Abstract
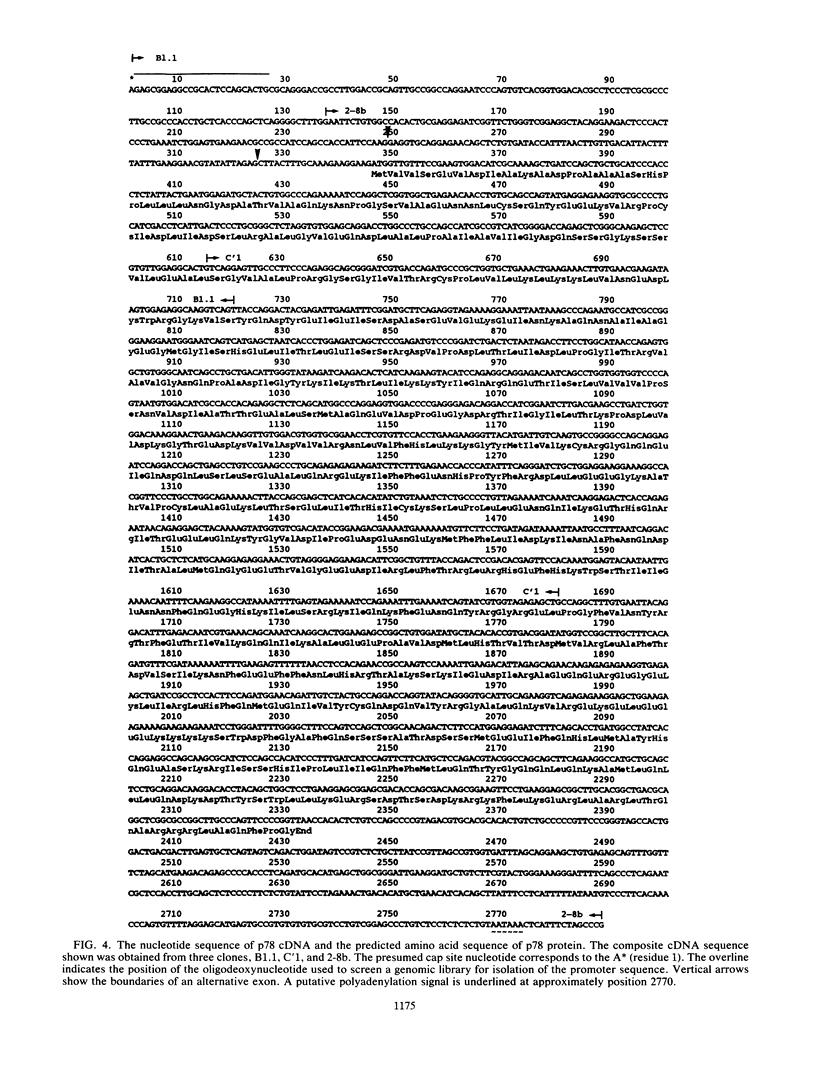
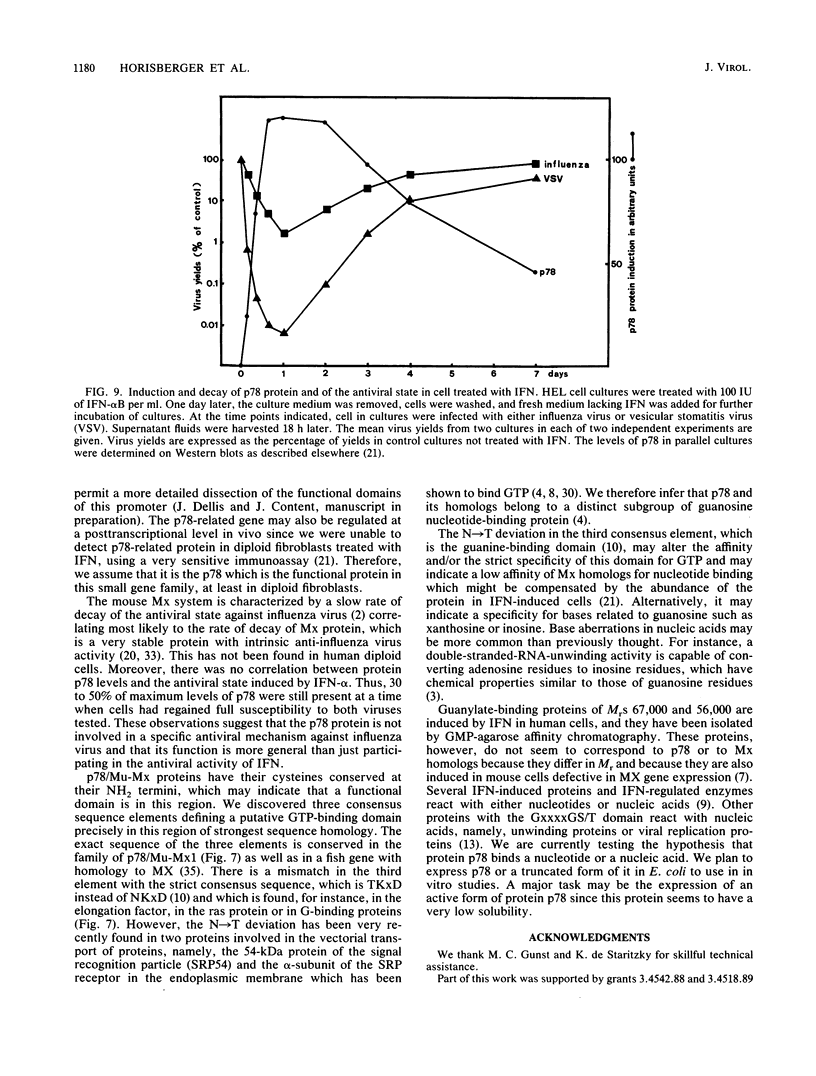
The human protein p78 is induced and accumulated in cells treated with type I interferon or with some viruses. It is the human homolog of the mouse Mx protein involved in resistance to influenza virus. A full-length cDNA clone encoding the human p78 protein was cloned and sequenced. It contained an open reading frame of 662 amino acids, corresponding to a polypeptide with a predicted molecular weight of 75,500, in good agreement with the Mr of 78,000 determined on sodium dodecyl sulfate gels for the purified natural p78 protein. The cloned gene was expressed in vitro and corresponded in size, pI, antigenic determinant(s), and NH2 terminus sequence to the natural p78 protein. A second cDNA was cloned which encoded a 633-amino-acid protein sharing 63% homology with human p78. This p78-related protein was translated in reticulocyte lysates where it shared an antigenic determinant(s) with p78. A putative 5' regulatory region of 83 base pairs contained within the gene promoter region upstream of the presumed p78 mRNA cap site conferred human alpha interferon (IFN-alpha) inducibility to the cat reporter gene. The p78 protein accumulated to high levels in cells treated with IFN-alpha. In contrast, the p78-related protein was not expressed at detectable levels. The rate of decay of p78 levels in diploid cells after a 24-h treatment with IFN-alpha was much slower than the rate of decay of the antiviral state against influenza A virus and vesicular stomatitis virus, suggesting that the p78 protein is probably not involved in an antiviral mechanism. Furthermore, we showed that these proteins, as well as the homologous mouse Mx protein, possess three consensus elements in proper spacing, characteristic of GTP-binding proteins.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnheiter H., Haller O. Mx gene control of interferon action: different kinetics of the antiviral state against influenza virus and vesicular stomatitis virus. J Virol. 1983 Sep;47(3):626–630. doi: 10.1128/jvi.47.3.626-630.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass B. L., Weintraub H. An unwinding activity that covalently modifies its double-stranded RNA substrate. Cell. 1988 Dec 23;55(6):1089–1098. doi: 10.1016/0092-8674(88)90253-x. [DOI] [PubMed] [Google Scholar]
