Abstract
We previously demonstrated that rice (Oryza sativa) SECRETORY CARRIER MEMBRANE PROTEIN1 (OsSCAMP1)-yellow fluorescent protein in transgenic tobacco (Nicotiana tabacum) Bright Yellow-2 cells locates to the plasma membrane and to motile punctate structures, which represent the trans-Golgi network/early endosome and are tubular-vesicular in nature. Here, we now show that SCAMPs are diverted to the cell plate during cytokinesis dividing Bright Yellow-2 cells. As cells progress from metaphase to cytokinesis, punctate OsSCAMP1-labeled structures begin to collect in the future division plane. Together with the internalized endosomal marker FM4-64, they then become incorporated into the cell plate as it forms and expands. This was confirmed by immunogold electron microscopy. We also monitored for the Golgi apparatus and the prevacuolar compartment (PVC)/multivesicular body. Golgi stacks tend to accumulate in the vicinity of the division plane, but the signals are clearly separate to the cell plate. The situation with the PVC (labeled by green fluorescent protein-BP-80) is not so clear. Punctate BP-80 signals are seen at the advancing periphery of the cell plate, which was confirmed by immunogold electron microscopy. Specific but weak labeling was observed in the cell plate, but no evidence for a fusion of the PVC/multivesicular body with the cell plate could be obtained. Our data, therefore, support the notion that cell plate formation is mainly a secretory process involving mass incorporation of domains of the trans-Golgi network/early endosome membrane. We regard the involvement of multivesicular late endosomes in this process to be equivocal.
Cytokinesis normally takes place immediately after the separation of daughter nuclei, except in special cases like the endosperm, where cellularization follows numerous nuclear divisions (Brown and Lemmon, 2007). In higher plants, cytokinesis involves the formation of a cell plate through the fusion of vesicles at its centrifugally expanding periphery (Jürgens, 2005; Hong and Verma, 2008). It is generally considered to be a different cell cycle event from that occurring in mammals, although this notion has recently been challenged (Dhonukshe et al., 2007). Cytokinesis is a complex of several interconnecting processes, including a positioning machinery that determines the plane of division, regulatory factors involved in the formation and movement of the phragmoplast, and membrane trafficking events bringing membrane to and from the developing cell plate (Reichardt et al., 2007; Walker et al., 2007). In tobacco (Nicotiana tabacum) Bright Yellow-2 (BY-2) cells, over 20 genes are switched on during cytokinesis (Yu et al., 2007), including those encoding kinesins (Lee et al., 2007), dynamin-type GTPases (Konopka et al., 2006), and a number of specific SNARE molecules such as KNOLLE, KEULE, and SNAP33 (Waizenegger et al., 2000; Assaad et al., 2001; Heese et al., 2001).
A current debate exists regarding the relative contributions of the endocytic and secretory pathways to cell plate formation. The conventional view was that the cell plate arose as a consequence of anterograde post-Golgi vesicle transport (Bednarek and Falbel, 2002; Jürgens, 2005). However, a number of observations suggest that there might also be a significant contribution from endosomal compartments. The evidence for this is both direct and indirect in nature. First, the endocytosis tracer FM4-64 accumulates in the cell plate, as do cell wall polysaccharides that are apparently internalized and recycled (Baluska et al., 2005; Dhonukshe et al., 2006). Second, the Rab GTPase RAB-F2b (Ara7), which is a prevacuolar compartment (PVC) marker (Lee et al., 2004; Ueda et al., 2004; Otegui et al., 2006), has been shown to be present at the cell plate during cytokinesis (Dhonukshe et al., 2006). Third, Arabidopsis (Arabidopsis thaliana) elch mutants are defective in cytokinesis, and the ELCH gene encodes VPS23, a component of the ESCRTI complex, which is supposed to locate to multivesicular bodies (MVBs; Spitzer et al., 2006). Thus, it has been proposed that cell plate growth is achieved by the fusion of MVB and PVC (Baluska et al., 2006). Two recent studies, however, have challenged this idea. Reichardt et al. (2007) have shown that wortmannin, which targets the MVB/PVC (Tse et al., 2004; Lam et al., 2007a, 2007b), does not inhibit cytokinesis, whereas the V-ATPase inhibitor concanamycin A, which blocks trafficking at the trans-Golgi network (TGN), severely impairs cell plate formation. In addition, both Reichardt et al. (2007) and Chow et al. (2008) were unable to detect PVC markers (BP-80 and RAB-F2A/2B) at the cell plate; on the other hand, Rab-A2/A3, which in interphase cells normally locates to a TGN-early endosomal compartment, was detected at the cell plate. Interestingly, this contrasts with the V-ATPase subunit (VHA-1a), which also locates to the TGN-early endosomal compartment (Dettmer et al., 2006) but is not incorporated into the cell plate (Reichardt et al., 2007).
