Abstract
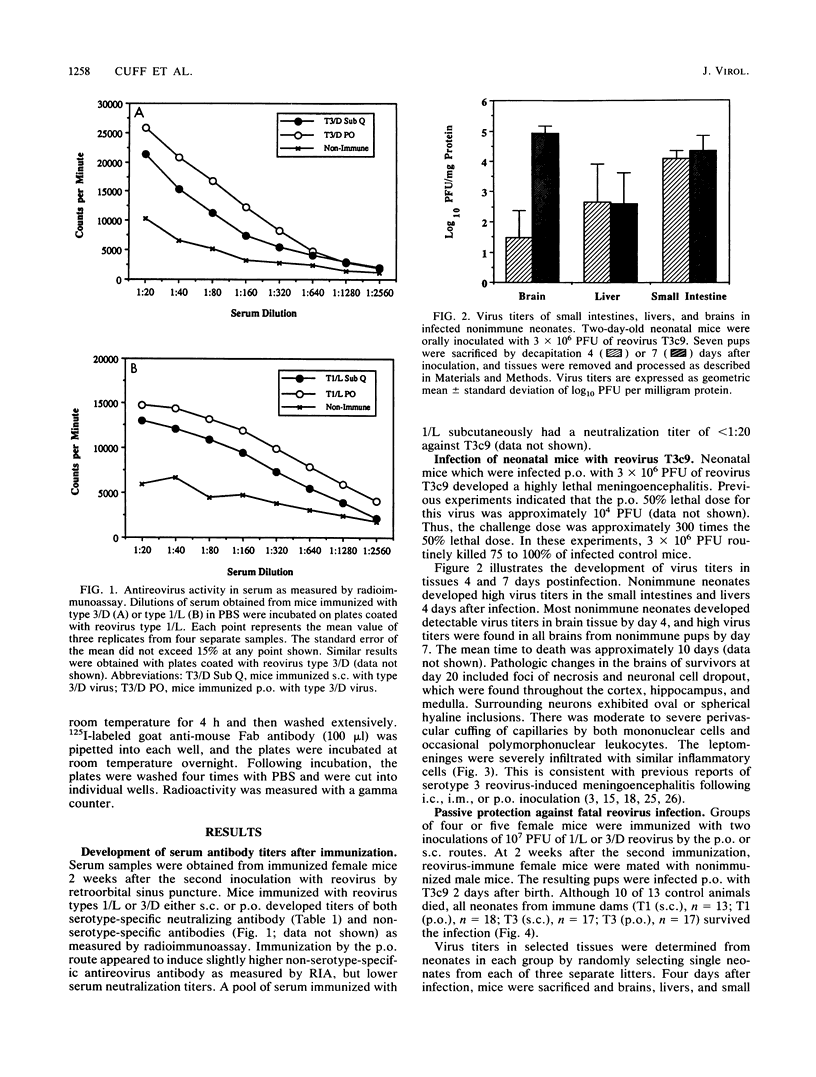
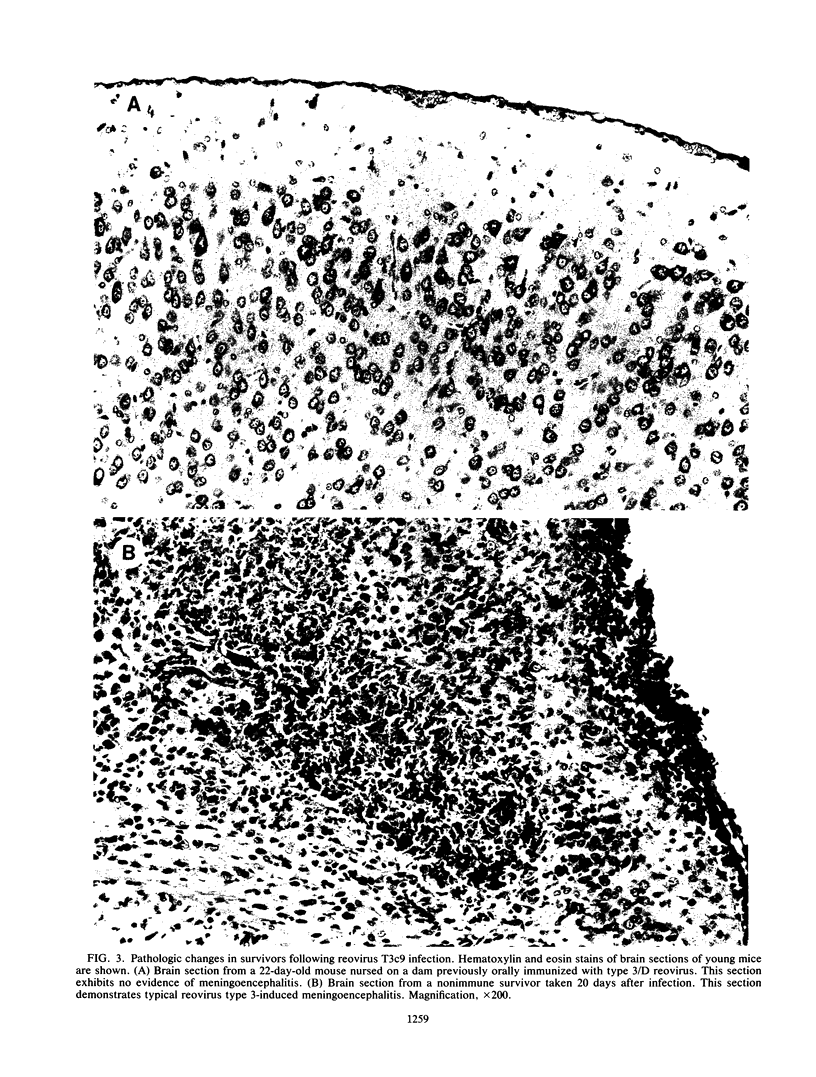
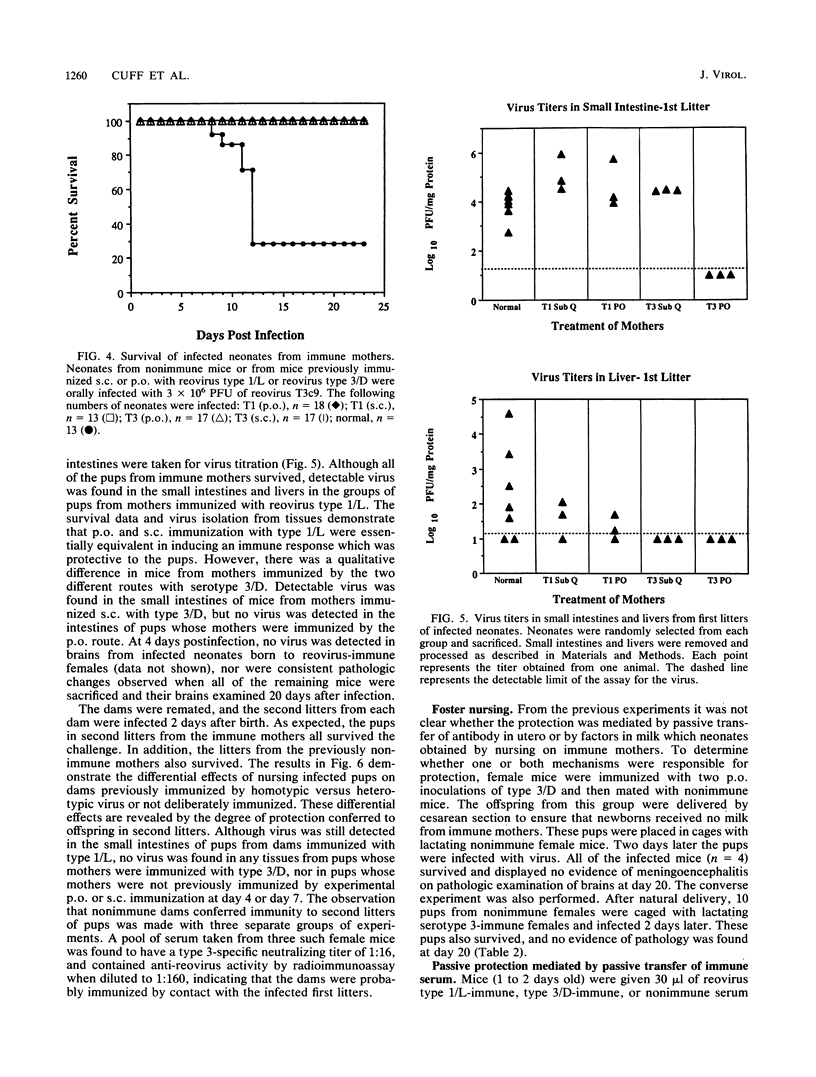
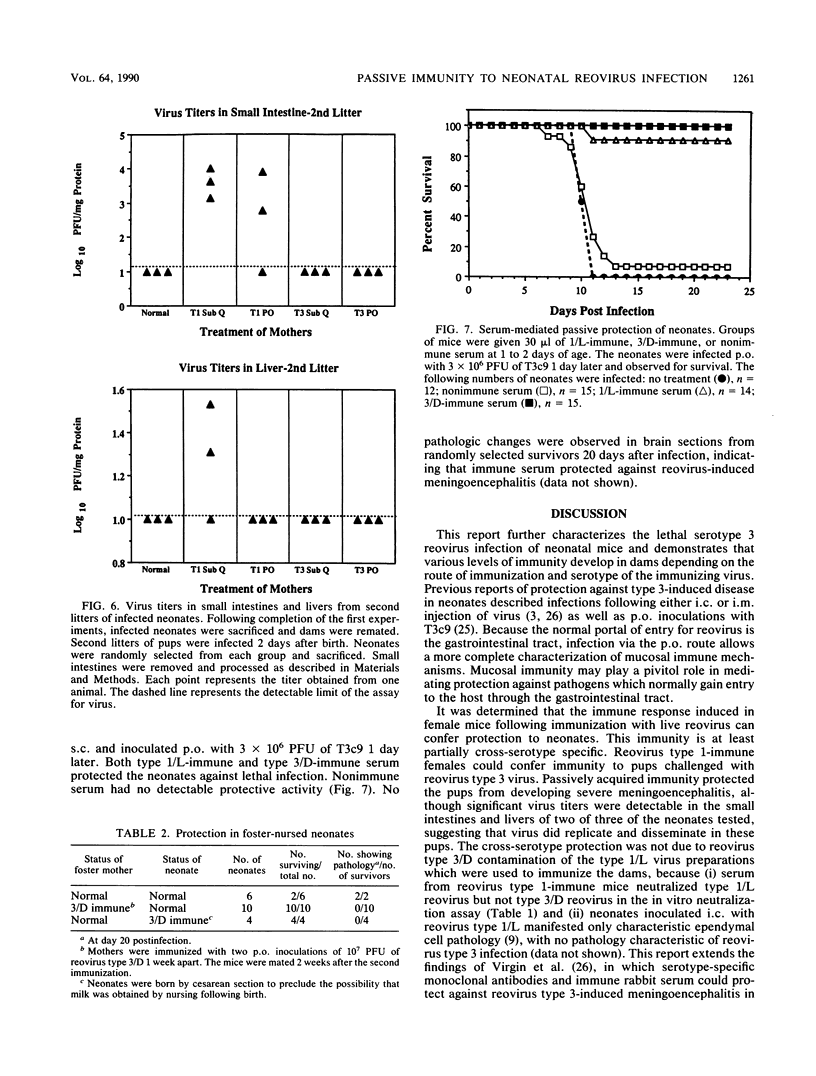
The role of passively acquired immunity to reovirus-induced meningoencephalitis in neonatal mice was examined. It was determined that female mice were capable of conferring protection against viral infection and meningoencephalitis in neonates depending on the route by which the dams were immunized and the serotype of the immunizing virus. Female mice immunized with homotypic virus via the oral route developed the most potent response. Infected neonates born and nursed by these females developed no signs of disease, and no virus was recoverable from their small intestines, livers, or brains following infection. Neonates born to females immunized with homotypic virus by the subcutaneous route manifested no evidence of meningoencephalitis or virus dissemination, yet virus was recovered from neonatal intestines. Mice immunized with heterotypic virus by either the subcutaneous or the oral route also conferred protection against disease; however, virus was recovered in small intestines and livers of infected neonates. Based on results from foster-nursing experiments, it appears that factors obtained both during suckling and by transplacental transfer contribute to protection. Passive transfer of reovirus-immune mouse serum also protected neonates from disease. These results demonstrate that passive immune mechanisms can mediate the protection of neonates against reovirus infection and provide further evidence of the importance of the mucosal immune response in protection against pathogens that invade the host via mucosal tissues.