Abstract
The switch from vegetative to reproductive growth is marked by the termination of vegetative development and the adoption of floral identity by the shoot apical meristem (SAM). This process is called the floral transition. To elucidate the molecular determinants involved in this process, we performed genome-wide RNA expression profiling on maize (Zea mays) shoot apices at vegetative and early reproductive stages using massively parallel signature sequencing technology. Profiling revealed significant up-regulation of two maize MADS-box (ZMM) genes, ZMM4 and ZMM15, after the floral transition. ZMM4 and ZMM15 map to duplicated regions on chromosomes 1 and 5 and are linked to neighboring MADS-box genes ZMM24 and ZMM31, respectively. This gene order is syntenic with the vernalization1 locus responsible for floral induction in winter wheat (Triticum monococcum) and similar loci in other cereals. Analyses of temporal and spatial expression patterns indicated that the duplicated pairs ZMM4-ZMM24 and ZMM15-ZMM31 are coordinately activated after the floral transition in early developing inflorescences. More detailed analyses revealed ZMM4 expression initiates in leaf primordia of vegetative shoot apices and later increases within elongating meristems acquiring inflorescence identity. Expression analysis in late flowering mutants positioned all four genes downstream of the floral activators indeterminate1 (id1) and delayed flowering1 (dlf1). Overexpression of ZMM4 leads to early flowering in transgenic maize and suppresses the late flowering phenotype of both the id1 and dlf1 mutations. Our results suggest ZMM4 may play roles in both floral induction and inflorescence development.
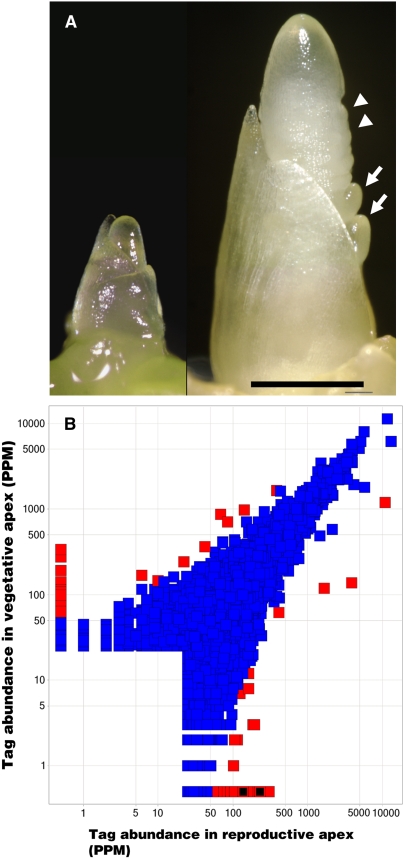
Growth of maize (Zea mays) is largely determined by the activity of the shoot apical meristem (SAM), which is a small, self-renewing organ positioned on the tip of the stem (Fig. 1A). During the first 3 to 4 weeks after germination, the SAM produces vegetative organs such as leaves and stem tissue. After about 4 to 5 weeks of growth, the transition from vegetative to reproductive development occurs. This period is called the floral transition and is distinguished in the SAM by the cessation of leaf initiation and its rapid increase in size, thereby changing morphology from a small symmetrical dome to elongated in shape (Irish and Nelson, 1991). The SAM continues to increase in size as it progresses through the floral transition. The transition terminates with the SAM acquiring inflorescence identity. An initial hallmark of inflorescence identity is the appearance of small ridges on the flanks of the elongated SAM, which are likely suppressed floral bract primordia (McSteen and Hake, 2001). Further specification of inflorescence identity is manifest by the initiation of branch (or spikelet pair) meristems (BMs) in the axils of the suppressed bracts (Fig. 1A). The total number of leaves produced by a plant is fixed by the floral transition and is one way to measure the timing of the transition. The appearance of bract primordia on the elongated SAM typifies an early stage inflorescence and can be used to mark the commencement of inflorescence development.
Figure 1.
Expression profiling of shoot apices before and after the floral transition. A, Representative image of maize shoot apices collected from V4-stage plants before (left) and V6-stage plants after (right) the floral transition. The vegetative meristem (left) is more symmetrical in shape, while the reproductive meristem (right) is much taller than it is wide. Suppressed bract primordia (arrowheads) and BMs (arrows) are evident on the flanks of the reproductive meristem. Scale bar = 1 mm. B, Scatter plot comparison of the abundance of 9,698 unique 17-mer sequence tags that matched maize EST sequences and accumulated to 25 ppm in at least one of the two apex samples detected by MPSS. Transcript abundance in parts per million is compared for each tag from the vegetative apex sample (y axis) to its level in the reproductive apex sample (x axis). Tags showing a z-score cutoff of 2.92 defining the top 1% expression differences are colored red. The expression levels of the two MADS-box genes, ZMM4 and ZMM15, only detected in the reproductive stage apex are red with a black center and lie near the x axis.
A large body of evidence supports the complex gene network established to explain the floral transition and floral development in Arabidopsis (Arabidopsis thaliana). To ensure favorable flowering time, plants sense environmental conditions such as day length, light quality, and temperature through the photoperiod and vernalization pathways (Boss et al., 2004; Bernier and Perilleux, 2005). Endogenous cues are transmitted through the hormone-signaling and autonomous pathways (Mouradov et al., 2002; Boss et al., 2004; Bernier and Perilleux, 2005; Razem et al., 2006). These complex signaling networks converge on key floral integrators such as LEAFY (LFY; Blazquez and Weigel, 2000; Boss et al., 2004), SUPPRESSOR OF OVEREXPRESSION OF CONSTANS1 (Lee et al., 2000), and FLOWERING LOCUS T (Samach et al., 2000; Michaels et al., 2005; Corbesier et al., 2007). From these integrators, the flowering signals are transmitted to early acting floral meristem identity MADS-box genes APETALA1 (AP1), CAULIFLOWER (CAL), and FRUITFUL (FUL) that promote formation of inflorescence meristems that ultimately produce flowers (Yanofsky, 1995; Ferrandiz et al., 2000). In addition to promoting floral identity, FUL also promotes the floral transition, since ful mutants are late flowering and FUL overexpression leads to early flowering (Ferrandiz et al., 2000).
A similar picture of the floral transition in rice (Oryza sativa) is also emerging due to the completion of its genome sequence and a significant number of cloned flowering time genes and quantitative trait loci (Yano et al., 2001; Izawa et al., 2003). Compared to Arabidopsis and rice, the genetic control of the floral transition and inflorescence specification in maize is less elaborated. Only three late flowering mutants are described in maize: Leafy (unrelated to Arabidopsis LFY), indeterminate1 (id1), and delayed flowering1 (dlf1); all delay the floral transition and therefore produce more leaves before flowering than wild-type plants (Neuffer et al., 1997; McSteen et al., 2000). The id1 gene has been cloned and encodes a zinc-finger transcription factor specifically expressed in immature leaf tissue and has been suggested to control a florigenic signal (Colasanti et al., 1998; Colasanti and Sundaresan, 2000; Kozaki et al., 2004). id1 is unique to grasses and a putative homolog was not found in Arabidopsis (Colasanti et al., 2006). The dlf1 gene was cloned recently and encodes a bZIP transcription factor homologous protein (Muszynski et al., 2006) that resembles the Arabidopsis floral integrator FLOWERING LOCUS D (FD; Abe et al., 2005). Two maize homologs of the Arabidopsis floral integrator LFY, zea floricaula/leafy1 (zf1l) and zfl2, seem to share a conserved role in regulating the floral transition and inflorescence architecture (Bomblies et al., 2003; Bomblies and Doebley, 2005). Comprehensive phylogenetic analyses have determined that of the AP1/CAL/FUL MADS-box gene clade, only FUL-like homologs exist within maize and other noneudicot species (Litt and Irish, 2003; Malcomber et al., 2006). The grass FUL-like genes fall into three subgroups; FUL1 and FUL2 are sister clades that are both sister to the FUL3 clade (Malcomber et al., 2006). FUL1 proteins are associated with specifying competence to flower after a cold treatment (vernalization) in barley (Hordeum vulgare), diploid wheat (Triticum monococcum), hexaploid wheat (Triticum aestivium), perennial ryegrass (Lolium perenne), and oats (Avena sativa; Danyluk et al., 2003; Murai et al., 2003; Trevaskis et al., 2003; Yan et al., 2003, 2005; Loukoianov et al., 2005; von Zitzewitz et al., 2005; Petersen et al., 2006; Preston and Kellogg, 2008). Additionally, expression of FUL1 and FUL2 within spikelet meristems and floral organs points to additional roles in specifying spikelet and/or floral meristem identity (Preston and Kellogg, 2007).
To identify new genes involved in specifying the floral transition and early inflorescence development in maize, we conducted genome-wide expression profiling of shoot apices collected at vegetative and early reproductive stages. The rationale for this experiment is based on previous findings that several Arabidopsis floral regulators are differentially expressed before and after the floral transition (Blazquez et al., 1997; Cardon et al., 1997; Borner et al., 2000; Yu et al., 2002; Boss et al., 2004; Vijayraghavan et al., 2005). Moreover, the differences in their level of expression can be detected using global transcript profiling techniques (Schmid et al., 2003). To identify genes differentially expressed before and after the floral transition, we used the open-ended massively parallel signature sequencing (MPSS) technology that quantifies gene expression levels by generating millions of gene-specific 17-mer sequence tags per mRNA library (Brenner et al., 2000; Reinartz et al., 2002). We identified two closely related MADS-box genes, ZMM4 and ZMM15, which were significantly up-regulated in posttransitional apices. ZMM4 and ZMM15 are linked to other MADS-box genes and form duplicate gene pairs ZMM4-ZMM24 and ZMM15-ZMM31 that are syntenic to the wheat vernalization1 (vrn1) locus that controls the floral transition in winter wheat varieties in response to a cold treatment (Danyluk et al., 2003; Trevaskis et al., 2003; Yan et al., 2005; Petersen et al., 2006). Previous work has shown ZMM4 and ZMM15 belong to the FUL1 family of MADS-box genes that controls the floral transition in temperate cereals and ZMM24 and ZMM31 are phylogenetically related to SEPALLATA MADS-box genes, a class of organ-identity genes that is required for flower development (Pelaz et al., 2000; Malcomber and Kellogg, 2005; Malcomber et al., 2006). Expression analyses of ZMM24 and ZMM31 are consistent with a role for these genes in inflorescence development after the acquisition of inflorescence identity. All four MADS-box genes are coordinately expressed in the developing inflorescence after the floral transition in wild-type plants, while expression is delayed in both late flowering id1 and dlf1 mutants. In situ hybridizations and analysis of PromoterZMM4:GUS transgenic (TG) plants revealed expression of ZMM4 is first detected in shoot apices near the time of the floral transition. Overexpression of ZMM4 induced early flowering in TG maize plants and was able to suppress the late flowering phenotype of both id1 and dlf1 mutants. Collectively, our results suggest ZMM4 may be involved in multiple roles including floral induction and inflorescence development.