- Bernstein H. D., Poritz M. A., Strub K., Hoben P. J., Brenner S., Walter P. Model for signal sequence recognition from amino-acid sequence of 54K subunit of signal recognition particle. Nature. 1989 Aug 10;340(6233):482–486. doi: 10.1038/340482a0. [DOI] [PubMed] [Google Scholar]
- Caput D., Beutler B., Hartog K., Thayer R., Brown-Shimer S., Cerami A. Identification of a common nucleotide sequence in the 3'-untranslated region of mRNA molecules specifying inflammatory mediators. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1670–1674. doi: 10.1073/pnas.83.6.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J. Y., Herbst H., Aebersold R., Braun D. G. A new isotype sequence (V kappa 27) of the variable region of kappa-light chains from a mouse hybridoma-derived anti-(streptococcal group A polysaccharide) antibody containing an additional cysteine residue. Application of the dimethylaminoazobenzene isothiocyanate technique for the isolation of peptides. Biochem J. 1983 Apr 1;211(1):173–180. doi: 10.1042/bj2110173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y. S., Colonno R. J., Yin F. H. Interferon induction of fibroblast proteins with guanylate binding activity. J Biol Chem. 1983 Jun 25;258(12):7746–7750. [PubMed] [Google Scholar]
- Connolly T., Gilmore R. The signal recognition particle receptor mediates the GTP-dependent displacement of SRP from the signal sequence of the nascent polypeptide. Cell. 1989 May 19;57(4):599–610. doi: 10.1016/0092-8674(89)90129-3. [DOI] [PubMed] [Google Scholar]
- Dever T. E., Glynias M. J., Merrick W. C. GTP-binding domain: three consensus sequence elements with distinct spacing. Proc Natl Acad Sci U S A. 1987 Apr;84(7):1814–1818. doi: 10.1073/pnas.84.7.1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
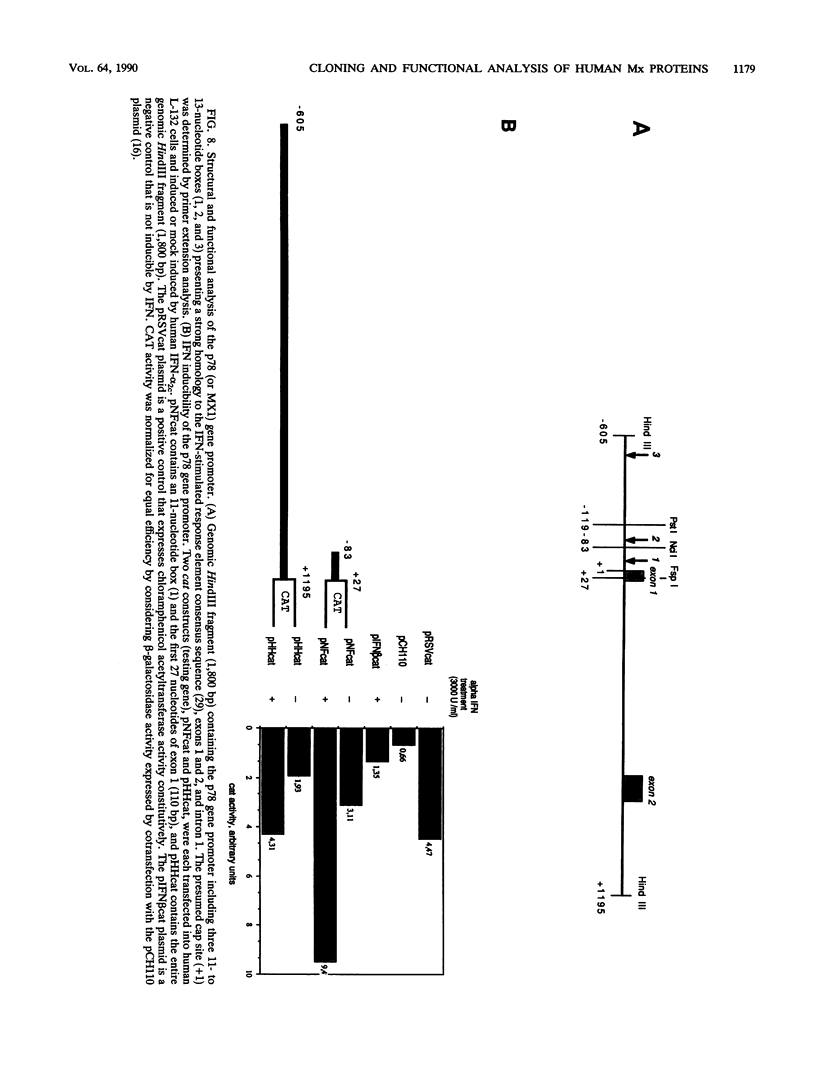
- Goetschy J. F., Zeller H., Content J., Horisberger M. A. Regulation of the interferon-inducible IFI-78K gene, the human equivalent of the murine Mx gene, by interferons, double-stranded RNA, certain cytokines, and viruses. J Virol. 1989 Jun;63(6):2616–2622. doi: 10.1128/jvi.63.6.2616-2622.