Membrane-delimited compartments in eukaryotic cells provide physical scaffolds to localize biochemical reactions, confining proteins and their activities as well as soluble compounds within cells. This structural differentiation is supported through the biosynthesis of membrane lipid and protein at the endoplasmic reticulum and Golgi apparatus as well as the shuttling of membrane vesicles and their contents between endomembrane compartments and the plasma membrane. The traffic of vesicles and their fusion at these various target membranes is critical for nervous signal transmission across the synaptic junctions of nerves, for cell wall delivery and budding in yeast, and for maintaining cell polarity, growth, and development in plants (Pratelli et al., 2004; Surpin and Raikhel, 2004; Sutter et al., 2006a). These are highly dynamic processes that, even in relatively quiescent plant tissues, contribute to a rapid turnover of large areas of membrane surface and, in certain specialized cell types, such as pollen and root hairs, drive the turnover of plasma membrane at rates in excess of 0.01 cm2 min−1 at the growing tip (Campanoni and Blatt, 2007).
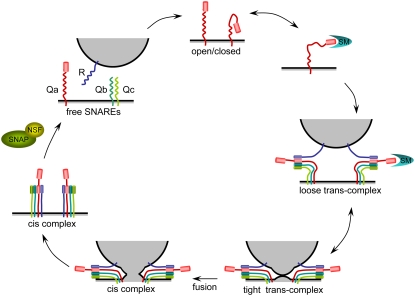
SNARE (for soluble N-ethylmaleimide-sensitive fusion protein attachment protein receptor) proteins facilitate vesicle traffic by overcoming the immense dehydration forces associated with bringing two lipid bilayers together in an aqueous environment (Rand and Parsegian, 1989) and by matching vesicles with their destinations to ensure efficient targeting and delivery of specific membrane proteins and soluble cargo. Subsets of SNAREs are found at both vesicle and target membranes, and it is the pairing of the complementary SNARE partners to form a tetrameric bundle of coiled helices that draws the membrane surfaces together for docking and fusion (Fig. 1). Elements of this SNARE complex differ widely in size and structure, but they share common structural motifs, notably those contributing to interactions at the core of the SNARE complex. Within the bundled α-helices of the SNARE core complex, at least one helix each is derived from a membrane-anchored protein associated with the target membrane (hence a t-SNARE) and with the vesicle (hence a v-SNARE), and in every case functional SNARE complexes are built of one element of each of four submotif domains, designated Qa, Qb, Qc, and R (Bock et al., 2001). These submotifs extend over roughly 60 amino acid residues and confer an amphipathicity to the helices that strongly favors their assembly, like a semicrystalline “zipper” (Sutton et al., 1998; Misura et al., 2000). Nominally, the Qa, Qb, and Qc domains center about a Gln residue within the SNARE motif, while the R domain centers about an Arg residue of the complementary SNARE motif. At the nerve synapse, t-SNAREs contribute three α-helices, one from syntaxin (Qa) and two from the N-terminal (Qb) and C-terminal (Qc) halves of SNAP-25. Intriguingly, the tripartite assembly of the neuronal SNARE complex is far less common by comparison with the model characterized by a single SNARE coil per polypeptide (Linial, 2001). The latter appears to be the norm also for plants. For example, Arabidopsis (Arabidopsis thaliana) has 18 syntaxin-like Qa-SNAREs, 18 Qb- and Qc-SNAREs, but only three SNAP-25-like (Qb+Qc)-SNAREs, and a similar distribution of SNARE groupings is found in rice (Oryza sativa; Sutter et al., 2006a).
Figure 1.
The canonical SNARE cycle and its regulation. SNARE components as indicated: red, R-SNARE; blue, syntaxin-like Qa-SNARE; yellow, Qb- and Qc-SNAREs. The cycle begins with the release of the closed conformation by Sec/Munc (SM) protein binding to the Ha/Hb/Hc domain to expose the Q-SNARE (H3) motif. Association in the trans-complex is accompanied by a large increase in core α-helical structure, which drives the transition to the cis-complex (Jahn et al., 2003). Dissociation of the cis-complex and repriming requires the energy input of ATP hydrolysis and is achieved through the binding of α-SNAP and the NSF ATPase. (Modified from Pratelli et al. [2004].)
The formation of a SNARE complex is sufficient to drive fusion (Weber et al., 1998; McNew et al., 2000; Hu et al., 2002) and will even facilitate fusion between cells when expressed with complementary SNARE motifs exposed outside the cells (Hu et al., 2003). These experiments provide strong evidence for SNARE function, but they do not rule out the role of lipids (Tamm et al., 2003) and other (possibly regulatory) proteins in fusion (Dennison et al., 2006; Vicogne et al., 2006). When assembled, the core complex of SNAREs shows a remarkable homology to viral type I fusion proteins, notably to the influenza hemagglutinin protein and gp41 of human immunodeficiency virus (Hughson, 1999; Jahn et al., 2003). Although each constitutes a single membrane protein, both hemagglutinin and gp41 tether viral and host membranes before undergoing a conformational change that brings the two membranes in close apposition and drives fusion. Furthermore, like the SNARE core complex, once inserted in the host membrane the viral peptide sequence not only anchors the two membranes but also deforms the bilayers to favor transition to a hemifusion structure and to force fusion (Colotto et al., 1996). There are similarities, too, in the stoichiometry of protein (complex) units between the two fusion processes. Fusion driven by hemagglutinin is cooperative, engaging two to three fusion initials to open the fusion pore (Bentz, 2000), while measurements of the stoichiometry associated with Ca2+-dependent vesicle fusion indicate a minimum of three to four SNARE complexes cooperating in neuronal exocytosis (Stewart et al., 2000) and that may oligomerize to form a supercomplex of bundles that open the fusion pore (Rickman et al., 2005).
The combinatorial model of SNARE interactions is largely sufficient to explain both the high specificity and the overlaps in function among the different SNAREs (McNew et al., 2000; Paumet et al., 2004) and is a key to understanding the functional relationships within the substantially expanded family of these proteins in plants (Sutter et al., 2006a; Sanderfoot, 2007). In fact, surprisingly little direct evidence of redundancy among these proteins has come to light. Among the Arabidopsis Q-SNAREs, which divide between eight classes (SYP1–SYP8, for syntaxins of plants), three of the Syp classes—Syp2x, Syp4x, and Syp1x (where x identifies the class member)—have shown gene disruptions and mutations of single members that give lethal phenotypes (Pratelli et al., 2004; Sutter et al., 2006a). Among members of the plasma membrane-like class, SYP1, T-DNA knockouts Atsyp121 and Atsyp122 have not yielded obvious differences from the wild type in the glasshouse, although the Atsyp121-Atsyp122 double knockout is severely stunted (Assaad et al., 2004). Both AtSYP121 and AtSYP122 interact in jasmonate and salicylate signaling (Zhang et al., 2007). However, several distinctions have surfaced between these and related plasma membrane-associated Q-SNAREs. Notably, AtSYP121 plays a role in nonhost resistance to fungal pathogens and its expression is sensitive to the plant hormone abscisic acid (ABA), while AtSYP122 appears to contribute to resistance to bacterial pathogens (Collins et al., 2003; Nühse et al., 2003). Furthermore, AtSYP121 failed to rescue the Atsyp111 (knolle) mutant when expressed under its promoter (Muller et al., 2003). AtSYP111 is essential for the formation of the cell plate during division (Jurgens, 2004). It has been suggested to localize in part to the plasma membrane (Dhonukshe et al., 2006), but the physiological significance of this latter finding is doubtful (Reichardt et al., 2007). Thus, a mixed picture of genetic overlaps as well as spatiotemporal overlaps and specializations is emerging.
Despite the growing body of data bearing on SNAREs in plants, remarkably little evidence is available that speaks directly to their functions in vesicle traffic within the living plant cell. Two different pharmacokinetic approaches have been utilized extensively in work on mammalian SNARE function, but they are only beginning to attract attention in plants. Clostridium botulinum (BotN/x, where x = A to G) neurotoxins have provided a powerful set of molecular tools with which to manipulate vesicle traffic in neuromuscular as well as other secretory tissues. These toxins act as endopeptidases to selectively cleave SNARE proteins, thereby blocking vesicle fusion and neurotransmitter release at the synapse (Humeau et al., 2000), and they have yielded substantial kinetic detail, including that of its coupling with Ca2+ channel control (Stanley and Mirotznik, 1997; Degtiar et al., 2000; Sakaba et al., 2005). In plants, to date, the neurotoxins have seen application in two studies only. Nonetheless, this work has confirmed a selective cleavage by BotN/A, BotN/C, and BotN/E toxins in Arabidopsis and tobacco (Nicotiana tabacum) and demonstrated neurotoxin action in suppressing ABA-mediated signaling (Leyman et al., 1999; Kargul et al., 2001). A second strategy, making use of dominant negative inhibitors complementary to a selected SNARE, was first employed by Leyman et al. (1999) to explore the role for the tobacco SNARE NtSYP121 in K+ and Cl− channel control and was subsequently used to examine the functioning of the same SNARE in membrane vesicle traffic and development (Geelen et al., 2002). The rationale of this method lies in the ability of a protein fragment genetically engineered from the full-length sequence to bind the native protein partners, thereby preventing completion of the normal SNARE function. The specificity of dominant negative SNARE fragments in vivo (Scales et al., 2000; Tyrrell et al., 2007) suggests that this approach will find much wider application in studies of trafficking in plants, although it should be noted that pairing of the dominant-negative fragment may also yield noncanonical SNARE complexes with similar functional consequences (Jahn et al., 2003; Tyrrell et al., 2007). Among the Arabidopsis SNAREs, Tyrrell et al. (2007) found that the targets of so-called Sp2 (dominant negative) fragments were exclusive to traffic at the membranes associated with the corresponding SNAREs. Furthermore, they were able to resolve quantitative differences in the efficacy of Sp2 fragments to the plasma membrane SNAREs AtSYP121, AtSYP122, and AtSYP71 in suppressing secretory traffic. Of course, not all traffic to a common target membrane need pass through the same vesicular pathway (Oufattole et al., 2005; Sutter et al., 2006a), a fact that must be borne in mind when comparing the traffic of different markers (Foresti et al., 2006; Tyrrell et al., 2007).