We previously used the rice (Oryza sativa) SECRETORY CARRIER MEMBRANE PROTEIN1 (OsSCAMP1) as a probe to study plant endocytosis (Lam et al., 2007a). In interphase transgenic tobacco BY-2 cells expressing the OsSCAMP1-yellow fluorescent protein (YFP) fusion construct, the fluorescent OsSCAMP1 was found to locate to the plasma membrane (PM) and a punctate cytosolic organelle. This was subsequently identified by immunogold electron microscopy (EM) with SCAMP1 and GFP antibodies as a tubular-vesicular structure resembling the TGN or a partially coated reticulum (Lam et al., 2007a). These TGN structures may also serve as an early endosome, because the internalized endosomal marker FM4-64 reached these SCAMP1-positive TGN organelles prior to PVC/MVB in uptake studies in BY-2 cells (Lam et al., 2007a), a result supporting similar findings in Arabidopsis root cells (Dettmer et al., 2006).
Here, we have continued our studies on the expression of OsSCAMP1-YFP in tobacco BY-2 cells. We show that during cytokinesis, SCAMP1 is dramatically concentrated at the cell plate. In contrast, the situation with the PVC markers BP-80 or vacuolar sorting receptors (VSRs) is ambiguous: although VSRs were detected at low levels in the cell plate by immunogold EM, a clear-cut labeling of the cell plate by immunofluorescence was not achieved. This indicates that during cytokinesis, the normal PM recycling function of the TGN is altered to channel biosynthetic cargo as well as cycling molecules such as OsSCAMP1 to the developing cell plate. Since SCAMPs are highly conserved among various plants (Lam et al., 2007a), this conclusion may represent a general mechanism for cell plate formation in plants.
RESULTS
Rice SCAMP1 Accumulates in the Developing Cell Plate
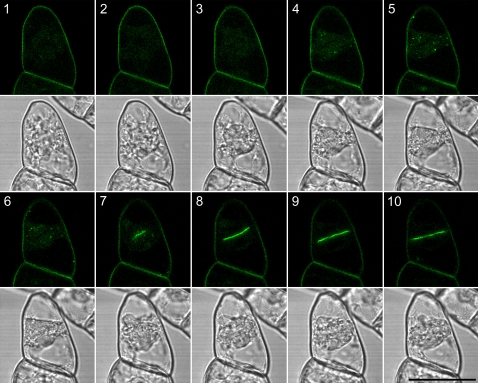
In addition to PM and TGN localization, we also noted that OsSCAMP1-YFP fluorescent signals were highly concentrated on the cell plate of dividing transgenic BY-2 cells. To study the dynamics of OsSCAMP1 during cytokinesis, therefore, we followed the distribution of fluorescent signals in a single dividing BY-2 cell expressing OsSCAMP1-YFP. Figure 1 depicts such a cell expressing OsSCAMP1-YFP as it progresses through mitosis and into cytokinesis. It clearly demonstrates the gradual collection of OsSCAMP1-YFP punctate structures into the region between the two nuclei (Fig. 1, panels 1–7). The signal then coalesces into a single thick line corresponding to the cell plate (Fig. 1, panels 8–10). These results indicated that OsSCAMP1-YFP highlights the cell plate formation during cytokinesis in transgenic BY-2 cells.
Figure 1.
Dynamics of OsSCAMP1-YFP in cell plate formation during cytokinesis in transgenic BY-2 cells. Shown is a continuous series of time-lapse confocal images of OsSCAMP1-YFP signals collected from a single transgenic tobacco BY-2 cell during cell plate formation. Bar = 50 μm. [See online article for color version of this figure.]
