the BBB limited the success of exogenous catalase suppression of EAE (19). While catalase had an overall suppressive effect on EAE, its lack of effectiveness during the initial stages of EAE was in part due to the BBB inhibition of CNS penetration of catalase. Only after extensive BBB disruption by the demyelinating inflammation of EAE, was catalase activity in the optic nerve significantly increased by the i.p. injections. In addition, catalase protein had to be administered daily to maintain this increased activity. Paradoxically, early restoration of BBB integrity allowed optic neuritis to recur due to the impaired ability of exogenous catalase protein to cross the once again intact BBB. This effect made the optic nerve again vulnerable to the effects of ROS, thereby contributing to relapses. Gene therapy with catalase avoids these problems by maintaining increased levels of intracellular catalase in the CNS after a single inoculation. If long term therapeutic levels are maintained in transduced cells and tissues, viral gene transfer not only has the potential to suppress the relapses of EAE, but perhaps reduce the frequent recurrences of optic neuritis that often contribute to permanent and disabling visual loss (34). Since axonal loss associated with multiple recurrences limits recovery of visual and neurologic function (35), increased axonal levels of catalase following gene transfer may limit this irreversible sequela. While the effects of axonal rescue in demyelinated nerves by catalase remains to be evaluated, it is clear that viral mediated gene transfer of catalase suppressed demyelination and BBB disruption when administered prior to the onset of EAE.
For therapeutic efficacy, cellular expression of transgene product must persist for the duration of the disease process. Our results and those of others (36) show that either rAAV or Adenovirus may be used to drive short term gene expression in the optic nerve for at least 1 month. However, longer term studies with Adenoviral vectors have shown that transgene expression was undetectable 2 months after inoculation into skeletal muscle (37), but it may persist longer in animals with defective immune systems (38). In contrast, long term expression of rAAV transferred genes has been demonstrated in various tissues including brain (39) (3–4 months) and muscle (37, 40) (40 weeks to 1.5 years). We found transduction in the optic nerve with rAAV persisted for at least two years after a single inoculation (unpublished results). Since many advances in the therapy of MS were first tested in EAE (6, 41–44), our findings of suppression of EAE coupled with long term rAAV-mediated gene expression in the optic nerve suggest that rAAV may be the most suitable vector for investigative studies of the potential for anti-ROS gene therapy of optic neuritis and perhaps MS.
A variety of ROS play a role in the pathogenesis of EAE (45–49). Therefore, scavenging other ROS mediators, including peroxynitrite and nitric oxide, also suppresses EAE (45–47). In addition, passive transfer of inflammatory cells transfected with the immunomodulatory cytokines interleukin 4 (50) or interleukin-10 (51) that effect multiple mediators of tissue injury also suppresses EAE. Although our study evaluated only H2O2 scavenging by catalase in the optic nerve, this nerve is a frequently involved site in EAE and MS, with optic neuritis often being the first clinical sign of MS (4, 5). Therefore, the demonstration here of optic nerve protection against EAE by virally mediated catalase gene delivery suggests that analogous CNS application has the potential to protect the brain and spinal cord in MS patients, particularly in those with a first demyelinating event who are at great risk for developing MS. Improvements in tissue-specific gene targeting after systemic administration, rather than locally invasive gene delivery, may increase the likelihood of acceptance of gene therapy for CNS demyelinating disorders.
Acknowledgments
We thank Mabel Wilson for editing our manuscript. Supported by grants EY-07982 (to J.G.), EY-11123, EY-07864 (to W.W.H.), and Core Grant EY-08571 (to Department of Ophthalmology) from the National Eye Institute, and indirectly by an unrestricted departmental grant from Research to Prevent Blindness, Inc., New York, NY.