SNARES IN VACUOLAR TRAFFICKING AND GRAVITROPISM
The vacuole is a major destination for vesicle trafficking, and SNAREs have been identified that function in this branch of the secretory pathway. Soluble vacuolar proteins contain a vacuolar sorting signal that is recognized at the trans-Golgi network (TGN) by a receptor protein for diversion away from the secretory route toward the vacuole. Two major transport pathways exist between the TGN and vacuole, distinguished by different types of sorting signal, pharmacological sensitivities, and type of cargo transported (lytic or storage; Matsuoka et al., 1995; Ahmed et al., 2000; Shimada et al., 2003; Park et al., 2005). In some cell types, these pathways terminate in distinct vacuole types, lytic vacuoles, or protein storage vacuoles (Paris et al., 1996; Di Sansebastiano et al., 2001). In other cells, a single vacuole type exists and both pathways lead to this same vacuole (Hunter et al., 2007; Olbrich et al., 2007). Transport occurs via a prevacuolar compartment (PVC)/multivesicular body, a site from which trafficking components can be recycled to the TGN (Mo et al., 2006).
The SYP2 family of SNAREs probably functions in anterograde trafficking to the vacuole. Knockout mutations in the corresponding genes are lethal (Sanderfoot et al., 2001a), but overexpression and analysis of point mutants has now yielded some functional information about these proteins. SYP21 is found at the PVC (da Silva Conceicao et al., 1997) and most likely functions in the trafficking of lytic cargo, as overexpression leads to mistargeting of fusion proteins containing a lytic vacuole sorting signal (Foresti et al., 2006). The fusions are partially secreted and partially retained in the PVC, which becomes enlarged and also accumulates some tonoplast proteins. SYP22 localizes to both the vacuole and the PVC (Sato et al., 1997; Sanderfoot et al., 1999) and potentially functions in fusion with the tonoplast (Rojo et al., 2003). Point mutations in VAM3 cause defects in growth (Ohtomo et al., 2005) and in shoot gravitropism (Yano et al., 2003; see below).
Perhaps the best studied SNARE subfamily in vacuolar trafficking is the VTI1 group of v-SNAREs. VTI11 and VTI12 are partially redundant in function, as a double mutation is embryo lethal but the corresponding single mutants are viable, albeit with some growth defects (Surpin et al., 2003). The single mutants have quite distinct phenotypes: a vti11 knockout has defects in vascular patterning, auxin transport, and shoot gravitropism (Kato et al., 2002; Surpin et al., 2003), and vti12 is hypersensitive to starvation and senesces prematurely (Surpin et al., 2003), phenotypes characteristic of autophagy mutants. Upon analysis of the vacuolar trafficking pathways involving VTI11 and VTI12, it was revealed that a vti11 mutant is defective in trafficking of proteins to the lytic vacuole, whereas a vti12 mutant fails to correctly transport storage proteins to the protein storage vacuole (Sanmartin et al., 2007). The basis for the functional redundancy was seen from analysis of the SNARE complexes in which the two proteins participate. In wild-type plants, VTI11 and VTI12 form distinct SNARE complexes, with VTI11 interacting with t-SNAREs from the SYP2 and SYP5 classes and VTI12 interacting with the SYP4 and SYP6 classes (Bassham et al., 2000; Sanderfoot et al., 2001b). This specificity is relaxed in the vti11 and vti12 single mutants, and the proteins can at least partially substitute for one another in SNARE complexes (Surpin et al., 2003) and presumably in other complexes that may be required for function. For example, the EPSIN family of trafficking proteins in Arabidopsis show specificity in their interactions, with EPSIN1 interacting with VTI11 and being required for transport to the lytic vacuole (Song et al., 2006) and EPSINR2 interacting with VTI12 (Lee et al., 2007).
A particularly intriguing consequence of inefficient vacuolar trafficking is the loss of shoot gravitropism. This was first discovered when screens for mutants in shoot gravitropism identified the SNAREs SYP22 and VTI11, both predicted to function in vacuolar trafficking (Kato et al., 2002; Yano et al., 2003). A complex between SYP22, VTI11, and the t-SNARE SYP5 was shown to exist in the gravity-perceiving endodermal cells, and the mutant SYP22 protein had a decreased ability to form this SNARE complex (Yano et al., 2003). While the precise mechanism by which the SNAREs allow gravity responses is unknown, one possibility is that the mutant phenotype is due to an indirect effect on the vacuolar membrane structure or composition, rather than vesicle trafficking being required directly for gravitropism. Shoot gravitropism involves the sedimentation of amyloplasts in endodermal cells, and this sedimentation is disrupted in the SNARE mutants and other mutants predicted to affect vacuolar structure (Saito et al., 2005). Vacuolar structure is abnormal in these mutants, with loss of transvacuolar strands and accumulation of vesicle-like structures, possibly restricting amyloplast movement.
MEMBRANE TRAFFIC, SOLUTE TRANSPORT, AND RECEPTOR TURNOVER
Membrane vesicle traffic is intimately linked to transmembrane ion transport, and not only in coupling electrical signals of the nerve to synaptic transmission and neurotransmitter release (Jahn et al., 2003). During vesicle formation, the recruitment of membrane components, including small GTPases and coat proteins essential for budding, is tied to luminal acidification (Zeuzem et al., 1992; Aniento et al., 1996; Maranda et al., 2001). Homotypic SNARE fusion in yeast has been associated with vacuole acidification (Ungermann et al., 1999). Similarly, the sensitivity of vesicle traffic to the H+-ATPase inhibitor bafilomycin A can be understood in the context of energizing K+/H+ exchange to facilitate vesicle swelling and fusion (Cousin and Nicholls, 1997; Palokangas et al., 1998; Choi et al., 2007). Thus, it is notable that the VHA-a1 protein, a member of the vacuolar V-type H+-ATPases, localizes to the Golgi and the TGN in Arabidopsis, consistent with a projected role in vesicle acidification (Dettmer et al., 2006). Although more direct evidence associating vesicular pH with VHA-a1 activity is still lacking, pharmacological studies using the V-type H+-ATPase antagonist concanamycin A build up a convincing picture of its likely contribution to endocytosis from the plasma membrane as well as export from the Golgi.
Equally, the transport of ions and solutes across membranes is subject to membrane traffic, if only through its impact on the population of transport proteins present at the membrane surface. Thus, exocytosis and endocytosis of selected ion and solute transporters serve to regulate the transport capacity, albeit not necessarily the intrinsic kinetic characteristics for transport across the membrane. The best characterized model among mammalian cells is the traffic of the Na+-coupled Glc transporter GLUT4, which cycles between the apical membrane and a pool of cytosolic vesicles in intestinal epithelial cells (Simpson et al., 2001; Ishiki and Klip, 2005). GLUT4 exocytosis in these cells is stimulated by insulin and leads to a roughly 5-fold increase in the rate of Glc uptake within 10 to 20 min. Fusion of GLUT4 vesicles depends on SNARE complexes that include mammalian SNAP-23, Syntaxin 4, and VAMP2 within lipid rafts of the plasma membrane (Volchuk et al., 1996; Chamberlain and Gould, 2002; Williams and Pessin, 2008). In turn, GLUT4 transporters are recovered from the apical plasma membrane by endocytosis and sequestered in specialized GLUT4 vesicles before recycling.
In Arabidopsis, a number of integral membrane proteins have now been identified to traffic to, and be recovered from, the plasma membrane. Takano et al. (2005) have described the boron transporter BOR1 as an integral membrane protein essential for xylem loading and boron translocation to the shoot under nutrient limitation, and they note that its accumulation at the plasma membrane is strongly affected by boron resupply, leading to BOR1 endocytosis and degradation in the vacuole. A similar pattern of traffic appears to govern the plasma membrane residence of KOR1, which contributes to cell wall deposition (Robert et al., 2005), of the putative auxin transporter PIN1 at the apical membrane of root epidermal and cortical cells (Geldner et al., 2003), and of the brassinolide receptor-like kinase protein BRI1 (Russinovaa et al., 2004; Geldner et al., 2007). Intriguingly, endocytosis of PIN1 appears to be modulated by auxin itself (Paciorek et al., 2005), and as a variation on this theme, Robatzek et al. (2006) have reported that the endocytosis of the flagellin receptor FLS2 is evoked by its ligand flg22. Internalization of the GFP-tagged FLS2 was observed to lead initially to its accumulation in endosomes and was sensitive to the inhibitor wortmannin as well as to cytoskeletal antagonists, again consistent with concerted passage of the receptor protein en route to the vacuole and its degradation. Thus, each of these examples has described a traffic characterized to varying degrees by changes in the constitutive turnover of the integral membrane protein; unlike GLUT4 traffic, however, upon endocytosis these plant proteins enter a one-way path that leads to their sequestration in the vacuole and degradation. Almost nothing is known of the molecular mechanics of traffic, although SNAREs almost certainly play roles in delivery to, and removal from, the plasma membrane as well as endomembrane compartments.