RESULTS
ZMM4 and ZMM15 MADS-Box Genes Are Up-Regulated after the Floral Transition in Shoot Apices
To identify genes involved in the floral transition and early inflorescence development, we screened for genes that were differentially expressed by conducting genome-wide expression profiling of shoot apices before and after the floral transition. Vegetative shoot apices were sampled from seedlings with four fully expanded leaves (V4 stage), while early reproductive apices were sampled from seedlings with six fully expanded leaves (V6 stage; Fig. 1A). Plant developmental stages were identified according to Ritchie et al. (1997). Approximately 500 plants were dissected at each stage and apices with one to two leaf primordia attached were collected. mRNA isolated from V4 and V6 apices was submitted for MPSS profiling at Solexa, Inc. (Brenner et al., 2000). V4 and V6 samples produced 30,956 (out of 1,365,302 total tags) and 35,434 (out of 1,329,865 total tags) unique 17-mer tag sequences, respectively. Of those, 27,095 were matched to maize EST sequences and of these 9,698 had expression values in at least one of the two samples of 25 ppm or more, which was chosen as a threshold level of expression. From these 9,698 tags, we defined a z-score cutoff that established the top 1% of expression differences. A z-score of 2.92, or almost 3 sds, identified 99 unique tag sequences (Fig. 1B). Of the 99 tags, 45 tags had higher expression values in the V4 prefloral transition sample and 53 tags had higher expression values in the V6 postfloral transition sample compared to the average tag distribution in both samples (Fig. 1B).
Using the functional annotation of the sequences, we identified two MADS-box genes that were prominent among the sequences with higher expression after the floral transition. One MADS-box sequence tag (GATCGCGAGAAGCAGCA) had 0 ppm in the V4 sample and 206 ppm in the V6 sample. The other MADS-box sequence tag (GATCGCGAGAGCAGCAG) had 0 ppm in the V4 sample and 106 ppm in the V6 sample. We used the 17-mer sequence tags to identify full-length ESTs from the Pioneer/DuPont EST database. Shortly afterward, the corresponding cDNAs were deposited in GenBank and we adopted the GenBank names in our study. The first tag uniquely identified the cDNA for the maize MADS-box gene ZMM4 (GenBank accession no. AJ430641), while the second tag identified the cDNA for ZMM15 (GenBank accession no. AJ430632). Because MADS-box proteins are key regulators of various plant developmental processes (Becker et al., 2000; Becker and Theissen, 2003; Parenicova et al., 2003), and many are involved in the regulation of flowering time and floral development (Kang et al., 1997; Immink et al., 1999; Borner et al., 2000; Hartmann et al., 2000; Lee et al., 2000; Jang et al., 2002; Danyluk et al., 2003; Moon et al., 2003; Petersen et al., 2004), we focused our analysis on these two genes.
ZMM4 and ZMM15 Are Linked to the MADS-Box Genes ZMM24 and ZMM31
The ZMM4 and ZMM15 cDNAs share 95% sequence identity across their coding regions and 60% identity within their 5′ and 3′ untranslated regions (UTRs). Both cDNAs encode MADS-box proteins of the MIKC type with four characteristic domains: M (MADS), I (intervening), K (keratin like), and C (C terminal; Schwarz-Sommer et al., 1990). Their proteins are 93% identical, having only 17 amino acid differences within 245 amino acids of the entire protein. Eleven of the amino acid differences are all located in the C domain (Supplemental Fig. S1A).
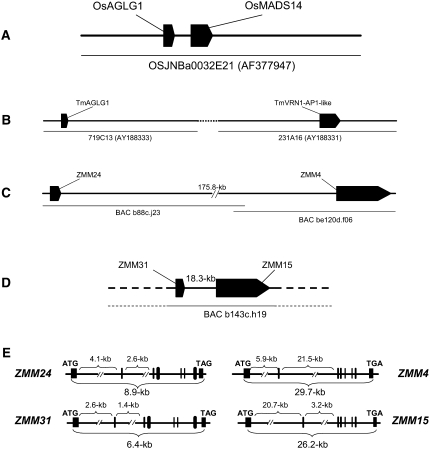
To identify genomic structures for ZMM4 and ZMM15, bacterial artificial chromosome (BAC) genomic libraries were screened with overgo probes unique to the 3′ UTRs of each gene. Several BAC clones were identified and assigned to two different BAC contigs on the DuPont maize physical map (Brunner et al., 2005). BAC b143c.h19 containing ZMM15 and BAC be120d.f06 containing ZMM4 with the adjacent overlapping BAC b88c.j23 were completely sequenced. The genomic structures of the corresponding BAC clones are shown in Figure 2, C and D. Subsequent annotation of the BAC sequences revealed the presence of two additional MADS-box genes; ZMM24 is approximately 175.8 kb upstream of ZMM4 (Fig. 2C) and ZMM31 is 18.3 kb upstream of ZMM15 (Fig. 2D). The ZMM24 and ZMM31 cDNAs share 84% identity to each other and also encode MADS-box proteins of the MIKC type. The ZMM24 and ZMM31 proteins are 93% identical, having 15 amino acid differences within 241 amino acids of the entire protein (Supplemental Fig. S1B).
Figure 2.
The genomic organization of the paired MADS-box loci in the grasses and gene structure in maize. A and B, Genome organization of the syntenic locus from rice (A) and wheat (B; Yan et al., 2003). C and D, Genome organization of the ZMM24-ZMM4 locus (C) and ZMM31-ZMM15 locus (D) from maize. E, Gene structure of each of the four MADS-box genes. Black boxes denote exons, thin black lines indicate introns, and the start codons (ATG) and stop codons (TAG or TGA) are marked. Large introns are reduced for clarity with their sizes indicated above each gene while overall coding sequence size is noted below each gene.
The gene pairs ZMM24-ZMM4 and ZMM31-ZMM15 show conservation of gene order (synteny) with segments of the rice and wheat genomes, in particular the wheat VRN1 locus (Yan et al., 2003; Fig. 2, A and B). Synteny with the wheat genome is especially significant because the VRN1 locus controls the floral transition in winter varieties of wheat and suggests a role for these maize loci in regulating flowering time. Because the maize duplication that gave rise to ZMM4 and ZMM15 occurred after the divergence of maize and wheat, both maize genes are equally distant from the wheat VRN1 gene.
The exon-intron organization of the four ZMM genes was deduced from alignment between their cDNA and genomic sequences. Similar to their rice and wheat counterparts, all four ZMM genes contain eight exons separated by seven introns of various lengths (Fig. 2E). This is the typical genomic structure of the MIKC type of MADS-box genes (Parenicova et al., 2003). However, one distinct feature of both ZMM4 and ZMM15 is the presence of a large intron. The second intron in ZMM4 is 21.5 kb and the first intron in ZMM15 is 20.7 kb. The large size of these introns expanded the overall length of both genes to 29.7 kb for ZMM4 and to 26.2 kb for ZMM15. This gene size is significantly above the 3 to 4 kb average size for plant genes (Bruggmann et al., 2006).
The duplicate ZMM loci were assigned to maize chromosomes according to the position of each BAC contig on the maize physical map. The ZMM24-ZMM4 pair was located on the long arm of chromosome 1 (bin 1.10) between markers umc1774 and csu261, while the ZMM31-ZMM15 pair was on the short arm of chromosome 5 (bin 5.01) between markers umc2036 and umc1781. Map positions were confirmed using the intermated B73/Mo17 (IBM2) mapping population. ZMM4 was linked to marker npi282b in bin 1.10 and ZMM15 was linked to npi282a in bin 5.01 (Supplemental Fig. S2).
The ZMM Genes Are Primarily Expressed during Reproductive Growth
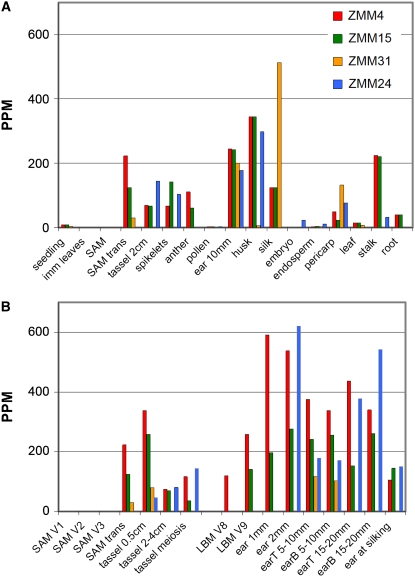
Expression levels were identified for the four ZMM genes across a wide range of tissues and stages through analysis of their 17-mer tag distribution in a variety of MPSS expression libraries. The site and level of expression for ZMM4 and ZMM15 are very similar (Fig. 3, A and B). Both transcripts are not detected at early stages of growth (V1–V3) before the floral transition in whole seedlings, immature leaves, and shoot apices. High transcript accumulation is detected after the floral transition in shoot apices and tassel primordia but declines in the mature tassel around the time of meiosis (Fig. 3B). The dynamics of their expression is similar during ear development (Fig. 3B). ZMM4 is detected first in lateral branch meristems at stage V8 followed by ZMM15 at stage V9. Transcript accumulation increases in 1 mm ears and then declines in ears at the time silks exsert (silking). Transcript for both genes is also detected in adult vegetative organs such as husk and stalk at moderate levels and in mature leaf and root at low levels. However, they are not detected in the embryo or endosperm. This pattern of expression indicates that ZMM4 and ZMM15 are developmentally coregulated genes that are not expressed in embryonic and juvenile tissues but primarily accumulate after the transition from vegetative to reproductive growth in developing apical and lateral inflorescences, and to a lesser extent in several other adult tissues.
Figure 3.
Transcript abundance of the four MADS-box genes in different maize tissues. A, Transcript abundance in various tissues. B, Transcript abundance in vegetative and reproductive stages of the shoot apex and lateral shoot. Abundance was measured by MPSS and is reported in parts per million. Bars for ZMM4 are red, for ZMM15 are green, for ZMM31 are orange, and for ZMM24 are blue. Imm, Immature; SAM, vegetative SAM; SAM trans, reproductive SAM; LBM, lateral branch meristem; earT, ear tip (2–3 mm section); earB, ear base (2–3 mm section). Lengths listed refer to the total length of the structure at the stage sampled.