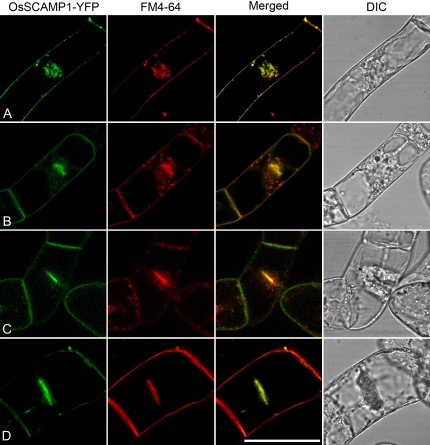
Since the internalized endosomal marker FM4-64 has been shown to accumulate in the cell plate during cytokinesis of various plant cells (Chow et al., 2008), we next carried out a FM4-64 uptake to study the relationship between the internalized FM4-64 and OsSCAMP1-YFP in transgenic BY-2 cells during cytokinesis. As shown in Figure 2, the accumulation of OsSCAMP1 fluorescence in the division plane is mirrored by internalized FM4-64 (Fig. 2A). This colocalization in the cell plate continues as the cell plate expands, although outside of the plate there are numerous noncolocalizing punctate FM4-64 structures (Fig. 2B). We interpret these as being late endosomes/PVCs, as reported previously (Tse et al., 2004; Lam et al., 2007a). As cytokinesis proceeds, the non-cell-plate FM4-64 punctate signals decrease markedly (Fig. 2, compare B with C and D).
Figure 2.
OsSCAMP1-YFP largely colocalizes with internalized endosomal marker FM4-64 in the cell plate during cytokinesis in transgenic BY-2 cells. Tobacco BY-2 cells expressing OsSCAMP1-YFP were allowed to take up FM4-64 for 30 min, followed by confocal image collection on cells in various stages of cytokinesis from A to D. DIC, Differential interference contrast. Bar = 50 μm.
Monitoring Golgi Stacks and the PVC during Cell Plate Formation
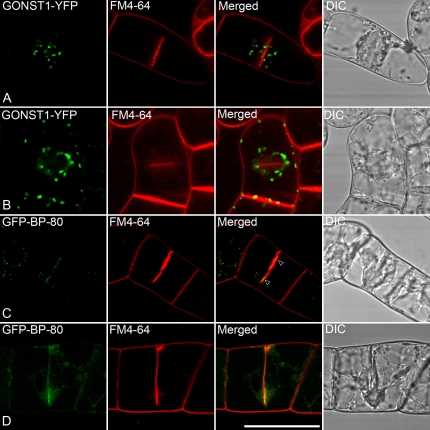
The Golgi apparatus in BY-2 cells can be visualized in a transgenic cell line stably expressing GONST1-YFP (Tse et al., 2004; Lam et al., 2007a), a nucleotide sugar transporter present in late Golgi compartments (Wee et al., 1998). As seen in Figure 3, some Golgi stacks are still present in the cortical cytoplasm, but the majority collect at the surface of the phragmoplast and do not enter it (Fig. 3, A and B). This confirms the observations of Nebenführ et al. (1999). In BY-2 cells, the multivesiculate PVC is characterized by high concentrations of VSRs (e.g. BP-80; Li et al., 2002; Tse et al., 2004; Miao et al., 2006). Using a stable transgenic cell line expressing GFP-BP-80, we visualized the position of the PVC during cytokinesis. At first glance, it appeared as if the PVC collects at the growing periphery of the cell plate (Fig. 3, C and D). This gives the impression that the PVCs are being lined up prior to fusion. Indeed, there does seem to be a colocalization with the younger portions of the cell plate (Fig. 3C, arrowheads). However, a closer examination shows that the fluorescent signals of GFP-BP-80 are still punctate, which would not be the case if a fusion had occurred. Therefore, we maintain that, in agreement with the data of Vermeer et al. (2006), who used a chimeric GFP-2XFYVE construct to label the late endosome/PVC in dividing BY-2 cells, while PVCs are closely associated with the developing cell plate they do not fuse with it.
Figure 3.
GONST1-YFP and GFP-BP-80 are largely separated from the internalized endosomal marker FM4-64 during cell plate formation in transgenic BY-2 cells. Tobacco BY-2 cells expressing either the Golgi marker GONST1-YFP (A and B) or the PVC marker GFP-BP-80 (C and D) were allowed to take up FM4-64 under conditions identical to those used for OsSCAMP1-YFP cells, followed by confocal image collection on cells at various stages of cytokinesis. Bar = 50 μm.