Traffic of the Kv-like K+ channel KAT1 presents a different picture (see also “Membrane Traffic, ABA, and Auxin” below). Turnover of KAT1 at the plasma membrane of intact epidermal and guard cells is tightly controlled through a mechanism evoked by ABA and leads to recycling in true exchange with an endomembrane pool distinct from known degratory pathways to the vacuole (Sutter et al., 2007). The close parallels to GLUT4 traffic and its role in transmembrane solute transport are self-evident. Furthermore, studies using dominant negative Sp2 fragments have indicated that export of KAT1 to the plasma membrane is dependent on SYP121 function. Sutter et al. (2006b) found that coexpression of the SYP121 Sp2 fragment selectively suppressed KAT1 delivery to the plasma membrane, but not that of the PMA2 H+-ATPase, and altered its local distribution within the plasma membrane. They engineered hemagglutinin epitopes within external loops of the K+ channel protein to demonstrate its localization within bona fide plasma membrane microdomains and observed that expressing the Sp2 fragment also led to a loss of the microdomain boundaries and “smearing” of the KAT1 channel over the surface of the plasma membrane. This loss of microdomain organization might be explained simply as a consequence of the Sp2 fragment interference with targeting of KAT1 delivery to the plasma membrane. However, Sutter et al. (2006b) also observed a roughly 100-fold increase in the lateral mobility of KAT1 within the plane of the plasma membrane in the presence of the Sp2 fragment, thus implicating an additional role for the SNARE in anchoring the KAT1 protein within the microdomains.
In fact, recent work from the same laboratory has yielded direct evidence for SYP121 as a key structural element determining the gating of another K+ channel and implicating the SNARE in an extensive scaffold of proteins associated with the membrane transport of K+. Honsbein et al. (2007) used a yeast mating-based split-ubiquitin assay (Obrdlik et al., 2004) to screen for protein partners that interact with SYP121 and reported a regulatory subunit that interacts selectively both in coimmunoprecipitation assays after heterologous expression and in vivo using a bimolecular fluorescence complementation assay (Walter et al., 2004). Significantly, they observed the interaction to be essential for K+ channel gating and K+ uptake. Thus, the SNARE may be a missing component essential for channel-mediated K+ nutrition, a function wholly distinct from any role in membrane traffic. In fact, a few SNARE proteins are known to interact with ion channels, notably mammalian Syntaxin 1A, which binds several different Ca2+ and K+ channels in nerves, subtly affecting channel gating to facilitate synaptic transmission (Rettig et al., 1996; Fili et al., 2001; Leung et al., 2007). However, such interactions have been thought to be restricted to mammalian tissues and to serve highly specialized functions in coupling membrane traffic and signaling. Thus, it will be of interest now to determine whether similar SNARE interactions contribute directly to other ion transport, signaling, and homeostatic functions.
MEMBRANE TRAFFIC, ABA, AND AUXIN
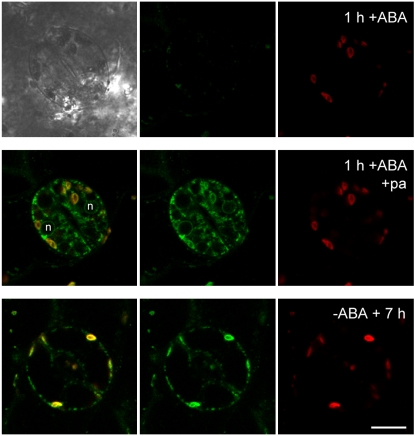
In addition to its role in balancing membrane transport activities to the need for nutrient acquisition, vesicle traffic in plants has been implicated in a variety of responses to hormonal and environmental stimuli (Sutter et al., 2006a). We touch on two examples here. Recent studies of the relationship of membrane traffic to ABA signaling have provided direct evidence not only for evoked endocytosis at the plasma membrane but also for its selectivity among integral membrane proteins and their subsequent recycling back to the plasma membrane. ABA acts as a drought stress signal to control ion transport in stomatal guard cells and suppress transpiration from leaf tissues (Blatt, 2000; Hetherington and Brownlee, 2004). It triggers rapid changes in the activities of three dominant K+ and Cl− channels at the guard cell plasma membrane that are, in part, coordinated through elevations in cytosolic-free Ca2+ concentration ([Ca2+]i), and it is also associated with longer term adaptive changes in the capacity for solute flux and stomatal responses to stress (Peng and Weyers, 1994; Allen et al., 2001). How these temporally different behaviors are related is still unresolved, but pieces of the puzzle are now falling into place. Last year, Sutter et al. (2007) reported that, concurrent with its action in ion channel gating, ABA initiates the endocytosis of the KAT1 K+ ion channel, which normally is active in K+ uptake for stomatal opening. Endocytosis was selective for the K+ channel over a similarly tagged H+-ATPase, and it led to sequestration of the protein within an endosomal membrane pool, from which it recycled back to the plasma membrane over 6 to 8 h after ABA washout (Fig. 2). Quite apart from offering the first unambiguous evidence for evoked endocytosis and membrane recycling in a plant cell, these data point to a role for channel traffic in adaptive changes in the capacity for solute flux, consistent with so-called “programmed closure” (Allen et al., 2001).
Figure 2.
KAT1 K+ channels recycle to plasma membrane-localized microdomains. Three-dimensional reconstructions (for clarity, omitting the upper and lower surfaces) of tobacco guard cells expressing KAT1 tagged with a photoactivatable GFP are shown. Epidermal peels were pretreated with 20 μm ABA for 60 min. Images were taken before (top) and after (middle) photoactivation (+pa) at the start of ABA washout and after a further 7-h continuous superfusion (bottom) with buffer (−ABA). Images are (left to right) overlay, GFP, and chloroplast (red) channels. A bright-field image overlay is included in the bottom set of frames. Nuclei (n) are labeled in middle frame left. Because only photoactivatable GFP photoactivated at the start of ABA washout will fluoresce, the GFP signal obtained 7 h later (bottom) reflects a true recycling of the KAT1 channel to the plasma membrane. (After Sutter et al. [2007].)
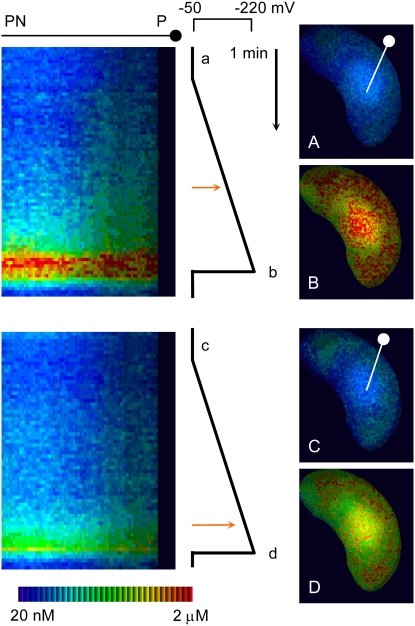
SYP121 (or a close homolog; Tyrrell et al., 2007) may also contribute to the early changes in ion channel gating in ABA. Indeed, the original observations of Leyman et al. (1999) were based on the finding that a dominant negative fragment of the tobacco homolog NtSYP121 (NtSYR1) blocked changes in K+ and Cl− channel gating in response to ABA. The recent work of Sokolovski et al. (2008) revisits these experiments to show that these changes can, in part, be understood by an action of the dominant negative fragment in suppressing Ca2+ channel gating, Ca2+ entry across the plasma membrane, and the consequent rise in [Ca2+]i (Fig. 3). Significantly, while the SNARE fragment blocked stomatal closure in response to ABA, the experiments show that closure could still be evoked by nitric oxide, which affects only Ca2+ release from endomembrane stores (Garcia-Mata et al., 2003). These findings offer primary evidence for the functional coupling of a SNARE with Ca2+ channels at the plant cell plasma membrane and, thus, add substance to a long-standing expectation that Ca2+ signaling may be tied to SNAREs in plants as it is in mammalian cells (Leung et al., 2007). Because [Ca2+]i plays a key role in the control of K+ and Cl− channel currents in guard cells, this underscores an important mechanism for SNARE integration with ion channel regulation during stomatal closure and raises a question about similar roles in Ca2+ signaling associated with other physiological responses in plants, notably in pathogen defense (Collins et al., 2003; Heese et al., 2005; Zhang et al., 2007).
Figure 3.
Expressing the inhibitory (dominant negative) Sp2 fragment of NtSyp121 shifts the voltage threshold, elevating [Ca2+]i. Kymographs (left) and individual images (right) of voltage-evoked [Ca2+]i rise were taken from image sequences of two intact guard cells before (above) and 24 h after inducing Sp2 fragment expression. The time line runs top to bottom, with voltage scale and ramps as indicated (center). [Ca2+]i was determined from fura2 fluorescence ratios, with selected ratio images (A–D, right) corresponding to the time points indicated adjacent the voltage scale. Kymographs were constructed from successive ratio images averaged over a two-pixel-wide band along the line indicated in the image frames (A and C) from cell exterior (left) and periphery (P) to the perinuclear region (PN). Note that the voltage threshold for [Ca2+]i rise (red arrows, center) shifted from approximately −130 mV before to −185 mV after inducing Sp2 fragment expression. (After Sokolovski et al. [2008].)