In contrast to expression of ZMM4 and ZMM15, the expression of the neighboring ZMM24 and ZMM31 genes is more restricted. ZMM31 displays the most distinctive developmental pattern. This gene is expressed only during a short period of time at early stages of tassel and ear development. ZMM31 transcripts are detected in the postfloral transition SAM, tassel primordia (approximately 5 mm), and in 5 to 10 mm developing ears (Fig. 3, A and B), but are not detected at later stages. This pattern of expression suggests ZMM31 plays a role in early tassel and ear development during this restricted time frame. Transcripts of ZMM24 are also detected in early stages of tassel and ear development but not in posttransitional apices. ZMM24 transcript is most abundant throughout ear development, with high levels at early stages (2 mm) and remaining relatively high until silking (Fig. 3B). This pattern suggests a putative function throughout female inflorescence development.
Expression of ZMM4 Initiates in Shoot Apices Near the Time of the Floral Transition and Increases in Developing Inflorescences
To determine more precisely the timing and localization of ZMM4 and ZMM15 expression, in situ hybridizations were performed. ZMM4 and ZMM15 digoxygenin-labeled probes were generated from 3′ UTR fragments specific for each gene. Hybridization experiments were performed on vegetative, transitional, and early reproductive shoot apices (V4–V6) and developing ears (V8–V10) in which many meristem types, namely, inflorescence, branch, spikelet pair, and spikelet meristems were present (McSteen et al., 2000).
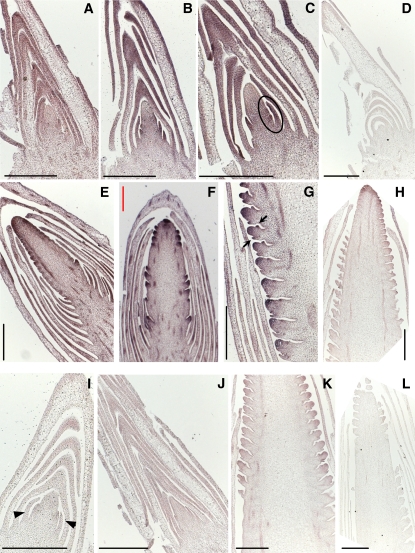
In vegetative stage shoot apices, ZMM4 expression was detected in immature leaf tissue surrounding the SAM but seemingly not in the SAM itself (Fig. 4A). Weak but reproducible signal was detected in the most recently initiated leaf primordium (plastochron 1) nearest the SAM and the other leaf primordia within the sample. The signal persisted and appeared to increase in the youngest leaves (plastochrons 1 and 2) of the shoot apex at transitional and early reproductive stages (Fig. 4, B and C). As the apical meristem initiated BMs, ZMM4 expression was more faithfully detected as a moderate hybridization signal throughout the base of the inflorescence meristem and in the newly arisen BMs (Fig. 4C). At later reproductive stages, ZMM4 signal was apparent in the inflorescence, spikelet pair, and spikelet meristems of the developing ear (Fig. 4, E to G). Signal was also detected in the vascular bundles of the axillary branch (shank), cob, and husk leaves (Fig. 4, E and F). Closer examination of developing ears showed signal within the spikelet meristem and inner and outer glume primordia (Fig. 4G). The pattern of ZMM15 expression in general was similar to ZMM4 but the hybridization signal was less intense, suggesting overall lower expression (Fig. 4, I–K). We could not reproducibly detect ZMM15 expression in the earliest vegetative shoot apices sampled but would sometimes detect weak signal in plastochron 1 leaves (Fig. 4I). Very weak signal was detected in later transitional apices within leaf primordia (Fig. 4J). The pattern of ZMM15 expression within developing ears was similar to ZMM4 but much less intense (Fig. 4K).
Figure 4.
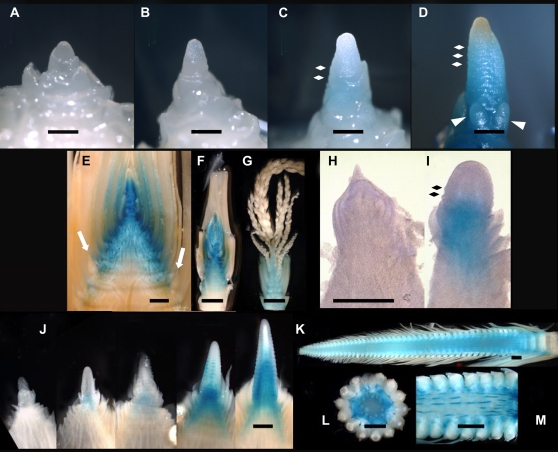
Transcript localization of ZMM4 and ZMM15. A to C and E to G, In situ hybridization with a ZMM4-specific 3′ UTR antisense probe. I to K, In situ hybridization with a ZMM15-specific 3′ UTR antisense probe. D, H, and L, Representative sense control hybridizations of ZMM4 (D and H) and ZMM15 (L). Hybridizations were to longitudinal sections of V3/4 vegetative shoot apex (A), V5 late transitional shoot apex (B), V5/6 early reproductive shoot apex (C), V5 shoot apex (D), V8 primary ear (E), V9 primary ear (F), V10 primary ear (G, H, K, and L), V5 vegetative shoot apex (I), and V4/5 transitional shoot apex (J). In all images, the black scale bar is 500 μm and the red scale bar is 400 μm. The initiating BMs are marked by the black oval in C, the inner and outer glume primordia are marked by the black arrows in G, and ZMM15 expression in the youngest leaf primordium is marked by the black arrowheads in I.
To extend our expression analyses of ZMM4 and ZMM15 during successive stages of growth, we made ProZMM4:GUS and ProZMM15:GUS reporter constructs. The reporter constructs were transformed into maize and outcrossed T1 generation TG plants were analyzed. For stages near the time of the floral transition (starting at V3), we collected and stained shoot apices in the morning and late afternoon every day until inflorescence development had noticeably commenced (Fig. 1A). From that point onward, we collected and stained shoot apices at every subsequent V stage. For ProZMM4:GUS plants, up to stage V4, GUS staining was not detected in vegetative SAMs, where its height varied between 120 to 130 microns (Fig. 5A). In late V4 and early V5 plants, the SAM began to elongate and GUS staining often became detectable, depending on the height of the elongated SAM. We considered plants with elongated SAMs to be transitioning. SAMs less than 180 microns in height did not reliably stain for GUS (Fig. 5B). In SAMs between 180 to 290 microns, GUS staining was either not detected or was extremely faint (Fig. 5C). As the SAM elongated beyond 290 microns, strong GUS staining was faithfully detected (Fig. 5D). Usually, less elongated meristems (290–300 microns) stained faintly, while meristems larger than 340 microns stained more intensely. Staining was characteristically detected in more basal regions of the meristem and subtending stem tissue and was absent from the very tip of the meristem. At the stage faint GUS staining was first detected, the first few suppressed bract primordia often became evident. We interpret this type of meristem to be exiting the period of the floral transition and adopting an inflorescence identity. After this stage, the SAM rapidly elongated beyond 650 microns and BMs initiated at its base, clearly beginning early inflorescence development (Fig. 5D). SAMs with this morphology stained intensely for GUS, although GUS staining was notably absent from the inflorescence meristem itself. For similarly staged SAMs, our GUS staining results corroborated the in situ hybridization patterns (Fig. 4). At V6, GUS staining persisted in all parts of the developing apical inflorescence except the inflorescence meristem (Fig. 5E). Staining was also seen in the abaxial sides of the three to four most tassel-adjacent leaves, the uppermost three to four internodes, and developing vascular system, patterns that are consistent with our in situ results. At this time, GUS staining was noticeably absent in the lateral shoots, which will give rise to the ear primordia (Fig. 5E, white arrows). At V7, GUS staining remained intense in upper internodes, the base of leaves attached to those internodes and most of the developing tassel (Fig. 5F). GUS staining did not persist in more mature tassels (Fig. 5G). Activation of ZMM4 expression at a very early reproductive stage was also demonstrated in the lateral shoot. At V8, the uppermost lateral inflorescence, which would ultimately become the grain-bearing ear, had visible suppressed bract primordia and its base stained intensely (Fig. 5I, black diamonds). Similar to the apical inflorescence at this early stage, the tip of the lateral inflorescence did not stain. Conversely, the second uppermost lateral shoot from the same plant was still vegetative and did not stain (Fig. 5H). The pattern of GUS staining persisted throughout all stages of early ear development as illustrated by the staining of the immature ears from the upper five lateral positions from the same plant (Fig. 5J). Consistently, GUS staining was more apparent at the middle and base of the inflorescence than the tip. This is in contrast to the in situ hybridizations where signal was detected in the inflorescence meristem (Fig. 4E). Finally, later V stages still showed GUS staining throughout the inner cob tissues, especially evident in the inner and outer vascular bundles and the vascular connections to the pistillate florets (Fig. 5, K–M). No GUS staining was detected in the mature florets (ovaries; Fig. 5L). The pattern of GUS staining for the ProZMM15:GUS construct was similar to the ProZMM4:GUS construct, but significantly weaker (Supplemental Fig. S3) with important exceptions during lateral inflorescence development. GUS staining was only detected in the lateral shoot at the V11 stage, after the ear had formed at least 10 spikelet pair meristems (Supplemental Fig. S3G). This indicates that ZMM15 is expressed in the lateral shoot well after inflorescence development initiated and thus is different from ZMM4.
Figure 5.
GUS staining of ProZMM4:GUS TG plant inflorescences at growth stages V4 to V15. A, V4 vegetative SAM. B, V5 transitional elongating SAM. C, V5 late transitional SAM (white diamonds mark the position of suppressed bract primordia). D, Late V5 early inflorescence (white diamonds mark bract primordia and white triangles mark initiating BMs); E, V6 tassel primordium (white arrows mark the lateral shoots). F, V8 developing tassel. G, V10 tassel. H, V8 penultimate lateral shoot. I, V8 ultimate lateral shoot (black diamonds mark bract primordia). J, V10 developing ear primordia from the fifth lateral position (left) to the uppermost lateral position (right). K, Longitudinal section of V15 ear. L, Transverse section of V15 ear. M, Close-up of basal part of ear in K. Scale bars are 200 microns in A to D, 1 mm in E, 5 mm in F and G, 500 microns in H and I, and 1 mm in J to M.
The Expression of All Four ZMM Genes Is Delayed in Late Flowering Mutants
Analysis of transcript accumulation and Pro:GUS staining indicated that expression of ZMM4 and ZMM15 are associated with the floral transition and inflorescence development. To extend this analysis, we determined transcript accumulation of the four MADS-box genes in genotypes with strong differences in flowering time. We chose three genotypes: the temperate inbred line B73 producing 13 to 15 leaves, the moderately late flowering mutant dlf1-N2461A producing 20 to 22 leaves, and the extremely late flowering mutant id1-m1 producing 28 to 30 leaves. Shoot apices were collected from these genotypes at 3 to 4 d intervals until they developed into immature tassels (similar to Fig. 5F). The total abundance of mRNA was measured and quantified using the GenomeLab GeXP multiplex reverse transcription (RT)-PCR system (Beckman Coulter) at Althea Technologies (San Diego). Quantitative levels of amplified PCR products were normalized to the internal control α-tubulin and expressed as a ratio of gene to tubulin.