ABBREVIATIONS
- EAE
experimental allergic encephalomyelitis
- MS
multiple sclerosis
- BBB
blood–brain barrier
- ROS
reactive oxygen species
- CNS
central nervous system
- rAAV
recombinant Adeno-associated virus
- gfp
green fluorescent protein
- CMV
cytomegalovirus
- TBS
Tris-buffered saline
- Cat
human gene for catalase
References
- 1.Raine C S. In: Handbook of Clinical Neurology. Vinken P J, Bruyn G W, Klawans H L, editors. Vol. 3. New York: Elsevier; 1985. [Google Scholar]
- 2.Paterson P Y, Day E D. Am J Pathol. 1982;52:251–263. [Google Scholar]
- 3.Rao N A. Invest Ophthalmol Visual Sci. 1981;20:159–172. [PubMed] [Google Scholar]
- 4.Beck R W, Cleary P A, Anderson M M, Jr, Keltner J L, Shults W T, Kaufman D I, Buckley E G, Corbett J J, Kupersmith M J, Miller N R, et al. N Engl J Med. 1992;326:581–588. doi: 10.1056/NEJM199202273260901. [DOI] [PubMed] [Google Scholar]
- 5.Rizzo J F, Lessell S. Neurology. 1988;38:185–190. doi: 10.1212/wnl.38.2.185. [DOI] [PubMed] [Google Scholar]
- 6.Steinman L. Adv Immunol. 1991;49:357–379. doi: 10.1016/s0065-2776(08)60779-8. [DOI] [PubMed] [Google Scholar]
- 7.Raine C S. J Neuroimmunol. 1997;77:135–152. doi: 10.1016/s0165-5728(97)00073-8. [DOI] [PubMed] [Google Scholar]
- 8.Daniel P M, Lam D K C, Pratt O E. J Neurol Sci. 1981;2:211–221. doi: 10.1016/0022-510x(81)90006-x. [DOI] [PubMed] [Google Scholar]
- 9.Claudio L, Kress Y, Factor J R, Norton W T, Brosnan C F. Am J Pathol. 1989;135:1157–1168. [PMC free article] [PubMed] [Google Scholar]
- 10.Lossinsky A S, Badmajew V, Robson J A, Moretz R C, Wisniewski H M. Acta Neuropathol. 1989;78:359–371. doi: 10.1007/BF00688172. [DOI] [PubMed] [Google Scholar]
- 11.Honegger C G, Krenger W, Langemann H. Neurosci Lett. 1989;98:327–332. doi: 10.1016/0304-3940(89)90423-0. [DOI] [PubMed] [Google Scholar]
- 12.Guy J R, Ellis E A, Hope G M, Rao N A. Arch Ophthalmol. 1989;107:1359–1363. doi: 10.1001/archopht.1989.01070020429048. [DOI] [PubMed] [Google Scholar]
- 13.Guy J R, Ellis E A, Hope G M, Rao N A. Curr Eye Res. 1989;8:467–477. doi: 10.3109/02713688909000027. [DOI] [PubMed] [Google Scholar]
- 14.Bowern N, Ramshaw I A, Clark I A, Doherty P C. J Exp Med. 1984;260:1532–1543. doi: 10.1084/jem.160.5.1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guy J, Ellis E A, Hope G M, Rao N A. J Free Rad Biol Med. 1986;2:349–357. doi: 10.1016/s0748-5514(86)80035-6. [DOI] [PubMed] [Google Scholar]
- 16.Ruuls S R, Bauer J, Sontrop K, Huitinga I, Hart B A, Dijkstra C D. J Neuroimmunol. 1995;56:207–217. doi: 10.1016/0165-5728(94)00154-g. [DOI] [PubMed] [Google Scholar]
- 17.Brett R, Rumsby M G. Neurochem Int. 1993;23:35–44. doi: 10.1016/0197-0186(93)90141-q. [DOI] [PubMed] [Google Scholar]
- 18.Abuchowski A, McCoy J R, Palczuk N C, Van Es T, Davis F F. J Biol Chem. 1977;252:3582–3586. [PubMed] [Google Scholar]
- 19.Guy J, McGorray S, Fitzsimmons J, Beck B, Mancuso A, Rao N A, Hamed L. Invest Ophthalmol Visual Sci. 1994;35:3456–3465. [PubMed] [Google Scholar]
- 20.Guy J, Fitzsimmons J, Beck B, Rao N A. Invest Ophthalmol Visual Sci. 1994;35:1114–1123. [PubMed] [Google Scholar]
- 21.Mulligan R. Science. 1993;260:926–932. doi: 10.1126/science.8493530. [DOI] [PubMed] [Google Scholar]
- 22.McLaughlin S K, Collis P, Hermonat P L, Muzyczka N. J Virol. 1988;62:1963–1973. doi: 10.1128/jvi.62.6.1963-1973.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Erzurum S C, Lemarchand P, Rosenfeld M A, Yoo J-H, Crystal R G. Nucleic Acids Res. 1993;21:1607–1612. doi: 10.1093/nar/21.7.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zolotukhin S, Potter M, Hauswirth W, Guy J, Muzyczka N. J Virol. 1996;70:4646–4654. doi: 10.1128/jvi.70.7.4646-4654.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Flannery J G, Zolotukhin S, Vaquero M I, LaVail M M, Muzyczka N, Hauswirth W W. Proc Natl Acad Sci USA. 1997;94:6916–6921. doi: 10.1073/pnas.94.13.6916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qi X, Guy J, Nick H, Valentine J, Rao N. Invest Ophthalmol Visual Sci. 1997;38:1203–1212. [PubMed] [Google Scholar]