Less is known of the contributions of SNAREs to trafficking associated with auxin and development per se. Nonetheless, an intriguing feature of the PIN (for PIN-formed) and AUX1 (for AUXIN1) proteins is their apical-basal polarity of distribution. Thus, quite apart from their presumptive roles in polar auxin transport, the subcellular targeting of these proteins, like the targeting of the KAT1 K+ channel to plasma membrane microdomains (Sutter et al., 2006a), makes these proteins attractive as models for study of the control of plant membrane traffic. Indeed, in many ways, the subcellular distribution of AUX1 and the PIN proteins may find analogs in traffic in mammalian epithelia. In the latter, distinct subsets of SNARE proteins are responsible for the differential targeting of solute transporters, including the Na+/K+-ATPase, gastric H+-ATPase, and coupled transporters such as the Glc transporter GLUT4, to the apical and basal cell membranes (Banerjee et al., 1999; Ishiki and Klip, 2005). AUX1, which is thought to function as an auxin uptake carrier, is localized to the apical ends of cell files, notably in epidermis and cortex of the Arabidopsis stem and root, while, conversely, the PIN1 protein, which is one of several presumptive auxin efflux carriers, has been found primarily at the opposite ends of the same cells. To date, however, attention to AUX1 and the PIN proteins has centered principally on their relationship to the phenomenology of polar auxin transport: these studies have yielded considerable evidence, if correlative, that their polar distribution is the molecular embodiment of the classic theories for auxin transport, plant growth, and development (Vieten et al., 2007).
If the analogy to mammalian epithelial polarity holds true (Muday et al., 2003; Vieten et al., 2007), we can expect that AUX1 and PIN traffic will depend on different subsets of Q- and R-SNAREs. Thus, it would be of interest to know which (if any) of the extant plasma membrane (Assaad et al., 2004; Zhang et al., 2007) or other SNARE mutants affect the distributions of these proteins, especially given the long-established role of auxin in gravitropism (Pickard, 1985; Hicks et al., 1989) and evidence that several SNAREs affect gravitropic responses (Kato et al., 2002; Surpin et al., 2003; Yano et al., 2003). What is known of the traffic of AUX1 and the PIN proteins has come largely from experiments with brefeldin A, a fungal toxin that affects traffic to the Golgi through its suppression of an ARF-GEF GTPase essential for vesicle formation (Jurgens, 2004; Teh and Moore, 2007). These studies have led to speculation that a constitutive trafficking and endocytosis, especially of the PIN proteins, is essential for the feedback control of polar auxin transport (Geldner et al., 2001; Vieten et al., 2007). At present, one difficulty with such speculation is that, although brefeldin A does effect a loss in polar distributions of these proteins (Steinmann et al., 1999; Paciorek et al., 2005), the concentrations required are more than one order of magnitude greater than those required to influence auxin transport per se (Petrasek et al., 2003). More still, the effects of various auxins in suppressing PIN endocytosis in root epidermis and cortex tissues are evident only at concentrations roughly two to three orders of magnitude higher than needed to determine the characteristics of auxin-stimulated growth in these tissues (Goldsmith, 1977; Trewavas, 1992). Clearly, there remain major gaps in our understanding of the mechanics of PIN and AUX1 traffic as well as its significance for auxin signaling and development. Closing these gaps is likely also to provide important discoveries that relate traffic to plant development.
SECRETORY SNARES ARE REQUIRED FOR PLANT DEFENSE AGAINST PATHOGENS
A specific role for several plasma membrane SNAREs in plant defense against pathogen attack has now been revealed. Possibly the best characterized example is that of the resistance of Arabidopsis to barley (Hordeum vulgare) powdery mildew. Barley powdery mildew infects barley and causes disease, but Arabidopsis is not normally a host plant for this species; spores are able to germinate on Arabidopsis, but they are not able to penetrate the plant cells and therefore are unable to establish an infection. This nonhost resistance is an active mechanism, in which cell wall deposits (known as papillae) are secreted at the site where penetration is attempted, providing a physical barrier to infection (Collins et al., 2003). A genetic screen for Arabidopsis mutants with increased susceptibility to barley powdery mildew, and therefore defective in nonhost resistance, led to the identification of pen mutants (for increased penetration). The pen1 mutant was discovered to have a mutation in the SYP121 gene encoding a plasma membrane syntaxin closely related to NtSyr1, a tobacco syntaxin required for secretion (Geelen et al., 2002). A barley homolog of PEN1/SYP121, named ROR2, is required for basal penetration resistance in barley, suggesting a relationship between the mechanisms of innate and nonhost immunity (Collins et al., 2003).
The precise function of SYP121 in resistance is not clear, but it most likely is related to cell wall structure and deposition as a physical barrier to fungal entry. Upon infection of barley, large vesicles filled with hydrogen peroxide are evident in the barley cells just below the site of infection; these were decreased in the ror2 mutant (Collins et al., 2003). These vesicles potentially function in cross-linking of cell wall components as a general response to infection attempts, thus leaving the mutant cells physically more susceptible to fungal penetration. The Arabidopsis syp121 mutant has a delay in the fungus-induced formation of papillae, which could also cause increased susceptibility to infection (Assaad et al., 2004). Recent work, however, suggests that the idea of a physical barrier deposited via a SYP121-mediated trafficking pathway may not be the whole story. The syp121 mutant also has increased expression of the pathogen response gene PR-1 and increased levels of salicylic acid (SA; Zhang et al., 2007), which acts as a signal in defense pathways, suggesting that SYP121 is a regulator of SA-mediated defense. These data indicate that SYP121 may have distinct and opposite roles in modulating different pathogen-responsive pathways.
Expression of the SYP121-related SNARE SYP122 is induced by fungal, bacterial, and viral infection (Assaad et al., 2004). It is also phosphorylated rapidly in response to the general bacterial elicitor flg22 (Nühse et al., 2003), suggesting that it may play a role in pathogen defense. In contrast, tobacco SYP121 is phosphorylated in response to the race-specific elicitor Avr9 and not flg22 (Heese et al., 2005). Unlike SYP121, mutants in SYP122 have no detectable defects in disease resistance. Despite this, double-mutant analysis indicates some overlap in their function, as unlike either single mutant, the double mutants are dwarfed and develop necrotic patches (Assaad et al., 2004) and have even higher levels of PR-1 and SA (Zhang et al., 2007). The primary functions of SYP121 and SYP122, therefore, are likely to be distinct, although it appears that they can partially substitute for one another.
Upon exposure of Arabidopsis to a nonhost fungus, both SYP121 and SYP122 are recruited to the sites of attempted penetration, although this is much more evident for SYP121 (Assaad et al., 2004). Barley ROR2 shows a similar redistribution within the plasma membrane to the proximity of fungal entry sites. It has been suggested that pathogen infection causes the formation of sterol-enriched plasma membrane microdomains, similar to lipid rafts in animal cells, and that components of the resistance response such as ROR2 move to these microdomains to combat the infection (Bhat et al., 2005). While this provides some intriguing corollaries to animal cells, the validity of this suggestion awaits a much more comprehensive biochemical analysis of the membrane structure.
A third plasma membrane syntaxin, SYP132, has been implicated in defense against bacterial infection. Arabidopsis SYP132, like SYP121 and SYP122, is phosphorylated upon elicitor treatment (Kalde et al., 2007), and it seems that phosphorylation may be a general method for regulating the function or localization of defense-related SNAREs (Nühse et al., 2003; Heese et al., 2005; Kalde et al., 2007). Tobacco plants in which tobacco SYP132 is silenced are more susceptible to bacterial infection, which may be due to decreased secretion of PR proteins. These results extend previous observations that a functional secretory pathway is required for plant defense via the secretion of proteins required for resistance to infection (Wang et al., 2005).
Additional components of the SNARE complex containing SYP121 have now been identified, lending credence to the idea that vesicle fusion, rather than other possible functions of the syntaxin-type SNAREs, is required for plant defense pathways. SNAP33 expression is induced by pathogen attack (Wick et al., 2003), and knockout mutants in SNAP33 are dwarfed and develop necrotic lesions (Heese et al., 2001), reminiscent of the syp121 syp122 double mutant combination. A syp121 mutant with a point mutation in the SNARE domain has a partial phenotype and, unlike wild-type SYP121, fails to form a SNARE complex with SNAP33 (Kwon et al., 2008). The v-SNARE for this complex is most likely a member of the VAMP72 family, with VAMP722 probably the most important but some functional redundancy present. While a complete vamp721 vamp722 knockout mutant is lethal, decreased expression of the VAMP72 family gave a similar phenotype to the syp121 mutant, with increased susceptibility to nonhost fungal infection. SNAP33 colocalizes with SYP121 at the plasma membrane, whereas GFP-VAMP722 labels structures that may be secretory carriers that move to the site of pathogen attack (Kwon et al., 2008). Together, these results suggest that many of the secretory SNAREs are multifunctional proteins, with a role in the general secretory pathway under normal conditions, and are recruited to defend the cell against pathogen attack by delivering cell wall material and defense proteins during challenge with pathogens.
SNARES IN ABIOTIC STRESS RESPONSES
An indication of a role for SNAREs in the tolerance of abiotic stress conditions came from a screen for mutants that were sensitive to osmotic and salt stress. One of the mutants (named osm1) was disrupted in the TGN-localized t-SNARE SYP61 (Sanderfoot et al., 2001b; Zhu et al., 2002). The SYP61 protein is able to function as a t-SNARE in membrane fusion (Chen et al., 2005), but whether the mutant phenotype reflects a defect in membrane fusion or in an additional process requiring SYP61 is not known, and it is not clear whether the osm1 mutant is a null mutant or may retain some function.
A plant-specific family of SNAREs, the NPSN group, may have multiple functions, including responses to the environment. Arabidopsis NPSN interacts with the cytokinesis-specific SNARE KNOLLE and may function in cell division (Zheng et al., 2002). By contrast, the rice NPSN gene family has been studied with respect to stress responses. OsNPSN11 expression increases upon hydrogen peroxide exposure and decreases in salt or mannitol (Bao et al., 2008). Overexpression of OsNPSN11 in tobacco gave rise to oxidative stress tolerance but salt and osmotic stress sensitivity, although the basis for this phenotype remains to be seen. Whether the Arabidopsis and rice NPSN proteins really have different functions is not clear, but it is possible that in both species the proteins function in both cell division and stress responses or that different members of the NPSN family have distinct functions.