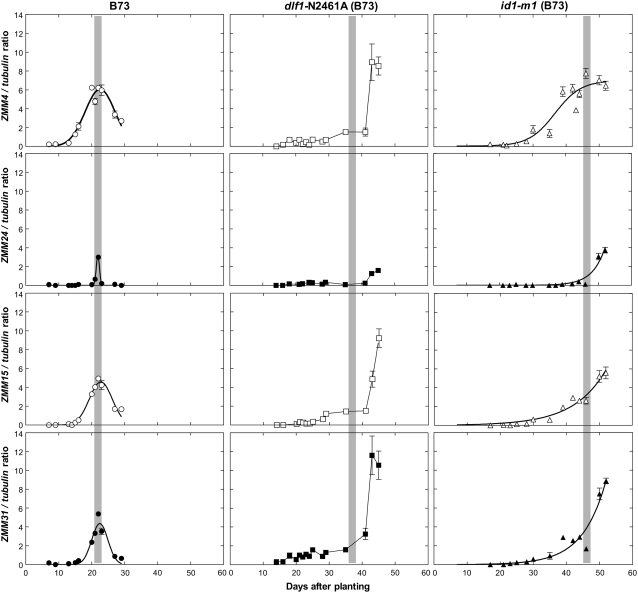
The pattern of transcript accumulation in B73 shoot apices is similar for all four MADS-box genes but the level of accumulation differs (Fig. 6). Transcript is undetectable at early growth stages, becomes detectable, increases rapidly, peaks near the time of BM initiation, and then declines as inflorescence development progresses. Transcript accumulation for ZMM24 varies from the other three genes in that transcript accumulation is not detected until BMs initiate, when it peaks and afterward diminishes rapidly. Relative transcript accumulation from the most to least abundant is: ZMM4, ZMM15, ZMM31, and ZMM24, which confirms our MPSS results. This basic pattern of transcript accumulation is altered in both late flowering mutants with all four genes responding similarly within each mutant. In the moderately late dlf1-N2461A mutant, transcript is detected and remains relatively low during the extended vegetative growth stage. Transcript accumulation increases moderately prior to BM initiation and then increases dramatically afterward. The increase in accumulation is steepest for ZMM4, ZMM15, and ZMM31. We did not detect a decline in transcript accumulation at the last stages sampled in dlf1-N2461A. In the extremely late flowering id1-m1 mutant, a similar trend is apparent. Transcripts for all four MADS-box genes are detected and accumulate very late during the greatly extended vegetative growth stage. The increase in accumulation is gradual, consistent with the very late and gradual transition of this mutant, and continues after BM initiation. Similar to dlf1-N2461A, in the id1-m1 mutant we did not find a decline in transcript accumulation in the latest samples tested, although transcript accumulation for ZMM4 appears to plateau (Fig. 6).
Figure 6.
Quantitative expression levels of ZMM4, ZMM24, ZMM15, and ZMM31 in shoot apices before, during, and after the floral transition in three genotypes. Quantitative expression of the four MADS-box genes is shown as a ratio in arbitrary units (y axis) at different days after planting (x axis) for each graph. Ratios are the average of three technical reps and the error bars are sds. Expression in normal flowering B73 is indicated by circles, in dlf1-N2461A mild late flowering by squares, and in id1-m1 very late flowering by triangles. ZMM4 and ZMM15 are unfilled, whereas ZMM24 and ZMM31 are filled black. All graphs are at the same scale for ease of comparison across genes and genotypes. The time of the first appearance of BM initiation, marking the onset of reproductive growth, is denoted by the gray vertical bar for each genotype.
Overexpression of ZMM4 Induces Early Flowering in TG Maize Plants
To test if ZMM4 or ZMM15 possess floral induction activity, we generated TG maize plants overexpressing each cDNA driven by the maize ubiquitin promoter (ProUBI; McElroya and Brettell, 1994). We first tested expression of ProUBI:ZMM4 and ProUBI:ZMM15 constructs in TG seedlings grown in the greenhouse. Green leaves, unexpanded immature leaves above the shoot apex, and shoot apices were sampled at 3 d intervals for both TG and nontransgenic (NTG) sibling plants of each construct. RT-PCR detected similar levels of transgene expression in all tissues for both constructs in the TG plants (Supplemental Fig. S4). This is consistent with the expected spatial expression pattern of the ubiquitin promoter. During seedling dissection we found that the ProUBI:ZMM4 TG plants transitioned to reproductive development earlier than the NTG plants of both constructs and TG ProUBI:ZMM15 plants (Supplemental Fig. S4). To determine a more precise effect on the floral transition, flowering was measured in seven independent events per construct of the T1 generation of ProUBI:ZMM4 and ProUBI:ZMM15 TG plants grown in the summer nursery. Three measures of flowering were collected for TG and NTG siblings: days to first pollen shed, days to first silk emergence, and total leaf number. Days to pollen shed and silk emergence were converted to the number of growing degree units (GDUs), which are standard units to measure corn maturity across different environments. A GDU is a more accurate measure of development, as it integrates both time (days) and the high and low temperature of each day that influences the growth rate of maize. Statistical analysis of the flowering data indicated that the ProUBI:ZMM4 TG plants flowered earlier than their NTG siblings (Table I). Although there was variation between the seven ProUBI:ZMM4 events used in the study, all events displayed earlier flowering of the TG plants. The ProUBI:ZMM4 TG plants flowered after an average of 1,337 GDU while their NTG siblings flowered after an average of 1,471 GDU. This was an average difference of about 5 d earlier flowering. The difference in GDU to first emerged silks (133 GDU) was nearly the same as the difference to first pollen shed (134 GDU), indicating that the anthesis-silking interval was not affected by the transgene. The ProUBI:ZMM4 TG plants produced on average two fewer leaves than their NTG siblings, confirming that the floral transition occurred earlier in TG plants. Unlike ProUBI:ZMM4, the ProUBI:ZMM15 TG plants showed no statistically significant variation for either flowering time or leaf number (Table I). We did not detect any obvious phenotypic abnormalities in ProUBI:ZMM4 or ProUBI:ZMM15 TG plants. Thus, the ZMM4 transgene specifically affects the timing of the floral transition and does not affect other aspects of floral development.
Table I.
Flowering phenotypes of TG maize plants
Seven independent events were analyzed for each construct. Phenotypic data were collected for 20 to 30 individual TG and NTG siblings per event. Mean values and sds were calculated by linear regression using SAS and the GLM procedure.
| TG Lines | GDU to Shed | GDU to Silk | Leaf No. |
|---|---|---|---|
| NTG siblings (ZMM4) | 1,471 ± 73 | 1,466 ± 71 | 17.9 ± 1.5 |
| TG plants ProUBI:ZMM4 | 1,337 ± 69 | 1,333 ± 60 | 15.7 ± 1.0 |
| NTG siblings (ZMM15) | 1,531 ± 49 | 1,534 ± 43 | 18.3 ± 1.0 |
| TG plants ProUBI:ZMM15 | 1,544 ± 25 | 1,538 ± 38 | 18.7 ± 1.1 |
Overexpression of ZMM4 Represses Two Late Flowering Mutants
Because the ProUBI:ZMM4 transgene induced an early floral transition in a wild-type background, we wondered if this effect would persist in mutant genotypes with a delayed transition. To determine the effect of ZMM4 overexpression on the floral transition in late flowering mutants, the ProUBI:ZMM4 transgene was crossed to both dlf1-mu453 and id1-m1 mutants in the same genetic background. Plants heterozygous for the late flowering allele and hemizygous for the transgene were self pollinated. Segregating F2 families were grown in the summer nursery, genotyped using PCR, and scored for total leaf number (Supplemental Table S1). For crosses with dlf1-mu453, the NTG dlf1-mu453 class produced an average of 23.9 leaves compared to the TG dlf1-mu453 class, which produced an average of 17.6 leaves (Table II). This result indicates the ProUBI:ZMM4 transgene can suppress the late flowering dlf1-mu453 phenotype. In fact, the TG dlf1-mu453 mutants produced the same number of leaves as the NTG wild-type class (17.7), suggesting that overexpression of ZMM4 can suppress the later floral transition of dlf1 mutants to wild-type timing (Supplemental Fig. S6). The TG wild-type class displayed significantly earlier flowering than the other three classes, demonstrating the transgene mediated an earlier transition in the wild-type segregants of this F2 (Table II). Even more striking was the effect of the ProUBI:ZMM4 transgene on the id1-m1 mutant phenotype. In the id1-m1 F2, the NTG id1-m1 class produced an average of 36.3 leaves compared to the TG id1-m1 class that produced an average of 19.0 leaves, nearly the same as the NTG wild-type class (Table III). This result indicates the ProUBI:ZMM4 transgene can also suppress the extremely late floral transition of the id1-m1 mutation to near wild type. Similar to results with dlf1, the TG wild-type class was early flowering, producing an average of 13.4 leaves (Supplemental Fig. S7). Taken together, these data indicate that the early floral transition promoted by the overexpression of ZMM4 is able to suppress the late floral transition of both dlf1 and id1 mutants to wild-type or near-wild-type timing.
Table II.
Flowering phenotypes of the four genotypic classes segregating in the F2 of ProUBI:ZMM4/-, Dlf1/dlf1-mu453
| Transgene Genotype | Flowering Locus Genotype | Mean Leaf No. |
|---|---|---|
| N | ±sd | |
| TGa | Dlf1/- (127)b | 14.0 (±1.90) Ac |
| NTG | Dlf1/- (51) | 17.7 (±1.00) B |
| TG | dlf1-mu453/dlf1-mu453 (50) | 17.6 (±2.47) B |
| NTG | dlf1-mu453/dlf1-mu453 (23) | 23.9 (±1.36) C |
Includes plants homozygous and hemizygous for the transgene.
Includes plants homozygous and heterozygous for the wild-type allele.
Means followed by the same letter are not significantly different by Tukey's multiple comparison test (P < 0.05).
Table III.
Flowering phenotypes of the four genotypic classes segregating in the F2 of ProUBI:ZMM4/-, Id1/id1-m1
| Transgene Genotype | Flowering Locus Genotype | Mean Leaf No. |
|---|---|---|
| ±sd | ||
| TGa | Id1/- (75)b | 13.4 (±1.50) Ac |
| NTG | Id1/- (14) | 18.0 (±1.04) B |
| TG | id1-m1/id1-m1 (10) | 19.0 (±1.89) B |
| NTG | id1-m1/id1-m1 (6) | 36.3 (±1.75) C |
Includes plants homozygous and hemizygous for the transgene.