- 27.Katz D, Taubenberger J K, Cannella B, McFarlin D E, Raine C S, McFarland H F. Ann Neurol. 1993;34:661–669. doi: 10.1002/ana.410340507. [DOI] [PubMed] [Google Scholar]
- 28.Guy J, Fitzsimmons J, Ellis E A, Beck B, Mancuso A. Ophthalmology. 1992;99:720–725. doi: 10.1016/s0161-6420(92)31905-0. [DOI] [PubMed] [Google Scholar]
- 29.Guy J, Fitzsimmons J, Ellis E A, Mancuso A. Ophthalmology. 1990;97:601–607. doi: 10.1016/s0161-6420(90)32536-8. [DOI] [PubMed] [Google Scholar]
- 30.Guy J, Ellis E A, Rao N A. Arch Ophthalmol. 1990;108:1614–1621. doi: 10.1001/archopht.1990.01070130116041. [DOI] [PubMed] [Google Scholar]
- 31.Kim Y S, Kim S U. J Neurosci Res. 1991;29:100–106. doi: 10.1002/jnr.490290111. [DOI] [PubMed] [Google Scholar]
- 32.Chen H, McCarty D M, Bruce A T, Suzuki K, Suzuki K. Gene Ther. 1998;5:50–58. doi: 10.1038/sj.gt.3300547. [DOI] [PubMed] [Google Scholar]
- 33.Peel A L, Zolotukhin S, Schrimsher G W, Muzyczka N, Rier P J. Gene Ther. 1997;4:16–24. doi: 10.1038/sj.gt.3300358. [DOI] [PubMed] [Google Scholar]
- 34.Optic Neuritis Study Group. Arch Ophthalmol. 1997;115:1545–1552. [PubMed] [Google Scholar]
- 35.Trapp B D, Peterson J, Ransohoff R M, Rudick R, Mork S, Bo L. N Engl J Med. 1998;338:278–285. doi: 10.1056/NEJM199801293380502. [DOI] [PubMed] [Google Scholar]
- 36.Cayouette M, Gravel C. Invest Ophthalmol Visual Sci. 1996;37:2022–2028. [PubMed] [Google Scholar]
- 37.Xiao X, Li J, Samulski R J. J Virol. 1996;70:8098–8108. doi: 10.1128/jvi.70.11.8098-8108.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoffman L M, Maquire A M, Bennett J. Invest Ophthalmol Visual Sci. 1997;38:2224–2233. [PubMed] [Google Scholar]
- 39.Kaplitt M G, Leone P, Samulski R J, Xiao X, Pfaff D W, O’Malley K L, During M J. Nat Genet. 1994;8:148–154. doi: 10.1038/ng1094-148. [DOI] [PubMed] [Google Scholar]
- 40.Kessler P D, Podsakoff G M, Chen X, McQuiston S A, Colosi P C, Matelis L A, Kurtzman G J, Byrne B J. Proc Natl Acad Sci USA. 1996;93:14082–14087. doi: 10.1073/pnas.93.24.14082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gaur A, Wiers B, Liu A, Rothbard J, Fathman C G. Science. 1992;258:1491–1494. doi: 10.1126/science.1279812. [DOI] [PubMed] [Google Scholar]
- 42.Weiner H L, Mackin G A, Matsui M, Orav E J, Khour S J, Dawson D M, Hafler D A. Science. 1993;259:1321–1324. doi: 10.1126/science.7680493. [DOI] [PubMed] [Google Scholar]
- 43.Panitch H S, Hirsch R L, Schindler J, Johnson K P. Neurology. 1987;37:1097–1102. doi: 10.1212/wnl.37.7.1097. [DOI] [PubMed] [Google Scholar]
- 44.Karussis D M, Lehmann D, Slavin S, Vourka-Karussis U, Mizrachi-Koll R, Ovadia H, Kalland T, Abramsky O. Proc Natl Acad Sci USA. 1993;90:6400–6404. doi: 10.1073/pnas.90.14.6400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Okuda Y, Sakoda S, Fujimura H, Yanagihara T. J Neuroimmunol. 1998;81:201–210. doi: 10.1016/s0165-5728(97)00180-x. [DOI] [PubMed] [Google Scholar]
- 46.Hooper D C, Spitsin S, Kean R B, Champion J M, Dickson G M, Chaudhry I, Koprowski H. Proc Natl Acad Sci USA. 1998;95:675–680. doi: 10.1073/pnas.95.2.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gold D P, Schroder K, Powell H C, Kelly C J. Eur J Immunol. 1997;27:2863–2869. doi: 10.1002/eji.1830271118. [DOI] [PubMed] [Google Scholar]
- 48.Cross A H, Manning P T, Stern M K, Misko T P. J Neuroimmunol. 1997;80:121–130. doi: 10.1016/s0165-5728(97)00145-8. [DOI] [PubMed] [Google Scholar]
- 49.van der Veen R C, Hinton D R, Incardonna F, Hofman F M. J Neuroimmunol. 1997;77:1–7. doi: 10.1016/s0165-5728(97)00013-1. [DOI] [PubMed] [Google Scholar]
- 50.Shaw M K, Lorens J B, Dhawan A, DalCanto R, Tse H Y, Tran A B, Bonpane C, Eswaran S L, Brocke S, Sarvetnick N, et al. J Exp Med. 1997;185:1711–1714. doi: 10.1084/jem.185.9.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mathisen P M, Yu M, Johnson J M, Drazba J A, Tuohy V K. J Exp Med. 1997;186:159–164. doi: 10.1084/jem.186.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]