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burstin S. J., Spriggs D. R., Fields B. N. Evidence for functional domains on the reovirus type 3 hemagglutinin. Virology. 1982 Feb;117(1):146–155. doi: 10.1016/0042-6822(82)90514-1. [DOI] [PubMed] [Google Scholar]
- Finberg R., Weiner H. L., Fields B. N., Benacerraf B., Burakoff S. J. Generation of cytolytic T lymphocytes after reovirus infection: role of S1 gene. Proc Natl Acad Sci U S A. 1979 Jan;76(1):442–446. doi: 10.1073/pnas.76.1.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaulton G. N., Sharpe A. H., Chang D. W., Fields B. N., Greene M. I. Syngeneic monoclonal internal image anti-idiotopes as prophylactic vaccines. J Immunol. 1986 Nov 1;137(9):2930–2936. [PubMed] [Google Scholar]
- Hauser S. L., Che M., Fallis R. J., Weiner H. L. Immunoregulatory abnormalities induced by experimental reovirus infection: functional alterations in T-cell subpopulations. Clin Immunol Immunopathol. 1987 Dec;45(3):481–490. doi: 10.1016/0090-1229(87)90099-7. [DOI] [PubMed] [Google Scholar]
- Hayes E. C., Lee P. W., Miller S. E., Joklik W. K. The interaction of a series of hybridoma IgGs with reovirus particles. Demonstration that the core protein lambda 2 is exposed on the particle surface. Virology. 1981 Jan 15;108(1):147–155. doi: 10.1016/0042-6822(81)90534-1. [DOI] [PubMed] [Google Scholar]
- Hrdy D. B., Rubin D. H., Fields B. N. Molecular basis of reovirus neurovirulence: role of the M2 gene in avirulence. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1298–1302. doi: 10.1073/pnas.79.4.1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauffman R. S., Lee S., Finberg R. Cytolytic T-cell mediated lysis of reovirus-infected cells: requirements for infectious virus, viral particles, and viral proteins in infected target cells. Virology. 1983 Dec;131(2):265–273. doi: 10.1016/0042-6822(83)90495-6. [DOI] [PubMed] [Google Scholar]