Includes plants homozygous and heterozygous for the wild-type allele.
Means followed by the same letter are not significantly different by Tukey's multiple comparison test (P < 0.05).
DISCUSSION
Duplicated MADS-Box Gene Pair Loci Are Colinear with Loci Regulating the Floral Transition in Temperate Grasses
Open-ended transcript profiling of shoot apices before and after the floral transition identified two paralogous MADS-box genes, ZMM4 and ZMM15, which were significantly up-regulated after the floral transition. Sequencing of their genomic regions identified two other MADS-box genes, ZMM24 and ZMM31, linked upstream, defining duplicate MADS-box gene pair loci in the maize genome (Fig. 2, C and D). The ZMM4-ZMM24 and ZMM15-ZMM31 gene pairs mapped to chromosome 1.10 and 5.01, respectively, which are segmental duplications of the maize genome (Gaut, 2001). Like other chromosome duplications, ZMM4-ZMM24 and ZMM15-ZMM31 appear to originate from an ancient maize tetraploid event (Gaut and Doebley, 1997; Lai et al., 2004; Swigonova et al., 2004). This event is thought to have occurred before 4.8 Mya (Swigonova et al., 2004). Duplicated regions of the maize genome are often unstable and can undergo complete or partial deletions (Ilic et al., 2003). Due to this process, the modern maize genome has lost more than half of its duplicated genes (Messing et al., 2004; Messing and Dooner, 2006). The presence of both ZMM genes at the duplicate loci suggests that each gene may have a function that was maintained by selection (Walsh, 2003). The evolutionary trajectory of such gene duplications may lead to neofunctionalization, subfunctionalization, or redundant gene function (Preston and Kellogg, 2006). With no null mutations to unambiguously define functions for any of these four ZMM genes, it is difficult to conclusively determine their evolutionary fate.
The gene order within each locus is colinear with the wheat VRN1 locus and corresponding regions of rice and sorghum (Sorghum bicolor) that are composed of two MADS-box genes in the same order (Yan et al., 2003). In temperate cereals, the major flowering activators responding to vernalization are the MADS-box VRN1/FUL-like genes whose transcription is activated upon cold treatment in the SAM and leaves (Danyluk et al., 2003; Trevaskis et al., 2003; Yan et al., 2003; von Zitzewitz et al., 2005; Preston and Kellogg, 2008). The VRN1 MADS activators are negatively regulated by the VRN2 CONSTANS-like repressors (Yan et al., 2004; Trevaskis et al., 2006). The repressive VRN2 gene is deleted in spring and facultative flowering barley varieties creating a facultative growth habit (vernalization unresponsive and cold tolerant; von Zitzewitz et al., 2005). However, maize has a tropical origin and does not require vernalization for flowering but instead relies on as yet unknown endogenous signals to trigger the floral transition. Like the spring varieties of barley, maize appears to not have the VRN2 repressor function but retains the VRN1 activator function whose transcription is tied to the floral transition. Synteny between the maize ZMM loci and the wheat and barley VRN1 loci implicates ZMM4 and ZMM15 as potentially having similar functions.
Expression Patterns of Duplicated Genes Suggest Multiple Functions
The temporal and spatial expression patterns of ZMM4 and ZMM15 suggest they function near the time of the floral transition and during inflorescence development. These patterns were determined and confirmed by several different methods: MPSS profiling, in situ hybridizations, quantitative RT-PCR, and Promoter:GUS analysis. The similarity in expression of ZMM4 and ZMM15 in multiple tissues through several stages of development indicates that both genes are coregulated and thus may be under the control of identical or overlapping determinants. ZMM4 and ZMM15 are not expressed in seedlings at early vegetative stages but ZMM4 is dramatically up-regulated in apices near the time of the floral transition (Figs. 4, A–C, 5, A–D, and 6) while ZMM15 is up-regulated later and to a lesser magnitude (Figs. 4, I and J, and 6; Supplemental Fig. S3, A and B). Expression of ZMM4 in leaf primordia preceding and during the transition is consistent with a potential role as a floral inducer. Expression of ZMM4 within the initiating branch (spikelet pair) meristems of the newly formed apical inflorescence and in multiple meristem types within the developing lateral inflorescence suggests additional roles during inflorescence development, perhaps in specification of meristem and/or organ identity (Fig. 4, C and E–G). ZMM4 expression was also notable in both the in situ hybridizations and GUS staining in the vascular bundles of the stem bearing the apical and lateral inflorescence, the husk leaves, and cob tissue (Figs. 4, E–G, and 5, E and M). Such a pattern hints at additional roles in vascular development. ZMM15 is expressed to a lesser extent and a later stage than ZMM4 in both the apical and lateral shoot. Thus, ZMM15 may play a partially overlapping or redundant role with ZMM4. The expression of ZMM4 is reminiscent of the expression pattern of the wheat, barley, and oat flowering time VRN1/FUL-like genes, which are repressed in seedling meristems and leaves before the floral transition but are up-regulated in shoot apices and developing inflorescences after vernalization (Danyluk et al., 2003; Trevaskis et al., 2003; Yan et al., 2003, 2004; Preston and Kellogg, 2008). The expression patterns of the VRN1/FUL-like genes together with ZMM4 and ZMM15 are most similar to the Arabidopsis FUL gene that is expressed in inflorescence meristems and leaves whereas the functionally redundant genes AP1 and CAL are preferentially expressed in inflorescence and floral meristems (Mandel et al., 1992; Kempin et al., 1995; Mandel and Yanofsky, 1995; Ferrandiz et al., 2000).
The ZMM Genes Function Late within the id1-dlf1 Pathway
The expression of four ZMM genes is delayed in the late flowering mutants id1 and dlf1 (Fig. 6). This implies that all four MADS-box genes are within the id1-dlf1 pathway and are positioned downstream of the floral inducer dlf1. In particular, dlf1 spatial expression overlaps closely with ZMM4 and ZMM15 expression in shoot apices but dlf1 expression precedes activation of ZMM4 and ZMM15. This pattern of expression supports the notion that ZMM4 and ZMM15 are possible targets of dlf1. Accordingly, we find canonical and noncanonical bZIP DNA-binding motifs in the promoter regions of ZMM4 and ZMM15 (Supplemental Fig. S5). Although activation of the ZMM genes in id1 and dlf1 mutants is late, it does occur, suggesting their activity is also controlled by an alternative pathway functioning in parallel to the id1-dlf1 pathway. The convergence of multiple pathways on a few key integrators is a hallmark of the flowering network in Arabidopsis. The meristem identity genes CAL, FUL, and AP1 work redundantly to integrate signals conveyed by different flowering pathways to trigger floral development (Ferrandiz et al., 2000). Despite their redundant function, AP1, CAL, and FUL are regulated by overlapping but distinct sets of transcriptional regulators. AP1 is a target of LFY (Parcy et al., 1998) and FD (Abe et al., 2005; Wigge et al., 2005). CAL is a direct target of LFY and the class I HD-Zip transcription factor LATE MERISTEM IDENTITY1 (Saddic et al., 2006). FUL is activated by FD but not LFY in leaves (Teper-Bamnolker and Samach, 2005). Candidates for alternative regulators of the ZMM genes may be zfl1 and zfl2; duplicate genes that have activities orthologous to Arabidopsis LFY (Bomblies et al., 2003). In support of this idea, we also found a canonical LFY-binding site in the ZMM15 promoter. Whether the LFY-binding site or other DNA-binding motifs found upstream of ZMM4 and ZMM15 are functional, will require additional experimentation.
Overexpression of ZMM4 Promotes the Floral Transition in Wild-Type and Late Flowering Mutant Genotypes
Since we were unable to obtain validated null alleles for any of the ZMM genes, we elected to overexpress ZMM4 and ZMM15 in TG maize to help clarify their role in flowering. Only ZMM4 mediated early flowering with a direct effect on the floral transition (Table I). To our knowledge, this is the first report of transgene-mediated early flowering in maize with no pleiotropic floral defects. Overexpression of AP1, CAL, or FUL leads to early flowering in Arabidopsis. AP1 is known to feedback regulate a number of its regulators, such as LFY, TERMINAL FLOWER, and AGAMOUS-LIKE24 (Liu et al., 2007). How ZMM4 mediates early flowering is not clear. Perhaps, it functions similar to AP1 by feedback regulating one or more of its regulators but this idea will require additional molecular characterization of the ZMM4 overexpression transgenics. Although ZMM15 is similar in many respects to ZMM4, its expression is delayed relative to ZMM4, is significantly weaker than ZMM4, and does not induce early flowering when overexpressed. Therefore, ZMM15 may have a function similar to but weaker than ZMM4 or it may have a function different than ZMM4.
The suppression of late flowering in both dlf1 and id1 mutant backgrounds is also significant (Tables II and III). The restoration of a wild-type floral transition for both mutations implies ZMM4 overexpression can substitute for both dlf1 and id1 function. This idea is compatible with the suggested placement and interactions of id1, dlf1, and ZMM4 in the emerging maize floral transition pathway (Muszynski et al., 2006). Within the proposed pathway, ZMM4 is activated by dlf1 that is activated by id1. Therefore, overexpression of ZMM4, the gene most downstream in the pathway, should compensate for any mutation further upstream. Moreover, since TG wild-type plants flowered earlier (13–14 leaves) than TG mutant plants (18–19 leaves), it appears the magnitude of earliness mediated by ZMM4 overexpression requires either id1 or dlf1 activity. This observation supports the hypothesis that TG ZMM4-mediated early flowering may function through feedback regulation of a floral inducer, perhaps dlf1. This can be easily tested and studies are under way to validate this hypothesis.
In conclusion, we have identified the MADS-box gene ZMM4 as being significantly up-regulated in maize shoot apices after the floral transition. ZMM4 is initially expressed in leaf primordia of vegetative apices and its expression persists and increases throughout the floral transition. ZMM4 is also expressed in multiple meristem types during reproductive development in both apical and lateral inflorescences. In addition, ZMM4 functions downstream of dlf1 in the id1-dlf1 flowering pathway and overexpression of ZMM4 has floral inductive activity in both wild-type and late flowering id1 and dlf1 mutant genotypes. Taken collectively, these data suggest that ZMM4 may possess AP1/CAL/FUL activity and might play a role in the maize floral transition similar to the VRN1/FUL-like genes in temperate cereals. It also may have additional roles in some aspect of inflorescence development. Future molecular studies on the ZMM4 overexpression transgenics and characterization of the phenotypic effects of loss of ZMM4 function will help clarify its role in flowering and inflorescence development.