SCAMP Immunogold EM of Cell Plate Formation
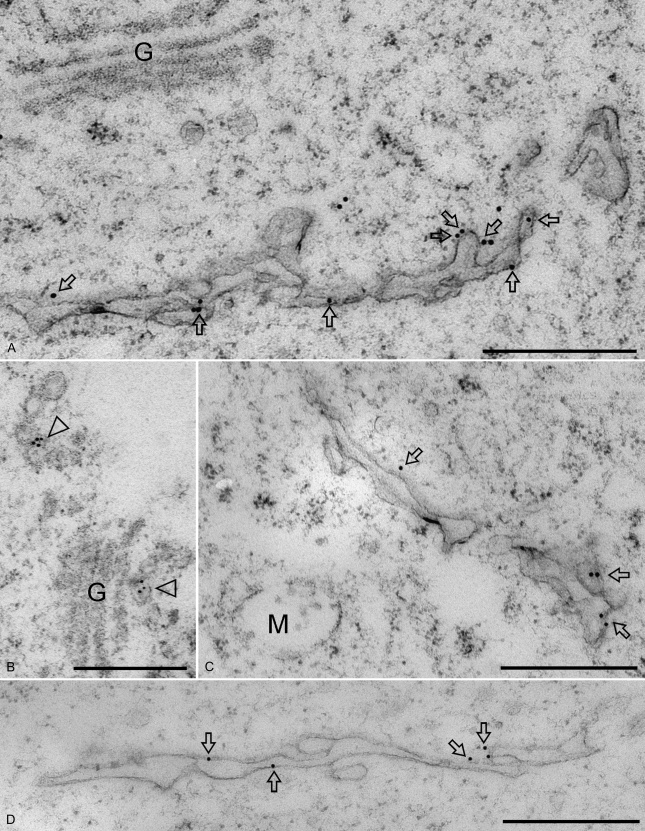
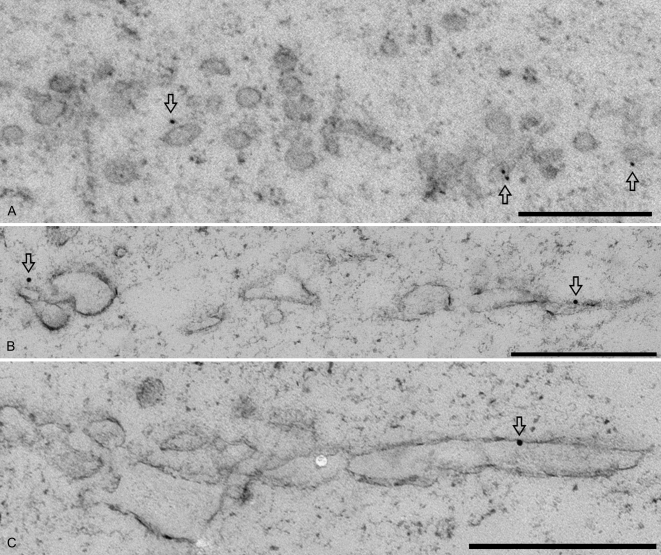
To morphologically identify the SCAMP1-positive cell plates in relation to PVC/MVB during cytokinesis, using both GFP antibodies and SCAMP1 antibodies (Lam et al., 2007a), we performed immunogold EM on ultrathin sections of high-pressure frozen/freeze-substituted samples of synchronized BY-2 cells expressing OsSCAMP1-YFP as well as synchronized wild-type BY-2 cells. As shown in Figure 4, developing cell plates labeled positively for SCAMPs in both the transgenic cells (Fig. 4, A and C, arrows) and wild-type cells (Fig. 4D). Label was also seen over vesicle clusters at and near the trans face of Golgi stacks (Fig. 4B, arrowheads). In contrast, the multivesicular PVC that was seen near the cell plate was not labeled (Fig. 4C, M). Similarly, Golgi stacks were not labeled (Fig. 4, A and B, G).
Figure 4.
Immunogold EM of SCAMP1 during cell plate formation in transgenic and wild-type BY-2 cells. Ultrathin sections prepared from high-pressure frozen/freeze-substituted synchronized transgenic (A–C) or wild type (D) BY-2 cells were labeled with either anti-GFP antibodies to detect OsSCAMP1-YFP (A–C) or anti-SCAMP1 antibodies to detect the endogenous SCAMP1 proteins (D). Arrows point to gold particles labeling the cell plates; arrowheads indicate examples of TGN labeling or TGN vesicle labeling. G, Golgi apparatus; M, MVB. Bars = 500 nm.
To find out whether elements of the PVC/MVB are incorporated into the cell plate during BY-2 cytokinesis, we performed immunogold EM labeling with VSRat-1 antibodies (Tse et al., 2004) on ultrathin sections of high-pressure frozen/freeze-substituted samples of synchronized wild-type BY-2 cells. As shown in Figure 5, cell plates were specifically labeled for VSRs, albeit at a much lower density than that of SCAMPs (compare Fig. 4). Interestingly, very little labeling of VSRs on PVC/MVB was observed in dividing BY-2 cells with clear cell plates (data not shown), indicating a possible redistribution of VSRs from PVC/MVB into the TGN or cell plate during cytokinesis. Direct evidence for the incorporation of MVB/PVC into the developing cell plate was not obtained, indicating that the presence of VSR on the cell plate might be due to vesicle transport rather than PVC fusion with the cell plate in BY-2 cells.