- Keroack M., Fields B. N. Viral shedding and transmission between hosts determined by reovirus L2 gene. Science. 1986 Jun 27;232(4758):1635–1638. doi: 10.1126/science.3012780. [DOI] [PubMed] [Google Scholar]
- Kilham L., Margolis G. Hydrocephalus in hamsters, ferrets, rats, and mice following inoculations with reovirus type I. I. Virologic studies. Lab Invest. 1969 Sep;21(3):183–188. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lee P. W., Hayes E. C., Joklik W. K. Characterization of anti-reovirus immunoglobulins secreted by cloned hybridoma cell lines. Virology. 1981 Jan 15;108(1):134–146. doi: 10.1016/0042-6822(81)90533-x. [DOI] [PubMed] [Google Scholar]
- Letvin N. L., Kauffman R. S., Finberg R. T lymphocyte immunity to reovirus: cellular requirements for generation and role in clearance of primary infections. J Immunol. 1981 Dec;127(6):2334–2339. [PubMed] [Google Scholar]
- London S. D., Cebra J. J., Rubin D. H. Intraepithelial lymphocytes contain virus-specific, MHC-restricted cytotoxic cell precursors after gut mucosal immunization with reovirus serotype 1/Lang. Reg Immunol. 1989 Mar-Apr;2(2):98–102. [PubMed] [Google Scholar]
- London S. D., Rubin D. H., Cebra J. J. Gut mucosal immunization with reovirus serotype 1/L stimulates virus-specific cytotoxic T cell precursors as well as IgA memory cells in Peyer's patches. J Exp Med. 1987 Mar 1;165(3):830–847. doi: 10.1084/jem.165.3.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis G., Kilham L., Gonatas N. K. Reovirus type 3 encephalitis: observations of virus-cell interactions in neural tissues. I. Light microscopy studies. Lab Invest. 1971 Feb;24(2):91–100. [PubMed] [Google Scholar]