MATERIALS AND METHODS
Plant Material and Phenotype Data Collection
The public inbred line B73 was used for tissue collection and RNA isolation. Plants were grown in the greenhouse at 25°C under 16-h long days. Vegetative growth stages (V1–V9) were defined according to the appearance of the leaf collar of the uppermost leaf (Ritchie et al., 1997). TG flowering time notes were collected in the field. Measurement of total leaf number was done by marking the fifth leaf and the 10th leaf when they first fully expanded. The time of first pollen shed and first silk emerged were recorded in the field and converted to GDU according to the formula GDU = (H + L)/2 − B, where H and L are the daily high and low temperatures and B = 50°F (Ritchie et al., 1997).
Tissue Sampling and Data Analysis of MPSS RNA Profiling
Shoot apices with one to two leaf primordia attached were dissected from seedlings at V4 (vegetative stage, 15 d after planting) and V6 (reproductive stage, 26 d after planting) grown in the greenhouse. Approximately 500 seedlings were dissected from each developmental stage. A total of 1.1 and 0.9 μg of polyA RNA were isolated from V4 and V6 samples, respectively. Samples were submitted to Solexa, Inc., for RNA profiling using MPSS. The data from the two experiments and a previously defined function relating expression and variance for MPSS data (defined from replicate samples; Stolovitzky et al., 2005) were used to calculate t-statistic values for each tag. Because the two samples were very similar in overall gene expression and the true variance in the experiments was not known, a ranking test was used to identify genes that were likely to be differentially expressed. Using the absolute value of t statistics, the 100 tags with the largest t statistics were chosen for further analysis. Although this number was arbitrary, it captured all of the most extreme differences (Fig. 1B).
RNA Isolation and RT-PCR
Six shoot apices per a sample were homogenized in 300 μL of TRIzol Reagent (Roche Diagnostics Corporation) using a 1.5-μL pestle (VWR KT479521-1590). Immature and mature leaves were ground with a mortar and pestle in liquid nitrogen. Fifty milligrams of ground tissue was treated with 300 μL of TRIzol. Total RNA was isolated with TRIzol Reagent in combination with Phase Lock gel (Brinkmann Instruments Inc.) according to the manufacturer's instructions. cDNA synthesis was performed with Superscript first-strand synthesis system (Invitrogen). RT-PCR amplification was performed using Expand Long Template DNA polymerase (Roche) with the following primers: ZMM4 forward, 5′-AGCAAGTGCAACGGGACCAAACTCA-3′; ZMM15 forward, 5′-AGAAGCAGAAAGCCCAGCGGAAGCAA-3′; and PINII reverse, 5′-CACATAACACACAACTTTGATGCCCAC-3′ (for transgene detection).
Two microliters of the cDNA reaction was used for PCR amplification in a 50-μL volume. The PCR conditions were 95°C for 2 min, followed by 35 cycles at 94°C for 45 s, 58°C for 45 s, 72°C for 1 min, and a final extension of 72°C for 10 min.
Multiplexed Quantitative RT-PCR by Althea Technologies
Multiplex gene expression analysis was carried out using the GenomeLab GeXP analysis system (Beckman Coulter) as described previously (Chen et al., 2007). The gene-specific sequences of the chimeric primers were as follows: ZMM4 forward 5′-GGCGAAGGTCGAGACAATAC-3′ and reverse 5′-GCTGGCTCTTCCTTGTTCTG-3′, ZMM15 forward 5′-GCTAGCTGTGACGTTATGCT-3′ and reverse 5′-CCTCTCCGCTAGGACAACCT-3′, ZMM24 forward 5′-GCTCCTGTGTGCAGTGTGTC-3′ and reverse 5′-GCGCTATCAGCGACCTCTT-3′, ZMM31 forward 5′-AGCATATAGCCCAGCACCAC-3′ and reverse 5′-CCCTGGGTTTTTTCGAGCT-3′, and α-tubulin forward 5′-GTTCAATGCTGTTGGTGGTG-3′ and reverse 5′-GTCCAGGAGGACTGCAACAT-3′. The resultant raw data were normalized against α-tubulin as the internal control within the same reaction.
In Situ Hybridization
In situ hybridization was performed according the protocol of Jackson (1991) with a few modifications according to Bradley et al. (1993). The templates for RNA synthesis were generated by PCR using primers for ZMM4 forward 5′-AGCATCTTCCCTGTGGCA and reverse 5′-CGTAAAGTACCGTGCGG; and ZMM15 forward 5′-ATAAGGGAAGCTGCCCCAA and reverse 5′-GATCAAGAACGTCTTATGGTC. Sense and antisense riboprobes were generated using the T7 and SP6 Megascript in vitro transcription kits (Ambion) with digoxygenin-labeled UTP (Roche) according to the manufacturer's instructions. Photography was performed with a Zeiss Axioplan II compound microscope equipped with an AxioCam color digital camera.
T-DNA Constructs and Plant Transformation
GATEWAYTECHNOLOGY (Invitrogen) was used for vector construction. Full-length cDNA sequences of ZMM4 and ZMM15 were integrated between the ubiquitin promoter and PinII terminators and cointegrated with JT vectors as previously described (Unger et al., 2001; Cigan et al., 2005). Plasmids were introduced into Agrobacterium strain LBA4404 and used to transform Hi-Type II maize (Zea mays) embryos as previously described (Unger et al., 2001; Cigan et al., 2005). Typically 20 independent events were generated for each construct. Events with single-copy T-DNA integrations were used for further characterization and crosses. Segregating populations of T1 generation from the primary transformed lines (T0 generation) were grown in the field. TG plants were identified by leaf painting with herbicide (2% Liberty). Seven independent events were analyzed for each constructs. Phenotypic data were collected for 20 to 30 individual plants per event.
For generation of promoter:GUS reporter constructs, the 1,722- and 1,941-bp genomic DNA fragments upstream of the start codon were amplified by PCR to clone the ZMM4 and ZMM15 promoters, respectively. The ZMM4 promoter was amplified from B73 genomic DNA using PCR primers ZMM4-F 5′-AACGAACCTCTATCAAACAAGC and ZMM4-R 5′-CCTTCTCCCTCTCCTGATCTC, whereas the ZMM15 promoter was amplified from the Mo17 BAC clone using primers ZMM15-F 5′-ATACAACCGGTATCCTCGAA and ZMM15-R 5′-CGAGAGCATAACGTCACAGC. These PCR fragments were flanked by appropriate restriction sites for cloning into the pENTRY multisite Gateway vector (Invitrogen). All the pENTRY vectors were quality checked by DNA sequencing. To generate JT vectors for Promoter:GUS reporters, the LR clonase reaction was performed with pENTRY vectors containing promoter, GUS, and PINII terminator and pDESTINATION vector containing the herbicide resistant selection marker between the right and left border sequences. All the JT vectors were quality checked by restriction digestion mapping and transferred into Agrobacterium tumefaciens LB4404JT by electroporation. The cointegrated DNA from transformed Agrobacterium was transferred in Escherichia coli DH10B and the plasmid DNA from this strain was used to check quality by restriction digestions. TG maize plants were generated as described earlier in this section. GUS staining was performed as described previously (Stangeland and Salehian, 2002).
Statistical Analysis of TG Data
For analysis of ProUBI:ZMM4 and ProUBI:ZMM15 TG plants, mean values and sds were calculated by linear regression using SAS Enterprise Guide 3.0 (SAS Institute Inc.) and the GLM procedure (the linear model ANOVA procedure in SAS). The difference in flowering time was tested by a two-way ANOVA taking the events and the presence or absence of the transgene as the two potential sources of variation. Analysis for the ProUBI:ZMM4 dlf1-mu453 and ProUBI:ZMM4 id1-m1 F2s were done in Minitab 14 (Minitab Inc.) using a one-way ANOVA with response as leaf number and factor as genotype. Tukey's family error rate was chosen for one-way multiple comparisons with a P value level of significance (α-level) equal to 0.05.
BAC Shotgun Sequencing and Assembly
The B73 genomic BAC library was screened with overgo probes specific for ZMM4 (5′-TGGATGCTTAGCCATCTGAGCTGC and 5′-TGAGGGCAAACCTTCAGCAGCTCA) and for ZMM15 (5′-ACCACCATGGATGCTTAGCCACCT and 5′-AACCTTCAGCTGCTCAGGTGGCTA). BAC clones were sequenced using the double-stranded random shotgun approach. Briefly, after the BAC was isolated via a double-acetate cleared lysate protocol and sheared by nebulizing at 18 psi, the resulting fragments were end repaired and subcloned into pBluescript II SK(+). After transformation into DH-10B electrocompetent E. coli cells (Invitrogen), the plasmid DNA was isolated, using the Templiphi DNA sequencing template amplification kit method (GE Healthcare), and quantified with the PicoGreen dsDNA quantitation reagent (Molecular Probes). The amplified products were denatured at 95°C for 10 min and end sequenced using vector-primed M13 oligonucleotides and the ABI BigDye version 3.1 Prism sequencing kit. After ethanol-based cleanup, cycle sequencing reaction products were resolved and detected on Perkin-Elmer ABI 3730xl automated sequencers. Individual sequences from each BAC clone were combined into a single project and assembled with the Phred/Phrap/Consed package (see http://www.phrap.org/phredphrapconsed.html). The resulting assembly was confirmed with Exgap (http://www.genome.ou.edu/informatics.html), a graphic tool that uses read pair information to order contigs and confirm the accuracy of the Phrap-based assembly. A unique super-contig sequence was generated manually, with randomly chosen strings of 40 consecutive Ns to link sequences from adjacent contigs, using information from the Exgap output.
Computer Software and Sequence Analysis
Prior to analysis, noncoding repetitive sequences in the 220,139-bp super-contig sequence were masked with the RepeatMasker program (http://www.genome.washington.edu/UWGC/analysistools/RepeatMasker.cfm), using release 3.0 version of The Institute for Genomic Research maize repeat database (http://www.tigr.org/tdb/tgi/maize/repeat_db.shtml). Potential protein-coding region recognition in the genomic sequences was done using the monocot plant dataset version of the programs FGENESH (Softberry). Gene annotation was performed using BLAST (at default stringency) and BLAT (minimal sequence identity of 80%) analysis against the GenBank and the DuPont maize EST databases, respectively. DNA sequence comparisons using CROSSMATCH (P. Green) were done on local Sun workstation.
MPSS Data
Information regarding the sequence identity of the MPSS tags not reported in this work is proprietary and may be obtained at the discretion of DuPont Crop Genetics.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers AF377947 (rice syntenic region), AY188331 and AY188333 (wheat vernalization 1 locus, chromosome 5), EU012444 (BAC b143c.h19), EU012446 (BAC be120d.f06), EU012445 (BAC b88c.j23), AJ430632 (ZMM15 cDNA), AJ430638 (ZMM24 cDNA), AJ430640 (ZMM31 cDNA), AJ430641 (ZMM4 cDNA), and X63178 (α-tubulin).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Amino acid alignments of the duplicate ZMM MADS-box proteins.