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbalenya A. E., Koonin E. V., Donchenko A. P., Blinov V. M. A novel superfamily of nucleoside triphosphate-binding motif containing proteins which are probably involved in duplex unwinding in DNA and RNA replication and recombination. FEBS Lett. 1988 Aug 1;235(1-2):16–24. doi: 10.1016/0014-5793(88)81226-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Herbomel P., Bourachot B., Yaniv M. Two distinct enhancers with different cell specificities coexist in the regulatory region of polyoma. Cell. 1984 Dec;39(3 Pt 2):653–662. doi: 10.1016/0092-8674(84)90472-0. [DOI] [PubMed] [Google Scholar]
- Horisberger M. A., De Staritzky K. Expression and stability of the Mx protein in different tissues of mice, in response to interferon inducers or to influenza virus infection. J Interferon Res. 1989 Oct;9(5):583–590. doi: 10.1089/jir.1989.9.583. [DOI] [PubMed] [Google Scholar]
- Horisberger M. A., Hochkeppel H. K. IFN-alpha induced human 78 kD protein: purification and homologies with the mouse Mx protein, production of monoclonal antibodies, and potentiation effect of IFN-gamma. J Interferon Res. 1987 Aug;7(4):331–343. doi: 10.1089/jir.1987.7.331. [DOI] [PubMed] [Google Scholar]
- Horisberger M. A., Staeheli P., Haller O. Interferon induces a unique protein in mouse cells bearing a gene for resistance to influenza virus. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1910–1914. doi: 10.1073/pnas.80.7.1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horisberger M. A. The action of recombinant bovine interferons on influenza virus replication correlates with the induction of two Mx-related proteins in bovine cells. Virology. 1988 Jan;162(1):181–186. doi: 10.1016/0042-6822(88)90407-2. [DOI] [PubMed] [Google Scholar]
- Horisberger M. A., Wathelet M., Szpirer J., Szpirer C., Islam Q., Levan G., Huez G., Content J. cDNA cloning and assignment to chromosome 21 of IFI-78K gene, the human equivalent of murine Mx gene. Somat Cell Mol Genet. 1988 Mar;14(2):123–131. doi: 10.1007/BF01534397. [DOI] [PubMed] [Google Scholar]
- Hug H., Costas M., Staeheli P., Aebi M., Weissmann C. Organization of the murine Mx gene and characterization of its interferon- and virus-inducible promoter. Mol Cell Biol. 1988 Aug;8(8):3065–3079. doi: 10.1128/mcb.8.8.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. At least six nucleotides preceding the AUG initiator codon enhance translation in mammalian cells. J Mol Biol. 1987 Aug 20;196(4):947–950. doi: 10.1016/0022-2836(87)90418-9. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Meier E., Fäh J., Grob M. S., End R., Staeheli P., Haller O. A family of interferon-induced Mx-related mRNAs encodes cytoplasmic and nuclear proteins in rat cells. J Virol. 