- Offit P. A., Clark H. F. Maternal antibody-mediated protection against gastroenteritis due to rotavirus in newborn mice is dependent on both serotype and titer of antibody. J Infect Dis. 1985 Dec;152(6):1152–1158. doi: 10.1093/infdis/152.6.1152. [DOI] [PubMed] [Google Scholar]
- Offit P. A., Clark H. F. Protection against rotavirus-induced gastroenteritis in a murine model by passively acquired gastrointestinal but not circulating antibodies. J Virol. 1985 Apr;54(1):58–64. doi: 10.1128/jvi.54.1.58-64.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSEN L. Serologic grouping of reoviruses by hemagglutination-inhibition. Am J Hyg. 1960 Mar;71:242–249. doi: 10.1093/oxfordjournals.aje.a120107. [DOI] [PubMed] [Google Scholar]
- Raine C. S., Fields B. N. Reovirus type 3 encephalitis--a virologic and ultrastructural study. J Neuropathol Exp Neurol. 1973 Jan;32(1):19–33. doi: 10.1097/00005072-197301000-00002. [DOI] [PubMed] [Google Scholar]
- Rubin D. H., Kornstein M. J., Anderson A. O. Reovirus serotype 1 intestinal infection: a novel replicative cycle with ileal disease. J Virol. 1985 Feb;53(2):391–398. doi: 10.1128/jvi.53.2.391-398.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin D., Weiner H. L., Fields B. N., Greene M. I. Immunologic tolerance after oral administration of reovirus: requirement for two viral gene products for tolerance induction. J Immunol. 1981 Oct;127(4):1697–1701. [PubMed] [Google Scholar]