Supplemental Figure S2. Map locations of the ZMM MADS-box pairs.
Supplemental Figure S3. GUS staining of the ProZMM15:GUS TG plant inflorescences at growth stages V4 to V15.
Supplemental Figure S4. Expression analysis of ProUBI:ZMM4 and ProUBI:ZMM15 TG plants.
Supplemental Figure S5. Organization of cis-regulatory motifs in the promoters of ZMM4 and ZMM15.
Supplemental Figure S6. Suppression of the dlf1-mu453 phenotype by ProUBI:ZMM4.
Supplemental Figure S7. Suppression of the id1-m1 phenotype by ProUBI:ZMM4.
Supplemental Table S1. Primers used to genotype the ZMM4 transgene and the dlf1-mu453 and id1-m1 alleles.
Supplementary Material
Acknowledgments
The authors would like to thank Rayeann Archibald and David Shirbroun for sampling shoot apices, Victor Llaca for making the BAC shotgun libraries, Sunita Chilakamari for technical assistance, Lawrence Stiner for help with vector construction, Chris Zinselmeier for graphing the Althea data, Nancy Rizzo for help with in situ hybridizations, and the Iowa State University Microscopy and NanoImaging Facility for help with imaging the in situ hybridizations.
This article is dedicated to the memory of Evgueni Ananiev for his commitment to scientific rigor, tireless curiosity, and inspirational inquisitiveness.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Olga N. Danilevskaya (olga.danilevskaya@pioneer.com).
The online version of this article contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- Abe M, Kobayashi Y, Yamamoto S, Daimon Y, Yamaguchi A, Ikeda Y, Ichinoki H, Notaguchi M, Goto K, Araki T (2005) FD, a bZIP protein mediating signals from the floral pathway integrator FT at the shoot apex. Science 309 1052–1056 [DOI] [PubMed] [Google Scholar]
- Becker A, Theissen G (2003) The major clades of MADS-box genes and their role in the development and evolution of flowering plants. Mol Phylogenet Evol 29 464–489 [DOI] [PubMed] [Google Scholar]
- Becker A, Winter KU, Meyer B, Saedler H, Theissen G (2000) MADS-Box gene diversity in seed plants 300 million years ago. Mol Biol Evol 17 1425–1434 [DOI] [PubMed] [Google Scholar]
- Bernier G, Perilleux C (2005) A physiological overview of the genetics of flowering time control. Plant Biotechnol J 3 3–16 [DOI] [PubMed] [Google Scholar]
- Blazquez MA, Soowal LN, Lee I, Weigel D (1997) LEAFY expression and flower initiation in Arabidopsis. Development 124 3835–3844 [DOI] [PubMed] [Google Scholar]
- Blazquez MA, Weigel D (2000) Integration of floral inductive signals in Arabidopsis. Nature 404 889–892 [DOI] [PubMed] [Google Scholar]
- Bomblies K, Doebley JF (2005) Pleiotropic effects of the duplicate maize FLORICAULA/LEAFY genes zfl1 and zfl2 on traits under selection during maize domestication. Genetics 172 519–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bomblies K, Wang RL, Ambrose BA, Schmidt RJ, Meeley RB, Doebley J (2003) Duplicate FLORICAULA/LEAFY homologs zfl1 and zfl2 control inflorescence architecture and flower patterning in maize. Development 130 2385–2395 [DOI] [PubMed] [Google Scholar]
- Borner R, Kampmann G, Chandler J, Gleissner R, Wisman E, Apel K, Melzer S (2000) A MADS domain gene involved in the transition to flowering in Arabidopsis. Plant J 24 591–599 [DOI] [PubMed] [Google Scholar]
- Boss PK, Bastow RM, Mylne JS, Dean C (2004) Multiple pathways in the decision to flower: enabling, promoting, and resetting. Plant Cell (Suppl) 16 S18–S31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley D, Carpenter R, Sommer H, Hartley N, Coen E (1993) Complementary floral homeotic phenotypes result from opposite orientations of a transposon at the plena locus of Antirrhinum. Cell 72 85–95 [DOI] [PubMed] [Google Scholar]
- Brenner S, Johnson M, Bridgham J, Golda G, Lloyd DH, Johnson D, Luo S, McCurdy S, Foy M, Ewan M et al (2000) Gene expression analysis by massively parallel signature sequencing (MPSS) on microbead arrays. Nat Biotechnol 18 630–634 [DOI] [PubMed] [Google Scholar]
- Bruggmann R, Bharti AK, Gundlach H, Lai J, Young S, Pontaroli AC, Wei F, Haberer G, Fuks G, Du C et al (2006) Uneven chromosome contraction and expansion in the maize genome. Genome Res 16 1241–1251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner S, Fengler K, Morgante M, Tingey S, Rafalski A (2005) Evolution of DNA sequence nonhomologies among maize inbreds. Plant Cell 17 343–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardon GH, Höhmann S, Nettesheim K, Saedler H, Huijser P (1997) Functional analysis of the Arabidopsis thaliana SBP-box gene SPL3: a novel gene involved in the floral transition. Plant J 12 367–377 [DOI] [PubMed] [Google Scholar]
- Chen Q-R, Vansant G, Oades K, Pickering M, Wei JS, Song YK, Monforte J, Khan J (2007) Diagnosis of the small round blue cell tumors using multiplex polymerase chain reaction. J Mol Diagn 9 80–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cigan AM, Unger-Wallace E, Haug-Collet K (2005) Transcriptional gene silencing as a tool for uncovering gene function in maize. Plant J 43 929–940 [DOI] [PubMed] [Google Scholar]
- Colasanti J, Sundaresan V (2000) ‘Florigen’ enters the molecular age: long-distance signals that cause plants to flower. Trends Biochem Sci 25 236–240 [DOI] [PubMed] [Google Scholar]
- Colasanti J, Tremblay R, Wong AY, Coneva V, Kozaki A, Mable BK (2006) The maize INDETERMINATE1 flowering time regulator defines a highly conserved zinc finger protein family in higher plants. BMC Genomics 7 158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colasanti J, Yuan Z, Sundaresan V (1998) The indeterminate gene encodes a zinc finger protein and regulates a leaf-generated signal required for the transition to flowering in maize. Cell 93 593–603 [DOI] [PubMed] [Google Scholar]
- Corbesier L, Vincent C, Jang S, Fornara F, Fan Q, Searle I, Giakountis A, Farrona S, Gissot L, Turnbull C, et al (2007) FT protein movement contributes to long-distance signaling in floral induction of Arabidopsis. Science 316 1030–1033 [DOI] [PubMed] [Google Scholar]
- Danyluk J, Kane NA, Breton G, Limin AE, Fowler DB, Sarhan F (2003) TaVRT-1, a putative transcription factor associated with vegetative to reproductive transition in cereals. Plant Physiol 132 1849–1860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrandiz C, Gu Q, Martienssen R, Yanofsky MF (2000) Redundant regulation of meristem identity and plant architecture by FRUITFULL, APETALA1 and CAULIFLOWER. Development 127 725–734 [DOI] [PubMed] [Google Scholar]
- Gaut BS (2001) Patterns of chromosomal duplication in maize and their implications for comparative maps of the grasses. Genome Res 11 55–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaut BS, Doebley JF (1997) DNA sequence evidence for the segmental allotetraploid origin of maize. Proc Natl Acad Sci USA 94 6809–6814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann U, Hohmann S, Nettesheim K, Wisman E, Saedler H, Huijser P (2000) Molecular cloning of SVP: a negative regulator of the floral transition in Arabidopsis. Plant J 21 351–360 [DOI] [PubMed] [Google Scholar]
- Ilic K, SanMiguel PJ, Bennetzen JL (2003) A complex history of rearrangement in an orthologous region of the maize, sorghum, and rice genomes. Proc Natl Acad Sci USA 100 12265–12270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Immink RG, Hannapel DJ, Ferrario S, Busscher M, Franken J, Lookeren Campagne MM, Angenent GC (1999) A petunia MADS box gene involved in the transition from vegetative to reproductive development. Development 126 5117–5126 [DOI] [PubMed] [Google Scholar]
- Irish EE, Nelson TM (1991) Identification of multiple stages in the conversion of maize meristems from vegetative to floral development. Development 112 891–898 [Google Scholar]
- Izawa T, Takahashi Y, Yano M (2003) Comparative biology comes into bloom: genomic and genetic comparison of flowering pathways in rice and Arabidopsis. Curr Opin Plant Biol 6 113–120 [DOI] [PubMed] [Google Scholar]
- Jackson D (1991) In situ hybridization in plants. In DJ Bowles, SJ Gurr, M McPherson, eds, Molecular Plant Pathology: A Practical Approach. Oxford University Press, Oxford, pp 163–174
- Jang S, An K, Lee S, An G (2002) Characterization of tobacco MADS-box genes involved in floral initiation. Plant Cell Physiol 43 230–238 [DOI] [PubMed] [Google Scholar]
- Kang HG, Jang S, Chung JE, Cho YG, An G (1997) Characterization of two rice MADS box genes that control flowering time. Mol Cells 7 559–566 [PubMed] [Google Scholar]
- Kempin SA, Savidge B, Yanofsky MF (1995) Molecular basis of the cauliflower phenotype in Arabidopsis. Science 267 522–525 [DOI] [PubMed] [Google Scholar]
- Kozaki A, Hake S, Colasanti J (2004) The maize ID1 flowering time regulator is a zinc finger protein with novel DNA binding properties. Nucleic Acids Res 32 1710–1720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai J, Ma J, Swigonova Z, Ramakrishna W, Linton E, Llaca V, Tanyolac B, Park YJ, Jeong OY, Bennetzen JL, et al (2004) Gene loss and movement in the maize genome. Genome Res 14 1924–1931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Suh SS, Park E, Cho E, Ahn JH, Kim SG, Lee JS, Kwon YM, Lee I (2000) The AGAMOUS-LIKE 20 MADS domain protein integrates floral inductive pathways in Arabidopsis. Genes Dev 14 2366–2376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litt A, Irish VF (2003) Duplication and diversification in the APETALA1/FRUITFULL floral homeotic gene lineage: implications for the evolution of floral development. Genetics 165 821–833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Zhou J, Bracha-Drori K, Yalovsky S, Ito T, Yu H (2007) Specification of Arabidopsis floral meristem identity by repression of flowering time genes. Development 134 1901–1910 [DOI] [PubMed] [Google Scholar]