1988 Jul;62(7):2386–2393. doi: 10.1128/jvi.62.7.2386-2393.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen P. J., McMaster G. K., Trachsel H. Cloning of eukaryotic protein synthesis initiation factor genes: isolation and characterization of cDNA clones encoding factor eIF-4A. Nucleic Acids Res. 1985 Oct 11;13(19):6867–6880. doi: 10.1093/nar/13.19.6867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter A. C., Chernajovsky Y., Dale T. C., Gilbert C. S., Stark G. R., Kerr I. M. Interferon response element of the human gene 6-16. EMBO J. 1988 Jan;7(1):85–92. doi: 10.1002/j.1460-2075.1988.tb02786.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin B. Y., Anderson S. L., Lunn R. M., Hellermann G. R., Richardson N. K., Smith L. J. Production of a monoclonal antibody directed against an interferon-induced 56,000-dalton protein and its use in the study of this protein. J Virol. 1988 Jun;62(6):1875–1880. doi: 10.1128/jvi.62.6.1875-1880.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Römisch K., Webb J., Herz J., Prehn S., Frank R., Vingron M., Dobberstein B. Homology of 54K protein of signal-recognition particle, docking protein and two E. coli proteins with putative GTP-binding domains. Nature. 1989 Aug 10;340(6233):478–482. doi: 10.1038/340478a0. [DOI] [PubMed] [Google Scholar]
- Shaw G., Kamen R. A conserved AU sequence from the 3' untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell. 1986 Aug 29;46(5):659–667. doi: 10.1016/0092-8674(86)90341-7. [DOI] [PubMed] [Google Scholar]
- Staeheli P., Haller O., Boll W., Lindenmann J., Weissmann C. Mx protein: constitutive expression in 3T3 cells transformed with cloned Mx cDNA confers selective resistance to influenza virus. Cell. 1986 Jan 17;44(1):147–158. doi: 10.1016/0092-8674(86)90493-9. [DOI] [PubMed] [Google Scholar]
- Staeheli P., Sutcliffe J. G. Identification of a second interferon-regulated murine Mx gene. Mol Cell Biol. 1988 Oct;8(10):4524–4528. doi: 10.1128/mcb.8.10.4524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staeheli P., Yu Y. X., Grob R., Haller O. A double-stranded RNA-inducible fish gene homologous to the murine influenza virus resistance gene Mx. Mol Cell Biol. 1989 Jul;9(7):3117–3121. doi: 10.1128/mcb.9.7.3117. [DOI] [PMC free article] [PubMed] [Google Scholar]




