Abstract
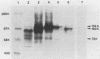
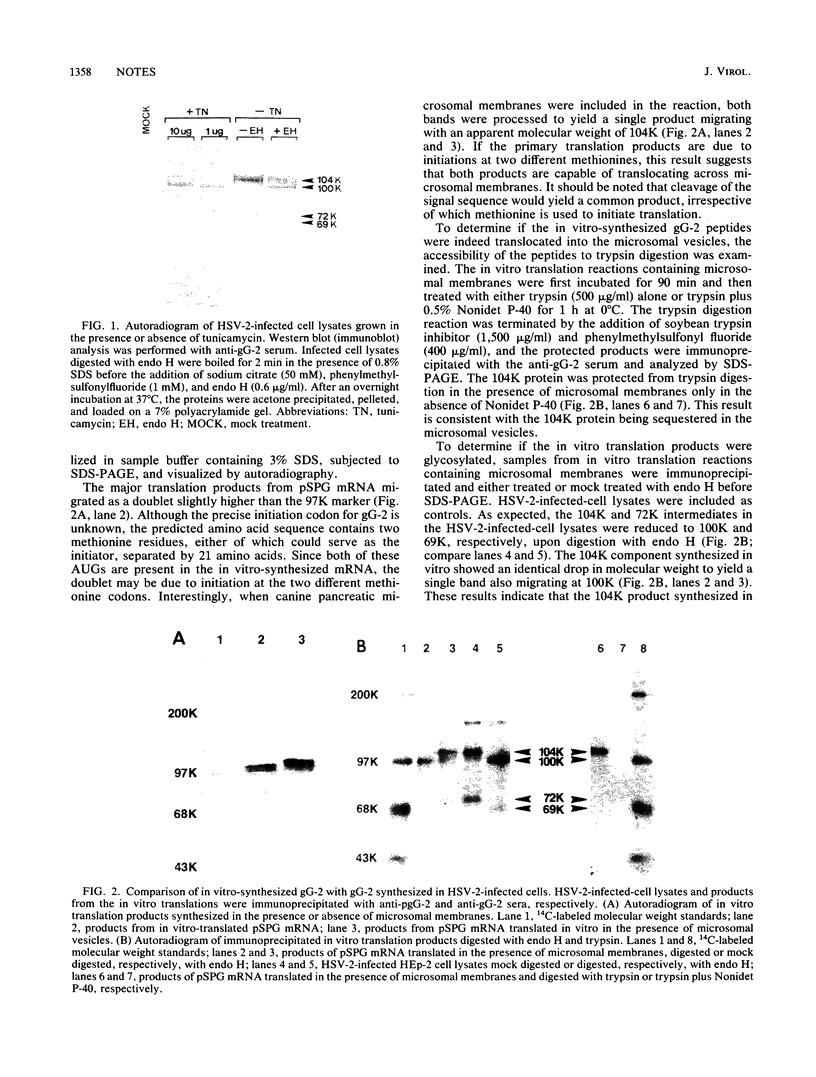
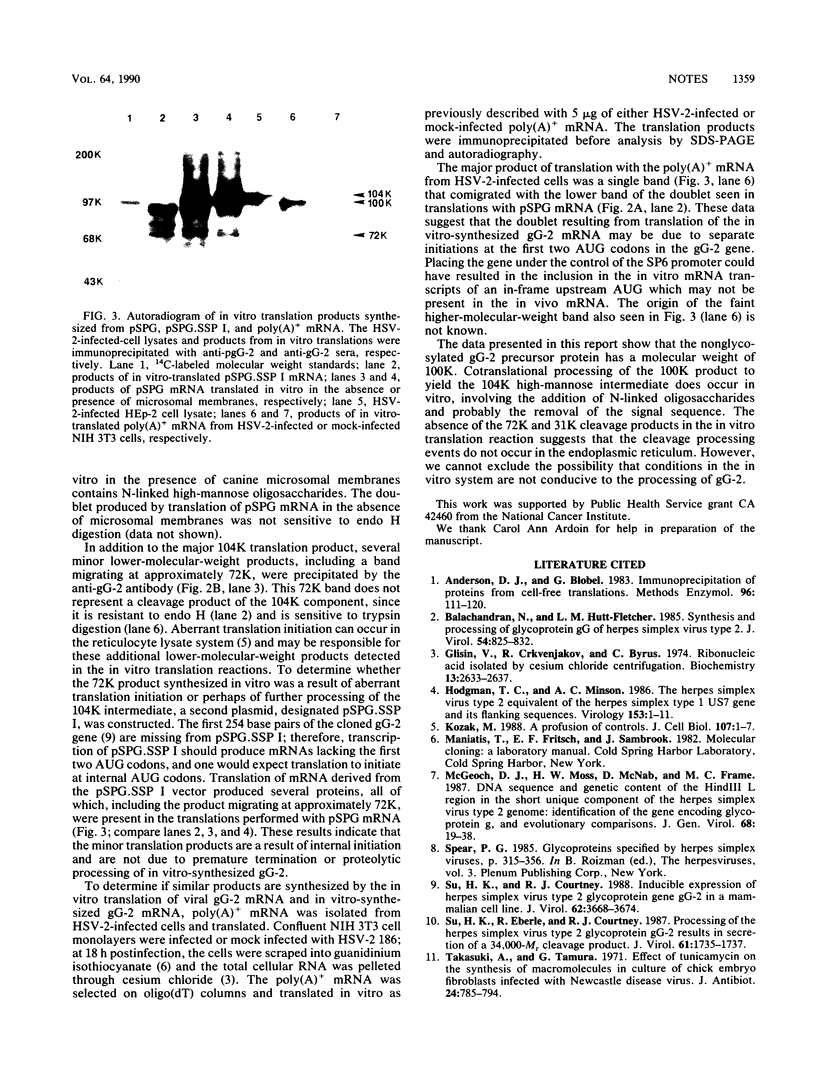
Translation of in vitro-synthesized herpes simplex virus type 2 (HSV-2) gG-2 mRNA in a reticulocyte lysate system was used to study the processing of HSV-2 gG-2. In the presence of canine pancreatic microsomal membranes, a single species that is protected from trypsin digestion was detected. This product comigrates with the 104,000-Mr (104K) high mannose intermediate seen in HSV-2-infected-cell lysates. Endo-beta-N-acetylglucosaminidase H treatment of the in vitro-synthesized 104K protein yielded a single product migrating at 100 K. The 72K and 31K cleavage products of gG-2 were not observed in the in vitro system. These data show that the molecular weight of the nonglycosylated form of the gG-2 protein is 100,000 and that the cotranslational processing of this protein in the endoplasmic reticulum yields the 104K high-mannose intermediate.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. J., Blobel G. Immunoprecipitation of proteins from cell-free translations. Methods Enzymol. 1983;96:111–120. doi: 10.1016/s0076-6879(83)96012-3. [DOI] [PubMed] [Google Scholar]
- Balachandran N., Hutt-Fletcher L. M. Synthesis and processing of glycoprotein gG of herpes simplex virus type 2. J Virol. 1985 Jun;54(3):825–832. doi: 10.1128/jvi.54.3.825-832.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glisin V., Crkvenjakov R., Byus C. Ribonucleic acid isolated by cesium chloride centrifugation. Biochemistry. 1974 Jun 4;13(12):2633–2637. doi: 10.1021/bi00709a025. [DOI] [PubMed] [Google Scholar]