- Loukoianov A, Yan L, Blechl A, Sanchez A, Dubcovsky J (2005) Regulation of VRN-1 vernalization genes in normal and transgenic polyploid wheat. Plant Physiol 138 2364–2373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malcomber ST, Kellogg EA (2005) SEPALLATA gene diversification: brave new whorls. Trends Plant Sci 10 427–435 [DOI] [PubMed] [Google Scholar]
- Malcomber ST, Preston JC, Reinheimer R, Kossuth J, Kellogg EA (2006) Developmental gene evolution and the origin of grass inflorescence diversity. Adv Bot Res 44 426–481 [Google Scholar]
- Mandel MA, Gustafson-Brown C, Savidge B, Yanofsky MF (1992) Molecular characterization of the Arabidopsis floral homeotic gene APETALA1. Nature 360 273–277 [DOI] [PubMed] [Google Scholar]
- Mandel MA, Yanofsky MF (1995) A gene triggering flower formation in Arabidopsis. Nature 377 522–524 [DOI] [PubMed] [Google Scholar]
- McElroya D, Brettell RIS (1994) Foreign gene expression in transgenic cereals. Trends Biotechnol 12 62–68 [Google Scholar]
- McSteen P, Hake S (2001) barren inflorescence2 regulates axillary meristem development in the maize inflorescence. Development 128 2881–2891 [DOI] [PubMed] [Google Scholar]
- McSteen P, Laudencia-Chingcuanco D, Colasanti J (2000) A floret by any other name: control of meristem identity in maize. Trends Plant Sci 5 61–66 [DOI] [PubMed] [Google Scholar]
- Messing J, Bharti AK, Karlowski WM, Gundlach H, Kim HR, Yu Y, Wei F, Fuks G, Soderlund CA, Mayer KF, et al (2004) Sequence composition and genome organization of maize. Proc Natl Acad Sci USA 101 14349–14354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J, Dooner HK (2006) Organization and variability of the maize genome. Curr Opin Plant Biol 9 157–163 [DOI] [PubMed] [Google Scholar]
- Michaels SD, Himelblau E, Kim SY, Schomburg FM, Amasino RM (2005) Integration of flowering signals in winter-annual Arabidopsis. Plant Physiol 137 149–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon J, Suh SS, Lee H, Choi KR, Hong CB, Paek NC, Kim SG, Lee I (2003) The SOC1 MADS-box gene integrates vernalization and gibberellin signals for flowering in Arabidopsis. Plant J 35 613–623 [DOI] [PubMed] [Google Scholar]
- Mouradov A, Cremer F, Coupland G (2002) Control of flowering time: interacting pathways as a basis for diversity. Plant Cell (Suppl) 14 S111–S130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murai K, Miyamae M, Kato H, Takumi S, Ogihara Y (2003) WAP1, a wheat APETALA1 homolog, plays a central role in the phase transition from vegetative to reproductive growth. Plant Cell Physiol 44 1255–1265 [DOI] [PubMed] [Google Scholar]
- Muszynski MG, Dam T, Li B, Shirbroun DM, Hou Z, Bruggemann E, Archibald R, Ananiev EV, Danilevskaya ON (2006) delayed flowering1 encodes a basic leucine zipper protein that mediates floral inductive signals at the shoot apex in maize. Plant Physiol 142 1523–1536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuffer MG, Coe EH, Wessler SR (1997) Mutants of Maize. CSHL Press, Cold Spring Harbor, NY
- Parcy F, Nilsson O, Busch MA, Lee I, Weigel D (1998) A genetic framework for floral patterning. Nature 395 561–566 [DOI] [PubMed] [Google Scholar]
- Parenicova L, de Folter S, Kieffer M, Horner DS, Favalli C, Busscher J, Cook HE, Ingram RM, Kater MM, Davies B, et al (2003) Molecular and phylogenetic analyses of the complete MADS-box transcription factor family in Arabidopsis: new openings to the MADS world. Plant Cell 15 1538–1551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelaz S, Ditta GS, Baumann E, Wisman E, Yanofsky MF (2000) B and C floral organ identity functions require SEPALLATA MADS-box genes. Nature 405 200–203 [DOI] [PubMed] [Google Scholar]
- Petersen K, Didion T, Andersen CH, Nielsen KK (2004) MADS-box genes from perennial ryegrass differentially expressed during transition from vegetative to reproductive growth. J Plant Physiol 161 439–447 [DOI] [PubMed] [Google Scholar]
- Petersen K, Kolmos E, Folling M, Salchert K, Storgaard M, Jensen CS, Didion T, Nielsen KK (2006) Two MADS-box genes from perennial ryegrass are regulated by vernalization and involved in the floral transition. Physiol Plant 126 268–278 [Google Scholar]
- Preston JC, Kellogg EA (2006) Reconstructing the evolutionary history of paralogous APETALA1/FRUITFULL-like genes in grasses (Poaceae). Genetics 174 421–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston JC, Kellogg EA (2007) Conservation and divergence of APETALA1/FRUITFULL-like gene function in grasses: evidence from gene expression analyses. Plant J 52 69–81 [DOI] [PubMed] [Google Scholar]
- Preston JC, Kellogg EA (2008) Discrete developmental roles for temperate cereal grass VRN1/FUL-like genes in flowering competency and the transition to flowering. Plant Physiol 146 265–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razem FA, El-Kereamy A, Abrams SR, Hill RD (2006) The RNA-binding protein FCA is an abscisic acid receptor. Nature 439 290–294 [DOI] [PubMed] [Google Scholar]
- Reinartz J, Bruyns E, Lin JZ, Burcham T, Brenner S, Bowen B, Kramer M, Woychik R (2002) Massively parallel signature sequencing (MPSS) as a tool for in-depth quantitative gene expression profiling in all organisms. Brief Funct Genomics Proteomics 1 95–104 [DOI] [PubMed] [Google Scholar]
- Ritchie SW, Hanway JJ, Benson GO (1997) How a Corn Plant Develops. Special Report No. 48, Vol 48. Iowa State University of Science and Technology Cooperative Extension Service, Ames, IA
- Saddic LA, Huvermann B, Bezhani S, Su Y, Winter CM, Kwon CS, Collum RP, Wagner D (2006) The LEAFY target LMI1 is a meristem identity regulator and acts together with LEAFY to regulate expression of CAULIFLOWER. Development 133 1673–1682 [DOI] [PubMed] [Google Scholar]
- Samach A, Onouchi H, Gold SE, Ditta GS, Schwarz-Sommer Z, Yanofsky MF, Coupland G (2000) Distinct roles of CONSTANS target genes in reproductive development of Arabidopsis. Science 288 1613–1616 [DOI] [PubMed] [Google Scholar]
- Schmid M, Uhlenhaut NH, Godard F, Demar M, Bressan R, Weigel D, Lohmann JU (2003) Dissection of floral induction pathways using global expression analysis. Development 130 6001–6012 [DOI] [PubMed] [Google Scholar]
- Schwarz-Sommer Z, Huijser P, Nacken W, Saedler H, Sommer H (1990) Genetic control of flower development by homeotic genes in Antirrhinum majus. Science 250 931–936 [DOI] [PubMed] [Google Scholar]
- Stangeland B, Salehian Z (2002) An improved clearing method for GUS assay in Arabidopsis endosperm and seeds. Plant Mol Biol Rep 20 107–114 [Google Scholar]
- Stolovitzky GA, Kundaje A, Held GA, Duggar KH, Haudenschild CD, Zhou D, Vasicek TJ, Smith KD, Aderem A, Roach JC (2005) Statistical analysis of MPSS measurements: application to the study of LPS-activated macrophage gene expression. Proc Natl Acad Sci USA 102 1402–1407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swigonova Z, Lai J, Ma J, Ramakrishna W, Llaca V, Bennetzen JL, Messing J (2004) Close split of sorghum and maize genome progenitors. Genome Res 14 1916–1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teper-Bamnolker P, Samach A (2005) The flowering integrator FT regulates SEPALLATA3 and FRUITFULL accumulation in Arabidopsis leaves. Plant Cell 17 2661–2675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevaskis B, Bagnall DJ, Ellis MH, Peacock WJ, Dennis ES (2003) MADS box genes control vernalization-induced flowering in cereals. Proc Natl Acad Sci USA 100 13099–13104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevaskis B, Hemming MN, Peacock WJ, Dennis ES (2006) HvVRN2 responds to daylength, whereas HvVRN1 is regulated by vernalization and developmental status. Plant Physiol 140 1397–1405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger E, Betz S, Xu R, Cigan AM (2001) Selection and orientation of adjacent genes influences DAM-mediated male sterility in transformed maize. Transgenic Res 10 409–422 [DOI] [PubMed] [Google Scholar]
- Vijayraghavan U, Prasad K, Meyerowitz EM (2005) Specification and maintenance of the floral meristem: interactions between positively-acting promoters of flowering and negative regulators. Curr Sci 89 1835–1843 [Google Scholar]
- von Zitzewitz J, Szucs P, Dubcovsky J, Yan L, Francia E, Pecchioni N, Casas A, Chen TH, Hayes PM, Skinner JS (2005) Molecular and structural characterization of barley vernalization genes. Plant Mol Biol 59 449–467 [DOI] [PubMed] [Google Scholar]
- Walsh B (2003) Population-genetic models of the fates of duplicate genes. Genetica 118 279–294 [PubMed] [Google Scholar]
- Wigge PA, Kim MC, Jaeger KE, Busch W, Schmid M, Lohmann JU, Weigel D (2005) Integration of spatial and temporal information during floral induction in Arabidopsis. Science 309 1056–1059 [DOI] [PubMed] [Google Scholar]
- Yan L, Loukoianov A, Blechl A, Tranquilli G, Ramakrishna W, SanMiguel P, Bennetzen JL, Echenique V, Dubcovsky J (2004) The wheat VRN2 gene is a flowering repressor down-regulated by vernalization. Science 303 1640–1644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L, Loukoianov A, Tranquilli G, Helguera M, Fahima T, Dubcovsky J (2003) Positional cloning of the wheat vernalization gene VRN1. Proc Natl Acad Sci USA 100 6263–6268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L, von Zitzewitz J, Skinner JS, Hayes PM, Dubcovsky J (2005) Molecular characterization of the duplicated meristem identity genes HvAP1a and HvAP1b in barley. Genome 48 905–912 [DOI] [PubMed] [Google Scholar]
- Yano M, Kojima S, Takahashi Y, Lin H, Sasaki T (2001) Genetic control of flowering time in rice, a short-day plant. Plant Physiol 127 1425–1429 [PMC free article] [PubMed] [Google Scholar]
- Yanofsky MF (1995) Floral meristems to floral organs: genes controlling early events in Arabidopsis flower development. Annu Rev Plant Physiol Plant Mol Biol 46 167–188 [Google Scholar]
- Yu H, Xu Y, Tan EL, Kumar PP (2002) AGAMOUS-LIKE 24, a dosage-dependent mediator of the flowering signals. Proc Natl Acad Sci USA 99 16336–16341 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.