- Spriggs D. R., Bronson R. T., Fields B. N. Hemagglutinin variants of reovirus type 3 have altered central nervous system tropism. Science. 1983 Apr 29;220(4596):505–507. doi: 10.1126/science.6301010. [DOI] [PubMed] [Google Scholar]
- Tardieu M., Powers M. L., Weiner H. L. Age dependent susceptibility to Reovirus type 3 encephalitis: role of viral and host factors. Ann Neurol. 1983 Jun;13(6):602–607. doi: 10.1002/ana.410130604. [DOI] [PubMed] [Google Scholar]
- Tyler K. L., Bronson R. T., Byers K. B., Fields B. Molecular basis of viral neurotropism: experimental reovirus infection. Neurology. 1985 Jan;35(1):88–92. doi: 10.1212/wnl.35.1.88. [DOI] [PubMed] [Google Scholar]
- Tyler K. L., Virgin H. W., 4th, Bassel-Duby R., Fields B. N. Antibody inhibits defined stages in the pathogenesis of reovirus serotype 3 infection of the central nervous system. J Exp Med. 1989 Sep 1;170(3):887–900. doi: 10.1084/jem.170.3.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virgin H. W., 4th, Bassel-Duby R., Fields B. N., Tyler K. L. Antibody protects against lethal infection with the neurally spreading reovirus type 3 (Dearing). J Virol. 1988 Dec;62(12):4594–4604. doi: 10.1128/jvi.62.12.4594-4604.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALTERS M. N., JOSKE R. A., LEAK P. J., STANLEY N. F. MURINE INFECTION WITH REOVIRUS: I. PATHOLOGY OF THE ACUTE PHASE. Br J Exp Pathol. 1963 Aug;44:427–436. [PMC free article] [PubMed] [Google Scholar]
- Williams W. V., London S. D., Weiner D. B., Wadsworth S., Berzofsky J. A., Robey F., Rubin D. H., Greene M. I. Immune response to a molecularly defined internal image idiotope. J Immunol. 1989 Jun 15;142(12):4392–4400. [PubMed] [Google Scholar]