Figure 5.
Immunogold EM of VSRs during cell plate formation in BY-2 cells. Ultrathin sections prepared from high-pressure frozen/freeze-substituted synchronized wild-type BY-2 cells were labeled with anti-VSRat-1 antibodies to detect the endogenous tobacco VSR proteins. Arrows point to gold particles labeling the cell plates. Bars = 500 nm.
DISCUSSION
SCAMPs are characteristic of the membranes of the secretory granules in exocrine glands of mammalian cells (Castle and Castle, 2005). With four transmembrane domains and cytoplasmically located C and N termini, they belong to the large family of tetraspan vesicle membrane proteins (Hübner et al., 2002). Recently, a plant SCAMP ortholog, OsSCAMP1, was expressed in tobacco BY-2 cells and localized both to the PM and to mobile structures in the cytoplasm, which were identified as tubular-vesicular clusters that were associated with, but distinct from, the Golgi apparatus (Lam et al., 2007a, 2007b). These structures were enriched in V-ATPase and were clathrin positive and rapidly received internalized FM4-64 from the PM, pointing to their identity as a TGN-early endosomal compartment, as described previously for Arabidopsis root cells (Dettmer et al., 2006).
In interphase, it seems that OsSCAMP1 cycles between the PM and the TGN/early endosome, but during the later stages of mitosis in BY-2 cells, it begins to collect in increasing amounts in the division plane and then gets incorporated into the developing cell plate. Based on the relative signal strengths of the YFP signals, it accumulates to much higher extents in the cell plate than in the PM during interphase. This suggests both a redirection of membrane trafficking away from the PM toward the cell plate and a shift in the proportion of anterograde to retrograde post-Golgi membrane transport. Our results do not allow us to distinguish between an increase in secretory vesicle transport and the mass incorporation of TGN/early endosomes into the growing cell plate. However, a consideration of previously published data involving brefeldin A (BFA) on BY-2 cells (see below) suggests that the latter possibility is the more likely scenario.
In BY-2 cells, BFA at a concentration of 5 to 10 μg mL−1 causes the formation of endoplasmic reticulum-Golgi hybrids as intermediate structures on the way to absorption of the Golgi membranes into the endoplasmic reticulum (Ritzenthaler et al., 2002). However, a limited amount of grouped Golgi stacks with aggregated TGN were also observed (see figures 5B and 12 in Ritzenthaler et al., 2002). In contrast, at higher concentrations (50–100 μg mL−1), a situation is obtained that resembles that seen in roots of Arabidopsis, with partially deformed Golgi stacks surrounding a cloud of vesicles (Tse et al., 2006). In Arabidopsis, these vesicles are in part derived from the TGN/early endosome (see update by Robinson et al. [2008] in this volume). On the other hand, Yasuhara et al. (1995) showed that when BFA is applied at metaphase to synchronized BY-2 cells, the Golgi apparatus disassembles, yet a cell plate is initiated but does not go to completion. This suggests that this abortive cell plate is formed through the fusion of a BFA-insensitive Golgi remnant, which from the foregoing is most likely to have been derived from the TGN/early endosome. Nevertheless, based on the observation that the TGN marker VHA-a1-ATPase is absent from the developing cell plate (Reichardt et al., 2007), an en bloc incorporation of the TGN/early endosome into the cell plate seems unlikely. It would seem that only specific domains of the TGN are used for this.
Our results also provide new information on the current debate regarding whether MVB/PVC contributes to cell plate growth. A standard marker for the MVB/PVC in BY-2 cells is the VSR BP-80 from pea (Pisum sativum; Li et al., 2002; Tse et al., 2004; Miao et al., 2006). Although punctate GFP-BP-80 signals collect in the plane of cell division, and therefore are suggestive of being located at the cell plate, the fuzzy appearance is more like the situation seen with GFP-2xFYVE, a biosensor for phosphatidylinositol 3-phosphate (Vermeer et al., 2006). GFP-2xFYVE colocalizes with the PVC marker RABF2b, and the micrographs of Vermeer et al. (2006) clearly show that the labeled structures accumulate around the developing cell plate but do not fuse with it. Using confocal fluorescence microscopy, Chow et al. (2008) did not detect BP-80 on the growing cell plate in Arabidopsis roots. However, with immunogold EM, which has a higher degree of resolution, we have been able to detect low levels of VSRs in the growing cell plate of BY-2 cells. Although this observation suggests a contribution of the MVB/PVC to its development, we have no evidence for a direct fusion. The presence of VSRs on the cell plate might be due to direct vesicle transport from the PVC/MVB, but it could also reflect a redistribution of VSRs from the PVC/MVB to TGN/early endosome followed by their incorporation into the cell plate together with SCAMPs. Considering the difference in immunogold labeling densities for SCAMPs and VSRs, we feel that a contribution of the PVC/MVB to cell plate formation is small, if it occurs at all.