The VAMP71 family of tonoplast-localized SNAREs (Carter et al., 2004) also decreases in expression during salt and osmotic stress, and knockout mutants have increased salt tolerance (Leshem et al., 2006). Closer analysis of the phenotype indicated that during exposure to high-salt conditions, reactive oxygen species were produced by endosomes that fused with the vacuole, and this fusion was blocked in the absence of VAMP711. It was suggested that these oxidative species damaged the tonoplast in wild-type plants, whereas the damage was minimized in the mutants and thus vacuole function was maintained (Leshem et al., 2006). Validation of this hypothesis awaits further analysis, but the involvement of other types of vesicle-trafficking proteins in stress responses (Mazel et al., 2004) may point to a general role of vesicle transport or membrane fusion in stress tolerance.
PROSPECTS FOR BIOTECHNOLOGY
Many plant secondary metabolites are stored in vacuoles, including various compounds that have anticancer and other useful properties (Noble, 1990). These metabolites are often produced only in trace quantities in the native plants, opening the possibility for greatly enhancing production using genetic engineering (Verpoorte and Memelink, 2002). In the example of anticancer alkaloids produced by Catharanthus roseus, not only are the metabolites stored in the vacuole, but they are also synthesized there (Sottomayor et al., 1996; Costa et al., 2008). One of the enzymes involved in their synthesis, a class III vacuolar peroxidase, has been shown recently to have a C-terminal vacuolar sorting signal (Costa et al., 2008). As overexpression of vacuolar proteins has been shown to saturate the vacuolar targeting machinery, leading to secretion instead of vacuolar targeting (Frigerio et al., 1998), an increase in production of the useful metabolites may also require increases in the activity of vacuolar trafficking components.
Increasing interest is now evident in the use of plants for the production of high-value proteins such as pharmaceuticals, and the endomembrane system has been proposed to be a useful site for the targeting and accumulation of these proteins (Vitale and Pedrazzini, 2005). Production of antibodies is one potential application, and systems have been developed for the synthesis of high levels of either full-sized antibodies or Fv-Fc fragments in transgenic plants (Stoger et al., 2005; Giritch et al., 2006; Van Droogenbroeck et al., 2007). One concern when producing pharmaceutical proteins via the secretory pathway is the addition of glycans; while plant cells are able to glycosylate animal proteins, and glycosylation is often important for function, plant complex glycans have structures different from those present on animal proteins. One possibility for overcoming this problem is retention and accumulation of the recombinant proteins in the endoplasmic reticulum, leading to the presence of only high-Man glycans (Sriraman et al., 2004; Triguero et al., 2005). However, adding an endoplasmic reticulum retention signal to antibodies, although effective in leaves, was ineffective in retaining the protein in the endoplasmic reticulum in seeds, potentially the most useful site for protein accumulation (Petruccelli et al., 2006). Instead, the recombinant protein was partially secreted and partially transported to protein storage vacuoles. Production of single-chain Fv-Fc antibodies in Arabidopsis seeds was even less effective, as not only was the recombinant protein secreted but high-level expression also disrupted normal trafficking pathways, leading to the secretion of endoplasmic reticulum chaperones and seed storage proteins (Van Droogenbroeck et al., 2007). Clearly, interfering with trafficking could lead to detrimental effects on seed viability and plant growth and development. These experiments highlight the need to understand the trafficking of proteins through the endomembrane system and the roles of vesicle-trafficking components. It is possible that for efficient targeting of foreign proteins, the appropriate trafficking components, including the SNAREs and other proteins required for vesicle budding and fusion, will need to be up-regulated to compensate for the increase in cargo through the system.
OUTLOOK
Research over the last 10 years has shown that SNARE proteins in plants have critical roles in a wide range of cellular activities, and not only those related to homeostasis, growth, and development. SNAREs are almost certainly important “cogs” in the machinery that plants engage during pathogen defense, for example; but they also play roles in coordinating events of cellular stimulus-response coupling and show up in protein complexes that do not have any obvious functions associated with membrane traffic. Precisely how SNAREs integrate these different functions at the cellular and molecular levels has yet to be explored in much detail in most cases, and it will be necessary now to fill in these gaps in our understanding. Future work must also address questions of mechanistic overlap between stimulus-response coupling and vesicle trafficking, notably in cargo and selective membrane protein cycling within the cell.
This work was supported by the Biotechnology and Biological Sciences Research Council (grant nos. BB/D001528/1, BB/C500595/1, BB/F001630/1, and BB/F001673/1), by the Leverhulme Trust (grant no. F00179/T), and by a John Simon Guggenheim Fellowship to M.R.B. and by the National Science Foundation (grant no. IOB–0515998) and the Iowa State University Plant Sciences Institute to D.C.B.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Diane C. Bassham (bassham@iastate.edu).
References
- Ahmed SU, Rojo E, Kovaleva V, Venkataraman S, Dombrowski JE, Matsuoka K, Raikhel NV (2000) The plant vacuolar sorting receptor AtELP is involved in transport of NH(2)-terminal propeptide-containing vacuolar proteins in Arabidopsis thaliana. J Cell Biol 149 1335–1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen GJ, Chu SP, Harrington CL, Schumacher K, Hoffman T, Tang YY, Grill E, Schroeder JI (2001) A defined range of guard cell calcium oscillation parameters encodes stomatal movements. Nature 411 1053–1057 [DOI] [PubMed] [Google Scholar]
- Aniento F, Gu F, Parton RG, Gruenberg J (1996) An endosomal beta COP is involved in the pH-dependent formation of transport vesicles destined for late endosomes. J Cell Biol 133 29–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assaad F, Qiu J, Youngs H, Ehrhardt D, Zimmerli L, Kalde M, Wanner G, Peck S, Edwards H, Ramonell K, et al (2004) The PEN1 syntaxin defines a novel cellular compartment upon fungal attack and is required for the timely assembly of papillae. Mol Biol Cell 15 5118–5129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee A, Shih T, Alexander EA, Schwartz JH (1999) SNARE proteins regulate H+-ATPase redistribution to the apical membrane in rat renal inner medullary collecting duct cells. J Biol Chem 274 26518–26522 [DOI] [PubMed] [Google Scholar]
- Bao Y, Wang J, Huang J, Zhang H (2008) Cloning and characterization of three genes encoding Qb-SNARE proteins in rice. Mol Genet Genomics 279 291–301 [DOI] [PubMed] [Google Scholar]
- Bassham DC, Sanderfoot AA, Kovaleva V, Zheng H, Raikhel NV (2000) AtVPS45 complex formation at the trans-Golgi network. Mol Biol Cell 11 2251–2265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentz J (2000) Minimal aggregate size and minimal fusion unit for the first fusion pore of influenza hemagglutinin-mediated membrane fusion. Biophys J 78 227–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat R, Miklis M, Schmelzer E, Schulze-Lefert P, Panstruga R (2005) Recruitment and interaction dynamics of plant penetration resistance components in a plasma membrane microdomain. Proc Natl Acad Sci USA 102 3135–3140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatt MR (2000) Cellular signaling and volume control in stomatal movements in plants. Annu Rev Cell Dev Biol 16 221–241 [DOI] [PubMed] [Google Scholar]
- Bock JB, Matern HT, Peden AA, Scheller RH (2001) A genomic perspective on membrane compartment organization. Nature 409 839–841 [DOI] [PubMed] [Google Scholar]
- Campanoni P, Blatt MR (2007) Membrane trafficking and polar growth in root hairs and pollen tubes. J Exp Bot 58 65–74 [DOI] [PubMed] [Google Scholar]
- Carter C, Pan S, Zouhar J, Avila E, Girke T, Raikhel N (2004) The vegetative vacuole proteome of Arabidopsis thaliana reveals predicted and unexpected proteins. Plant Cell 16 3285–3303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain LH, Gould GW (2002) The vesicle- and target-SNARE proteins that mediate Glut4 vesicle fusion are localized in detergent-insoluble lipid rafts present on distinct intracellular membranes. J Biol Chem 277 49750–49754 [DOI] [PubMed] [Google Scholar]
- Chen Y, Shin YK, Bassham DC (2005) YKT6 is a core constituent of membrane fusion machineries at the Arabidopsis trans-Golgi network. J Mol Biol 350 92–101 [DOI] [PubMed] [Google Scholar]
- Choi YO, Park JH, Song YS, Lee W, Moriyama Y, Choe H, Leem CH, Jang YJ (2007) Involvement of vesicular H+-ATPase in insulin-stimulated glucose transport in 3T3-F442A adipocytes. Endocr J 54 733–743 [DOI] [PubMed] [Google Scholar]