- Hodgman T. C., Minson A. C. The herpes simplex virus type 2 equivalent of the herpes simplex virus type 1 US7 gene and its flanking sequences. Virology. 1986 Aug;153(1):1–11. doi: 10.1016/0042-6822(86)90002-4. [DOI] [PubMed] [Google Scholar]
- Kozak M. A profusion of controls. J Cell Biol. 1988 Jul;107(1):1–7. doi: 10.1083/jcb.107.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeoch D. J., Moss H. W., McNab D., Frame M. C. DNA sequence and genetic content of the HindIII l region in the short unique component of the herpes simplex virus type 2 genome: identification of the gene encoding glycoprotein G, and evolutionary comparisons. J Gen Virol. 1987 Jan;68(Pt 1):19–38. doi: 10.1099/0022-1317-68-1-19. [DOI] [PubMed] [Google Scholar]
- Su H. K., Courtney R. J. Inducible expression of herpes simplex virus type 2 glycoprotein gene gG-2 in a mammalian cell line. J Virol. 1988 Oct;62(10):3668–3674. doi: 10.1128/jvi.62.10.3668-3674.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su H. K., Eberle R., Courtney R. J. Processing of the herpes simplex virus type 2 glycoprotein gG-2 results in secretion of a 34,000-Mr cleavage product. J Virol. 1987 May;61(5):1735–1737. doi: 10.1128/jvi.61.5.1735-1737.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takatsuki A., Tamura G. Effect of tunicamycin on the synthesis of macromolecules in cultures of chick embryo fibroblasts infected with Newcastle disease virus. J Antibiot (Tokyo) 1971 Nov;24(11):785–794. doi: 10.7164/antibiotics.24.785. [DOI] [PubMed] [Google Scholar]