In addition, even though this study used a rice SCAMP1 and its YFP fusion to probe the cell plate formation in transgenic BY-2 cells, we feel that the results obtained are likely to represent the situation for the native tobacco SCAMPs in BY-2 cells, because the expressed OsSCAMP1-YFP fusion was shown to colocalize fully with the endogenous tobacco SCAMPs in transgenic tobacco BY-2 cells (Lam et al., 2007a). Given the highly similar sequences within the SCAMP and VSR family proteins in various plants, including Arabidopsis, rice, and tobacco (Paris and Neuhaus, 2002; Lam et al., 2007a), the dynamics of OsSCAMP-YFP incorporation into the cell plate observed in transgenic tobacco BY-2 cells may reflect a general picture of SCAMP-marked cell plate formation during cytokinesis in plants.
MATERIALS AND METHODS
General methods for the construction and characterization of recombinant plasmids, the maintenance of suspension cultured tobacco (Nicotiana tabacum) BY-2 cells, and the preparation and characterization of antibodies have been described previously (Tse et al., 2004; Lam et al., 2007a).
Culturing and Synchronization of Tobacco BY-2 Cells
Tobacco BY-2 suspension cells were grown in Murashige and Skoog (MS) medium and subcultured every 7 d. Cells were synchronized by the techniques described by Samuels et al. (1995). Briefly, 5 mL of 5- to 8-d-old cell suspension was transferred into 95 mL of MS medium containing 5 mg mL−1 aphidicolin and cultured for 24 h in a shaker set at 125 rpm. Cells were collected and washed four times with 250 mL of medium for 15 min each to remove the aphidicolin. After the washes, the cell suspension was cultured in medium for 3 h and followed by the addition of 6 mm pronamide for an additional 6 h of incubation. Treated cells were then collected and washed three times with 250 mL of MS medium for 10 min each. Within 90 min after washing, cells would enter the telophase.
FM4-64 Uptake Studies
Transgenic tobacco BY-2 cell lines expressing various reporters were used in the FM4-64 uptake studies. The suspension cultured BY-2 cells were first washed with MS liquid medium, followed by the addition of FM4-64 (from a 12 μm stock and diluted to working solution with MS liquid medium just prior to use) to 500 μL of cultured cells to reach the final concentration for 10 min. The FM4-64-treated cells were then washed with MS medium several times and transferred onto a slide with medium for time course observation and image collection using a 60× objective oil lens in a Bio-Rad Radiance 2100 system. The filter sets were used as follows: for YFP, excitation wavelength of 514 nm, dichroic mirror 560DCLPXR, and emission filter HQ545/40; for FM4-64, excitation wavelength of 543 nm and emission filter HQ660LP. Images were processed using Adobe Photoshop software as described previously (Jiang and Rogers, 1998).
Antibodies
The production and characterization of polyclonal antibodies specific for VSRat-1 and OsSCAMP1 have been described previously (Tse et al., 2004; Lam et al., 2007a). GFP antibodies were purchased from Molecular Probes or generated using recombinant GFP purchased from Molecular Probes as antigens to inject rabbits at the animal facilities of the Chinese University of Hong Kong. GFP antibodies were affinity purified using a cyanogen bromide-Sepharose (Sigma) column conjugated with recombinant GFP.
EM of Resin-Embedded Cells
The general procedures for transmission EM sample preparation and thin sectioning of samples of BY-2 cells were performed essentially as described previously (Tse et al., 2004; Lam et al., 2007a). For high-pressure freezing, BY-2 cells were harvested by filtering and immediately frozen in a high-pressure freezing apparatus (EMP2; Leica). For subsequent freeze substitution, the frozen samples were first kept at −85°C for 60 h, then gradually warmed up to −35°C over 18 h. Substitution was performed in an AFS freeze substitution unit (Leica). The substitution medium (dry acetone) was supplemented with 0.1% (w/v) uranyl acetate. When the samples reached −35°C, the medium was replaced with 100% ethanol, which was again changed to fresh 100% ethanol 10 min later. The cells were then infiltrated stepwise with HM20 at −20°C, embedded, and UV polymerized. Immunogold labeling on HM20 sections was done using standard procedures as described previously (Hohl et al., 1996; Tse et al., 2004; Lam et al., 2007a), with SCAMP1 or GFP antibodies at 40 to 80 μg mL−1 and gold-coupled secondary antibodies at 1:50. Aqueous uranyl acetate/lead citrate poststained sections were examined for image collections with a Hitachi H-7650 transmission electron microscope with a CCD camera (Hitachi High Technologies) operating at 80 kV.