- Collins NC, Thordal-Christensen H, Lipka V, Bau S, Kombrink E, Qiu JL, Huckelhoven R, Stein M, Freialdenhoven A, Somerville SC, et al (2003) SNARE-protein-mediated disease resistance at the plant cell wall. Nature 425 973–977 [DOI] [PubMed] [Google Scholar]
- Colotto A, Martin I, Ruysschaert JM, Sen A, Hui SW, Epand RM (1996) Structural study of the interaction between the SIV fusion peptide and model membranes. Biochemistry 35 980–989 [DOI] [PubMed] [Google Scholar]
- Costa M, Hilliou F, Duarte P, Pereira L, Almeida I, Leech M, Memelink J, Barceló A, Sottomayor M (2008) Molecular cloning and characterization of a vacuolar class III peroxidase involved in the metabolism of anticancer alkaloids in Catharanthus roseus. Plant Physiol 146 403–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousin MA, Nicholls DG (1997) Synaptic vesicle recycling in cultured cerebellar granule cells: role of vesicular acidification and refilling. J Neurochem 69 1927–1935 [DOI] [PubMed] [Google Scholar]
- da Silva Conceicao A, Marty-Mazars D, Bassham DC, Sanderfoot AA, Marty F, Raikhel NV (1997) The syntaxin homolog AtPEP12p resides on a late post-Golgi compartment in plants. Plant Cell 9 571–582 [PMC free article] [PubMed] [Google Scholar]
- Degtiar VE, Scheller RH, Tsien RW (2000) Syntaxin modulation of slow inactivation of N-type calcium channels. J Neurosci 20 4355–4367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennison SM, Bowen ME, Brunger AT, Lentz BR (2006) Neuronal SNAREs do not trigger fusion between synthetic membranes but do promote PEG-mediated membrane fusion. Biophys J 90 1661–1675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dettmer J, Hong-Hermesdorf A, Stierhof YD, Schumacher K (2006) Vacuolar H+-ATPase activity is required for endocytic and secretory trafficking in Arabidopsis. Plant Cell 18 715–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhonukshe P, Baluska F, Schlicht M, Hlavacka A, Samaj J, Friml J, Gadella TWJ (2006) Endocytosis of cell surface material mediates cell plate formation during plant cytokinesis. Dev Cell 10 137–150 [DOI] [PubMed] [Google Scholar]
- Di Sansebastiano G, Paris N, Marc-Martin S, Neuhaus J (2001) Regeneration of a lytic central vacuole and of neutral peripheral vacuoles can be visualized by green fluorescent proteins targeted to either type of vacuoles. Plant Physiol 126 78–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fili O, Michaelevski I, Bledi Y, Chikvashvili D, Singer-Lahat D, Boshwitz H, Linial M, Lotan I (2001) Direct interaction of a brain voltage-gated K+ channel with syntaxin 1A: functional impact on channel gating. J Neurosci 21 1964–1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foresti O, daSilva L, Denecke J (2006) Overexpression of the Arabidopsis syntaxin PEP12/SYP21 inhibits transport from the prevacuolar compartment to the lytic vacuole in vivo. Plant Cell 18 2275–2293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frigerio L, de Virgilio M, Prada A, Faoro F, Vitale A (1998) Sorting of phaseolin to the vacuole is saturable and requires a short C-terminal peptide. Plant Cell 10 1031–1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Mata C, Gay R, Sokolovski S, Hills A, Lamattina L, Blatt MR (2003) Nitric oxide regulates K+ and Cl− channels in guard cells through a subset of abscisic acid-evoked signaling pathways. Proc Natl Acad Sci USA 100 11116–11121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geelen D, Leyman B, Batoko H, Di Sansebastiano G, Moore I, Blatt M, Di Sansabastiano G (2002) The abscisic acid-related SNARE homolog NtSyr1 contributes to secretion and growth: evidence from competition with its cytosolic domain. Plant Cell 14 387–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geldner N, Anders N, Wolters H, Keicher J, Kornberger W, Muller P, Delbarre A, Ueda T, Nakano A, Jurgens G (2003) The Arabidopsis GNOM ARF-GEF mediates endosomal recycling, auxin transport, and auxin-dependent plant growth. Cell 112 219–230 [DOI] [PubMed] [Google Scholar]
- Geldner N, Friml J, Stierhof YD, Jurgens G, Palme K (2001) Auxin transport inhibitors block PIN1 cycling and vesicle trafficking. Nature 413 425–428 [DOI] [PubMed] [Google Scholar]
- Geldner N, Hyman DL, Wang XL, Schumacher K, Chory J (2007) Endosomal signaling of plant steroid receptor kinase BRI1. Genes Dev 21 1598–1602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giritch A, Marillonnet S, Engler C, van Eldik G, Botterman J, Klimyuk V, Gleba Y (2006) Rapid high-yield expression of full-size IgG antibodies in plants coinfected with noncompeting viral vectors. Proc Natl Acad Sci USA 103 14701–14706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith MHM (1977) The polar transport of auxin. Annu Rev Plant Physiol 28 439–478 [Google Scholar]
- Heese A, Ludwig A, Jones J (2005) Rapid phosphorylation of a syntaxin during the Avr9/Cf-9-race-specific signaling pathway. Plant Physiol 138 2406–2416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heese M, Gansel X, Sticher L, Wick P, Grebe M, Granier F, Jurgens G (2001) Functional characterization of the KNOLLE-interacting t-SNARE AtSNAP33 and its role in plant cytokinesis. J Cell Biol 155 239–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetherington AM, Brownlee C (2004) The generation of Ca2+ signals in plants. Annu Rev Plant Biol 55 401–427 [DOI] [PubMed] [Google Scholar]
- Hicks GR, Rayle DL, Lomax TL (1989) The diageotropica mutant of tomato lacks high specific activity auxin binding sites. Science 245 52–54 [DOI] [PubMed] [Google Scholar]
- Honsbein A, Campanoni P, Sokolovski S, Pratelli R, Paneque-Corralles M, Johansson I, Blatt MR (2007) A plasma membrane SNARE protein is essential for potassium transport in Arabidopsis root epidermis and growth. International Plant Membrane Biology Workshop 14 173 [Google Scholar]
- Hu C, Ahmed M, Melia TJ, Sollner TH, Mayer T, Rothman JE (2003) Fusion of cells by flipped SNAREs. Science 300 1745–1749 [DOI] [PubMed] [Google Scholar]
- Hu K, Carroll J, Fedorovich S, Rickman C, Sukhodub A, Davletov B (2002) Vesicular restriction of synaptobrevin suggests a role for calcium in membrane fusion. Nature 415 646–650 [DOI] [PubMed] [Google Scholar]
- Hughson FM (1999) Membrane fusion: structure snared at last. Curr Biol 9 R49–R52 [DOI] [PubMed] [Google Scholar]
- Humeau Y, Doussau F, Grant NJ, Poulain B (2000) How botulinum and tetanus neurotoxins block neurotransmitter release. Biochimie 82 427–446 [DOI] [PubMed] [Google Scholar]
- Hunter P, Craddock C, Di Benedetto S, Roberts L, Frigerio L (2007) Fluorescent reporter proteins for the tonoplast and the vacuolar lumen identify a single vacuolar compartment in Arabidopsis cells. Plant Physiol 145 1371–1382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiki M, Klip A (2005) Minireview. Recent developments in the regulation of glucose transporter-4 traffic: new signals, locations, and partners. Endocrinology 146 5071–5078 [DOI] [PubMed] [Google Scholar]
- Jahn R, Lang T, Sudhof TC (2003) Membrane fusion. Cell 112 519–533 [DOI] [PubMed] [Google Scholar]
- Jurgens G (2004) Membrane trafficking in plants. Annu Rev Cell Dev Biol 20 481–504 [DOI] [PubMed] [Google Scholar]
- Kalde M, Nühse T, Findlay K, Peck S (2007) The syntaxin SYP132 contributes to plant resistance against bacteria and secretion of pathogenesis-related protein 1. Proc Natl Acad Sci USA 104 11850–11855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kargul J, Gansel X, Tyrrell M, Sticher L, Blatt MR (2001) Protein-binding partners of the tobacco syntaxin NtSyr1. FEBS Lett 508 253–258 [DOI] [PubMed] [Google Scholar]
- Kato T, Morita MT, Fukaki H, Yamauchi Y, Uehara M, Niihama M, Tasaka M (2002) SGR2, a phospholipase-like protein, and ZIG/SGR4, a SNARE, are involved in the shoot gravitropism of Arabidopsis. Plant Cell 14 33–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon C, Neu C, Pajonk S, Yun H, Lipka U, Humphry M, Bau S, Straus M, Kwaaitaal M, Rampelt H, et al (2008) Co-option of a default secretory pathway for plant immune responses. Nature 451 835–840 [DOI] [PubMed] [Google Scholar]
- Lee G, Kim H, Kang H, Jang M, Lee D, Lee S, Hwang I (2007) EpsinR2 interacts with clathrin, adaptor protein-3, AtVTI12, and phosphatidylinositol-3-phosphate: implications for EpsinR2 function in protein trafficking in plant cells. Plant Physiol 143 1561–1575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leshem Y, Melamed-Book N, Cagnac O, Ronen G, Nishri Y, Solomon M, Cohen G, Levine A (2006) Suppression of Arabidopsis vesicle-SNARE expression inhibited fusion of H2O2-containing vesicles with tonoplast and increased salt tolerance. Proc Natl Acad Sci USA 103 18008–18013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung YM, Kwan EP, Ng B, Kang Y, Gaisano HY (2007) SNAREing voltage-gated K+ and ATP-sensitive K+ channels: tuning beta-cell excitability with syntaxin-1A and other exocytotic proteins. Endocr Rev 28 653–663 [DOI] [PubMed] [Google Scholar]