Sequence data for the rice SCAMP1 cDNA can be found in the GenBank/EMBL data libraries under accession number gi 7332504.
Supplemental Data
The following material is available in the online version of this article.
Supplemental Movie S1. Dynamic changes in OsSCAMP1-YFP distribution during cell plate formation in a transgenic BY-2 cell expressing OsSCAMP1-YFP.
Supplementary Material
Acknowledgments
We thank Dr. Gynheung An (Pohang University of Science and Technology, Korea) for sharing the rice SCAMP1 cDNA used in our original collaborative research.
This work was supported by grants from the Research Grants Council of Hong Kong (grant nos. CUHK4307/03M, CUHK4580/05M, and CUHK488707), UGC-AoE, Chinese University of Hong Kong Scheme C, National Science Foundation of China (grant no. 30529001), and the National 863 Program of China (grant no. 2007AA02Z102) to L.J. and from the Deutsche Forschungsgemeinschaft to D.G.R.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Liwen Jiang (ljiang@cuhk.edu.hk).
Some figures in this article are displayed in color online but in black and white in the print edition.
The online version of this article contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- Assaad FF, Huet Y, Mayer U, Jurgens G (2001) The cytokinesis gene KEULE encodes a Sec1 protein that binds the syntaxin KNOLLE. J Cell Biol 152 531–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baluska F, Liners F, Hlavacka A, Schlicht M, Van Cutsem P, McCurdy DW, Menzel D (2005) Cell wall pectins and xyloglucans are internalized into dividing root cells and accumulate within cell plates during cytokinesis. Protoplasma 225 141–155 [DOI] [PubMed] [Google Scholar]
- Baluska F, Menzel D, Barlow PW (2006) Cytokinesis in plant and animal cells: endosomes ‘shut the door’. Dev Biol 294 1–10 [DOI] [PubMed] [Google Scholar]
- Bednarek SY, Falbel TG (2002) Membrane trafficking during plant cytokinesis. Traffic 3 621–629 [DOI] [PubMed] [Google Scholar]
- Brown RC, Lemmon BE (2007) The developmental biology of cereal endosperm. In O-A Olsen, ed, Endosperm: Developmental and Molecular Biology. Plant Cell Monographs, Vol 8. Springer-Verlag, Heidelberg, pp 1–20
- Castle A, Castle D (2005) Ubiquitously expressed secretory carrier membrane proteins (SCAMPs) 1-4 mark different pathways and exhibit limited constitutive trafficking to and from the cell surface. J Cell Sci 118 3769–3780 [DOI] [PubMed] [Google Scholar]
- Chow CM, Neto H, Foucart C, Moore I (2008) Rab-A2 and Rab-A3 GTPases define a trans-Golgi endosomal membrane domain in Arabidopsis that contributes substantially to the cell plate. Plant Cell 20 101–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dettmer J, Hong-Hermesdorf A, Stierhof YD, Schumacher K (2006) Vacuolar H+-ATPase activity is required for endocytic and secretory trafficking in Arabidopsis. Plant Cell 18 715–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhonukshe P, Baluska F, Schlicht M, Hlavacka A, Samaj J, Friml J, Gadella TW Jr (2006) Endocytosis of cell surface material mediates cell plate formation during plant cytokinesis. Dev Cell 10 137–150 [DOI] [PubMed] [Google Scholar]
- Dhonukshe P, Samaj J, Baluska F, Friml J (2007) A unifying new model of cytokinesis for the dividing plant and animal cells. Bioessays 29 371–381 [DOI] [PubMed] [Google Scholar]
- Heese M, Gansel X, Sticher L, Wick P, Grebe M, Granier F, Jurgens G (2001) Functional characterization of the KNOLLE-interacting t-SNARE AtSNAP33 and its role in plant cytokinesis. J Cell Biol 155 239–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohl I, Robinson DG, Chrispeels MJ, Hinz G (1996) Transport of storage proteins to the vacuole is mediated by vesicles without a clathrin coat. J Cell Sci 109 2539–2550 [DOI] [PubMed] [Google Scholar]
- Hong Z, Verma DPS (2008) Molecular analysis of the cell plate forming machinery. In DPS Verma, Z Hong, eds, Cell Division Control in Plants. Plant Cell Monographs, Vol 9. Springer-Verlag, Heidelberg, pp 303–320