- Leyman B, Geelen D, Quintero FJ, Blatt MR (1999) A tobacco syntaxin with a role in hormonal control of guard cell ion channels. Science 283 537–540 [DOI] [PubMed] [Google Scholar]
- Linial M (2001) SNARE proteins: from membranes to genomes. Curr Genomics 2 337–347 [Google Scholar]
- Maranda B, Brown D, Bourgoin S, Casanova JE, Vinay P, Ausiello DA, Marshansky V (2001) Intra-endosomal pH-sensitive recruitment of the Arf-nucleotide exchange factor ARNO and Arf6 from cytoplasm to proximal tubule endosomes. J Biol Chem 276 18540–18550 [DOI] [PubMed] [Google Scholar]
- Matsuoka K, Bassham DC, Raikhel NV, Nakamura K (1995) Different sensitivity to wortmannin of two vacuolar sorting signals indicates the presence of distinct sorting machineries in tobacco cells. J Cell Biol 130 1307–1318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazel A, Leshem Y, Tiwari B, Levine A (2004) Induction of salt and osmotic stress tolerance by overexpression of an intracellular vesicle trafficking protein AtRab7 (AtRabG3e). Plant Physiol 134 118–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNew JA, Parlati F, Fukuda R, Johnston RJ, Paz K, Paumet F, Sollner TH, Rothman JE (2000) Compartmental specificity of cellular membrane fusion encoded in SNARE proteins. Nature 407 153–159 [DOI] [PubMed] [Google Scholar]
- Misura KMS, Scheller RH, Weis WI (2000) Three-dimensional structure of the neuronal-Sec1-syntaxin 1a complex. Nature 404 355–362 [DOI] [PubMed] [Google Scholar]
- Mo B, Tse Y, Jiang L (2006) Plant prevacuolar/endosomal compartments. Int Rev Cytol 253 95–129 [DOI] [PubMed] [Google Scholar]
- Muday GK, Peer WA, Murphy AS (2003) Vesicular cycling mechanisms that control auxin transport polarity. Trends Plant Sci 8 301–304 [DOI] [PubMed] [Google Scholar]
- Muller I, Wagner W, Volker A, Schellmann S, Nacry P, Kuttner F, Schwarz-Sommer Z, Mayer U, Jurgens G (2003) Syntaxin specificity of cytokinesis in Arabidopsis. Nat Cell Biol 5 531–534 [DOI] [PubMed] [Google Scholar]
- Noble R (1990) The discovery of the vinca alkaloids: chemotherapeutic agents against cancer. Biochem Cell Biol 68 1344–1351 [PubMed] [Google Scholar]
- Nühse T, Boller T, Peck S (2003) A plasma membrane syntaxin is phosphorylated in response to the bacterial elicitor flagellin. J Biol Chem 278 45248–45254 [DOI] [PubMed] [Google Scholar]
- Obrdlik P, El Bakkoury M, Hamacher T, Cappellaro C, Vilarino C, Fleischer C, Ellerbrok H, Kamuzinzi R, Ledent V, Blaudez D, et al (2004) K+ channel interactions detected by a genetic system optimized for systematic studies of membrane protein interactions. Proc Natl Acad Sci USA 101 12242–12247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtomo I, Ueda H, Shimada T, Nishiyama C, Komoto Y, Hara-Nishimura I, Takahashi T (2005) Identification of an allele of VAM3/SYP22 that confers a semi-dwarf phenotype in Arabidopsis thaliana. Plant Cell Physiol 46 1358–1365 [DOI] [PubMed] [Google Scholar]
- Olbrich A, Hillmer S, Hinz G, Oliviusson P, Robinson D (2007) Newly formed vacuoles in root meristems of barley and pea seedlings have characteristics of both protein storage and lytic vacuoles. Plant Physiol 145 1383–1394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oufattole M, Park JH, Poxleitner M, Jiang LW, Rogers JC (2005) Selective membrane protein internalization accompanies movement from the endoplasmic reticulum to the protein storage vacuole pathway in Arabidopsis. Plant Cell 17 3066–3080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paciorek T, Zazimalova E, Ruthardt N, Petrasek J, Stierhof YD, Kleine-Vehn J, Morris DA, Emans N, Jurgens G, Geldner N, et al (2005) Auxin inhibits endocytosis and promotes its own efflux from cells. Nature 435 1251–1256 [DOI] [PubMed] [Google Scholar]
- Palokangas H, Ying M, Vaananen K, Saraste J (1998) Retrograde transport from the pre-Golgi intermediate compartment and the Golgi complex is affected by the vacuolar H+-ATPase inhibitor bafilomycin A1. Mol Biol Cell 9 3561–3578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris N, Stanley C, Jones R, Rogers J (1996) Plant cells contain two functionally distinct vacuolar compartments. Cell 85 563–572 [DOI] [PubMed] [Google Scholar]
- Park M, Lee D, Lee G, Hwang I (2005) AtRMR1 functions as a cargo receptor for protein trafficking to the protein storage vacuole. J Cell Biol 170 757–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paumet F, Rahimian V, Rothman JE (2004) The specificity of SNARE-dependent fusion is encoded in the SNARE motif. Proc Natl Acad Sci USA 101 3376–3380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng ZY, Weyers JDB (1994) Stomatal sensitivity to abscisic acid following water-deficit stress. J Exp Bot 45 835–845 [Google Scholar]
- Petrasek J, Cerna A, Schwarzerova K, Elckner M, Morris DA, Zazimalova E (2003) Do phytotropins inhibit auxin efflux by impairing vesicle traffic? Plant Physiol 131 254–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petruccelli S, Otegui M, Lareu F, Tran Dinh O, Fitchette A, Circosta A, Rumbo M, Bardor M, Carcamo R, Gomord V, et al (2006) A KDEL-tagged monoclonal antibody is efficiently retained in the endoplasmic reticulum in leaves, but is both partially secreted and sorted to protein storage vacuoles in seeds. Plant Biotechnol J 4 511–527 [DOI] [PubMed] [Google Scholar]
- Pickard BG (1985) Role of hormones in phototropism. In RP Pharis, DM Reid, eds, Encyclopedia of Plant Physiology, New Series, Vol 11: Hormonal Regulation of Development III. Springer-Verlag, Heidelberg, pp 365–417
- Pratelli R, Sutter JU, Blatt MR (2004) A new catch to the SNARE. Trends Plant Sci 9 187–195 [DOI] [PubMed] [Google Scholar]
- Rand RP, Parsegian VA (1989) Hydration forces between phospholipid bilayers. Biochim Biophys Acta 988 351–376 [Google Scholar]
- Reichardt L, Stierhof YD, Mayer U, Richter S, Schwarz H, Schumacher K, Jurgens G (2007) Plant cytokinesis requires de novo secretory trafficking but not endocytosis. Curr Biol 17 2047–2053 [DOI] [PubMed] [Google Scholar]
- Rettig J, Sheng ZH, Kim DK, Hodson CD, Snutch TP, Catterall WA (1996) Isoform-specific interaction of the alpha(1a) subunits of brain Ca2+ channels with the presynaptic proteins syntaxin and SNAP-25. Proc Natl Acad Sci USA 93 7363–7368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickman C, Hu K, Carroll J, Davletov B (2005) Self-assembly of SNARE fusion proteins into star-shaped oligomers. Biochem J 388 75–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robatzek S, Chinchilla D, Boller T (2006) Ligand-induced endocytosis of the pattern recognition receptor FLS2 in Arabidopsis. Genes Dev 20 537–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert S, Bichet A, Grandjean O, Kierzkowski D, Satiat-Jeunemaitre B, Pelletier S, Hauser MT, Hofte H, Vernhettes S (2005) An Arabidopsis endo-1,4-beta-D-glucanase involved in cellulose synthesis undergoes regulated intracellular cycling. Plant Cell 17 3378–3389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojo E, Zouhar J, Kovaleva V, Hong S, Raikhel N (2003) The AtC-VPS protein complex is localized to the tonoplast and the prevacuolar compartment in Arabidopsis. Mol Biol Cell 14 361–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russinovaa E, Borst JW, Kwaaitaal M, Cano-Delgado A, Yin YH, Chory J, de Vries SC (2004) Heterodimerization and endocytosis of Arabidopsis brassinosteroid receptors BRI1 and AtSERK3 (BAK1). Plant Cell 16 3216–3229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito C, Morita M, Kato T, Tasaka M (2005) Amyloplasts and vacuolar membrane dynamics in the living graviperceptive cell of the Arabidopsis inflorescence stem. Plant Cell 17 548–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaba T, Stein A, Jahn R, Neher E (2005) Distinct kinetic changes in neurotransmitter release after SNARE protein cleavage. Science 309 491–494 [DOI] [PubMed] [Google Scholar]
- Sanderfoot A (2007) Increases in the number of SNARE genes parallels the rise of multicellularity among the green plants. Plant Physiol 144 6–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderfoot A, Kovaleva V, Zheng H, Raikhel N (1999) The t-SNARE AtVAM3p resides on the prevacuolar compartment in Arabidopsis root cells. Plant Physiol 121 929–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderfoot A, Pilgrim M, Adam L, Raikhel N (2001. a) Disruption of individual members of Arabidopsis syntaxin gene families indicates each has essential functions. Plant Cell 13 659–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderfoot AA, Kovaleva V, Bassham DC, Raikhel NV (2001. b) Interactions between syntaxins identify at least five SNARE complexes within the Golgi/prevacuolar system of the Arabidopsis cell. Mol Biol Cell 12 3733–3743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanmartin M, Ordonez A, Sohn EJ, Robert S, Sanchez-Serrano JJ, Surpin MA, Raikhel NV, Rojo E (2007) Divergent functions of VTI12 and VTI11 in trafficking to storage and lytic vacuoles in Arabidopsis. Proc Natl Acad Sci USA 104 3645–3650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M, Nakamura N, Ohsumi Y, Kouchi H, Kondo M, Hara-Nishimura I, Nishimura M, Wada Y (1997) The AtVAM3 encodes a syntaxin-related molecule implicated in the vacuolar assembly in Arabidopsis thaliana. J Biol Chem 272 24530–24535 [DOI] [PubMed] [Google Scholar]
- Scales SJ, Chen YA, Yoo BY, Patel SM, Doung YC, Scheller RH (2000) SNAREs contribute to the specificity of membrane fusion. Neuron 26 457–464 [DOI] [PubMed] [Google Scholar]
- Shimada T, Fuji K, Tamura K, Kondo M, Nishimura M, Hara-Nishimura I (2003) Vacuolar sorting receptor for seed storage proteins in Arabidopsis thaliana. Proc Natl Acad Sci USA 100 16095–16100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson F, Whitehead JP, James DE (2001) GLUT4: at the cross roads between membrane trafficking and signal transduction. Traffic 2 2–11 [DOI] [PubMed] [Google Scholar]
- Sokolovski S, Hills A, Gay R, Blatt MR (2008) Functional interaction of the SNARE protein NtSyp121 in Ca2+ channel gating, Ca2+ transients and ABA signalling of stomatal guard cells. Molecular Plant 1 347–358 [DOI] [PubMed] [Google Scholar]
- Song J, Lee M, Lee G, Yoo C, Hwang I (2006) Arabidopsis EPSIN1 plays an important role in vacuolar trafficking of soluble cargo proteins in plant cells via interactions with clathrin, AP-1, VTI11, and VSR1. Plant Cell 18 2258–2274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sottomayor M, Pinto MC, Salema R, DiCosmo F, Pedreoo MA, Ros Barcelo A (1996) The vacuolar localization of a basic peroxidase isoenzyme responsible for the synthesis of alpha-31,41-anhydrovinblastine in Catharanthus roseus (L.) G. Don leaves. Plant Cell Environ 19 761–767 [Google Scholar]
- Sriraman R, Bardor M, Sack M, Vaquero C, Faye L, Fischer R, Finnern R, Lerouge P (2004) Recombinant anti-hCG antibodies retained in the endoplasmic reticulum of transformed plants lack core-xylose and core-alpha(1,3)-fucose residues. Plant Biotechnol J 2 279–287 [DOI] [PubMed] [Google Scholar]
- Stanley EF, Mirotznik RR (1997) Cleavage of syntaxin prevents G-protein regulation of presynaptic calcium channels. Nature 385 340–343 [DOI] [PubMed] [Google Scholar]
- Steinmann T, Geldner N, Grebe M, Mangold S, Jackson CL, Paris S, Galweiler L, Palme K, Jurgens G (1999) Coordinated polar localization of auxin efflux carrier PIN1 by GNOM ARF GEF. Science 286 316–318 [DOI] [PubMed] [Google Scholar]
- Stewart BA, Mohtashami M, Trimble WS, Boulianne GL (2000) SNARE proteins contribute to calcium cooperativity of synaptic transmission. Proc Natl Acad Sci USA 97 13955–13960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoger E, Sack M, Nicholson L, Fischer R, Christou P (2005) Recent progress in plantibody technology. Curr Pharm Des 11 2439–2457 [DOI] [PubMed] [Google Scholar]
- Surpin M, Raikhel N (2004) Traffic jams affect plant development and signal transduction. Nat Rev Mol Cell Biol 5 100–109 [DOI] [PubMed] [Google Scholar]
- Surpin M, Zheng HJ, Morita MT, Saito C, Avila E, Blakeslee JJ, Bandyopadhyay A, Kovaleva V, Carter D, Murphy A, et al (2003) The VTI family of SNARE proteins is necessary for plant viability and mediates different protein transport pathways. Plant Cell 15 2885–2899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutter JU, Campanoni P, Blatt MR, Paneque M (2006. a) Setting SNAREs in a different wood. Traffic 7 627–638 [DOI] [PubMed] [Google Scholar]
- Sutter JU, Campanoni P, Tyrrell M, Blatt MR (2006. b) Selective mobility and sensitivity to SNAREs is exhibited by the Arabidopsis KAT1 K+ channel at the plasma membrane. Plant Cell 18 935–954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutter JU, Sieben C, Hartel A, Eisenach C, Thiel G, Blatt MR (2007) Abscisic acid triggers the endocytosis of the Arabidopsis KAT1 K+ channel and its recycling to the plasma membrane. Curr Biol 17 1396–1402 [DOI] [PubMed] [Google Scholar]
- Sutton RB, Fasshauer D, Jahn R, Brunger AT (1998) Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 angstrom resolution. Nature 395 347–353 [DOI] [PubMed] [Google Scholar]
- Takano J, Miwa K, Yuan LX, von Wiren N, Fujiwara T (2005) Endocytosis and degradation of BOR1, a boron transporter of Arabidopsis thaliana, regulated by boron availability. Proc Natl Acad Sci USA 102 12276–12281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamm LK, Crane J, Kiessling V (2003) Membrane fusion: a structural perspective on the interplay of lipids and proteins. Curr Opin Struct Biol 13 453–466 [DOI] [PubMed] [Google Scholar]
- Teh OK, Moore I (2007) An ARF-GEF acting at the Golgi and in selective endocytosis in polarized plant cells. Nature 448 493–496 [DOI] [PubMed] [Google Scholar]
- Trewavas AJ (1992) Growth substances in context: a decade of sensitivity. Biochem Soc Trans 20 102–108 [DOI] [PubMed] [Google Scholar]
- Triguero A, Cabrera G, Cremata J, Yuen C, Wheeler J, Ramírez N (2005) Plant-derived mouse IgG monoclonal antibody fused to KDEL endoplasmic reticulum-retention signal is N-glycosylated homogeneously throughout the plant with mostly high-mannose-type N-glycans. Plant Biotechnol J 3 449–457 [DOI] [PubMed] [Google Scholar]
- Tyrrell M, Campanoni P, Sutter JU, Pratelli R, Paneque-Corralles M, Blatt MR (2007) Selective targeting of plasma membrane and tonoplast traffic by inhibitory (dominant-negative) SNARE fragments. Plant J 51 1099–1115 [DOI] [PubMed] [Google Scholar]
- Ungermann C, Wickner W, Xu ZY (1999) Vacuole acidification is required for trans-SNARE pairing, LMA1 release, and homotypic fusion. Proc Natl Acad Sci USA 96 11194–11199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Droogenbroeck B, Cao J, Stadlmann J, Altmann F, Colanesi S, Hillmer S, Robinson D, Van Lerberge E, Terryn N, Van Montagu M, et al (2007) Aberrant localization and underglycosylation of highly accumulating single-chain Fv-Fc antibodies in transgenic Arabidopsis seeds. Proc Natl Acad Sci USA 104 1430–1435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verpoorte R, Memelink J (2002) Engineering secondary metabolite production in plants. Curr Opin Biotechnol 13 181–187 [DOI] [PubMed] [Google Scholar]
- Vicogne J, Vollenweider D, Smith JR, Huang P, Frohman MA, Pessin JE (2006) Asymmetric phospholipid distribution drives in vitro reconstituted SNARE-dependent membrane fusion. Proc Natl Acad Sci USA 103 14761–14766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieten A, Sauer M, Brewer PB, Friml J (2007) Molecular and cellular aspects of auxin-transport-mediated development. Trends Plant Sci 12 160–168 [DOI] [PubMed] [Google Scholar]
- Vitale A, Pedrazzini E (2005) Recombinant pharmaceuticals from plants: the plant endomembrane system as bioreactor. Mol Interv 5 216–225 [DOI] [PubMed] [Google Scholar]
- Volchuk A, Wang QH, Ewart HS, Liu Z, He LJ, Bennett MK, Klip A (1996) Syntaxin-4 in 3T3-L1 adipocytes: regulation by insulin and participation in insulin-dependent glucose transport. Mol Biol Cell 7 1075–1082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter M, Chaban C, Schutze K, Batistic O, Weckermann K, Nake C, Blazevic D, Grefen C, Schumacher K, Oecking C, et al (2004) Visualization of protein interactions in living plant cells using bimolecular fluorescence complementation. Plant J 40 428–438 [DOI] [PubMed] [Google Scholar]
- Wang D, Weaver N, Kesarwani M, Dong X (2005) Induction of protein secretory pathway is required for systemic acquired resistance. Science 308 1036–1040 [DOI] [PubMed] [Google Scholar]
- Weber T, Zemelman BV, McNew JA, Westermann B, Gmachl M, Parlati F, Sollner TH, Rothman JE (1998) SNAREpins: minimal machinery for membrane fusion. Cell 92 759–772 [DOI] [PubMed] [Google Scholar]
- Wick P, Gansel X, Oulevey C, Page V, Studer I, Dürst M, Sticher L (2003) The expression of the t-SNARE AtSNAP33 is induced by pathogens and mechanical stimulation. Plant Physiol 132 343–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D, Pessin JE (2008) Mapping of R-SNARE function at distinct intracellular GLUT4 trafficking steps in adipocytes. J Cell Biol 180 375–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano D, Sato M, Saito C, Sato MH, Morita MT, Tasaka M (2003) A SNARE complex containing SGR3/AtVAM3 and ZIG/VTI11 in gravity-sensing cells is important for Arabidopsis shoot gravitropism. Proc Natl Acad Sci USA 100 8589–8594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeuzem S, Feick P, Zimmermann P, Haase W, Kahn RA, Schulz I (1992) Intravesicular acidification correlates with binding of ADP-ribosylation factor to microsomal membranes. Proc Natl Acad Sci USA 89 6619–6623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZG, Feechan A, Pedersen C, Newman MA, Qiu JL, Olesen KL, Thordal-Christensen H (2007) A SNARE-protein has opposing functions in penetration resistance and defence signalling pathways. Plant J 49 302–312 [DOI] [PubMed] [Google Scholar]
- Zheng H, Bednarek S, Sanderfoot A, Alonso J, Ecker J, Raikhel N (2002) NPSN11 is a cell plate-associated SNARE protein that interacts with the syntaxin KNOLLE. Plant Physiol 129 530–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JH, Gong ZZ, Zhang CQ, Song CP, Damsz B, Inan G, Koiwa H, Zhu JK, Hasegawa PM, Bressan RA (2002) OSM1/SYP61: a syntaxin protein in Arabidopsis controls abscisic acid-mediated and non-abscisic acid-mediated responses to abiotic stress. Plant Cell 14 3009–3028 [DOI] [PMC free article] [PubMed] [Google Scholar]