- Hübner K, Windoffer R, Hutter H, Leube RE (2002) Tetraspan vesicle membrane proteins: synthesis, subcellular localization, and functional properties. Int Rev Cytol 214 103–159 [DOI] [PubMed] [Google Scholar]
- Jiang L, Rogers JC (1998) Integral membrane protein sorting to vacuoles in plant cells: evidence for two pathways. J Cell Biol 143 1183–1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jürgens G (2005) Cytokinesis in higher plants. Annu Rev Plant Biol 56 281–299 [DOI] [PubMed] [Google Scholar]
- Konopka CA, Schleede JB, Skop AR, Bednarek SY (2006) Dynamin and cytokinesis. Traffic 7 239–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam SK, Siu CL, Hillmer S, Jang S, An G, Robinson DG, Jiang L (2007. a) Rice SCAMP1 defines clathrin-coated, trans-Golgi-located tubular-vesicular structures as an early endosome in tobacco BY-2 cells. Plant Cell 19 296–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam SK, Tse YC, Robinson DG, Jiang L (2007. b) Tracking down the elusive early endosome. Trends Plant Sci 12 497–505 [DOI] [PubMed] [Google Scholar]
- Lee GJ, Sohn EJ, Lee MH, Hwang I (2004) The Arabidopsis rab5 homologs rha1 and ara7 localize to the prevacuolar compartment. Plant Cell Physiol 45 1211–1220 [DOI] [PubMed] [Google Scholar]
- Lee YR, Li Y, Liu B (2007) Two Arabidopsis phragmoplast-associated kinesins play a critical role in cytokinesis during male gametogenesis. Plant Cell 19 2595–2605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YB, Rogers SW, Tse YC, Lo SW, Sun SS, Jauh GY, Jiang L (2002) BP-80 and homologs are concentrated on post-Golgi, probable lytic prevacuolar compartments. Plant Cell Physiol 43 726–742 [DOI] [PubMed] [Google Scholar]
- Miao Y, Yan PK, Kim H, Hwang I, Jiang L (2006) Localization of green fluorescent protein fusions with the seven Arabidopsis vacuolar sorting receptors to prevacuolar compartments in tobacco BY-2 cells. Plant Physiol 142 945–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebenführ A, Gallagher LA, Dunahay TG, Frohlick JA, Mazurkiewicz AM, Meehl JB, Staehelin LA (1999) Stop-and-go movements of plant Golgi stacks are mediated by the acto-myosin system. Plant Physiol 121 1127–1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otegui MS, Herder R, Schulze J, Jung R, Staehelin LA (2006) The proteolytic processing of seed storage proteins in Arabidopsis embryo cells starts in the multivesicular bodies. Plant Cell 18 2567–2581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris N, Neuhaus JM (2002) BP-80 as a vacuolar sorting receptor. Plant Mol Biol 50 903–914 [DOI] [PubMed] [Google Scholar]
- Reichardt I, Stierhof YD, Mayer U, Richter S, Schwarz H, Schumacher K, Jurgens G (2007) Plant cytokinesis requires de novo secretory trafficking but not endocytosis. Curr Biol 17 2047–2053 [DOI] [PubMed] [Google Scholar]
- Ritzenthaler C, Nebenfuhr A, Movafeghi A, Stussi-Garaud C, Behnia L, Pimpl P, Staehelin LA, Robinson DG (2002) Reevaluation of the effects of brefeldin A on plant cells using tobacco Bright Yellow 2 cells expressing Golgi-targeted green fluorescent protein and COPI antisera. Plant Cell 14 237–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DG, Jiang L, Schumacher K (2008) The endosomal system of plants: charting new and familiar territories. Plant Physiol 147 1482–1492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels AL, Giddings TH Jr, Staehelin LA (1995) Cytokinesis in tobacco BY-2 and root tip cells: a new model of cell plate formation in higher plants. J Cell Biol 130 1345–1357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer C, Schellmann S, Sabovljevic A, Shahriari M, Keshavaiah C, Bechtold N, Herzog M, Muller S, Hanisch FG, Hulskamp M (2006) The Arabidopsis elch mutant reveals functions of an ESCRT component in cytokinesis. Development 133 4679–4689 [DOI] [PubMed] [Google Scholar]
- Tse YC, Lo SW, Hillmer S, Dupree P, Jiang L (2006) Dynamic response of prevacuolar compartments to brefeldin A in plant cells. Plant Physiol 142 1442–1459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse YC, Mo B, Hillmer S, Zhao M, Lo SW, Robinson DG, Jiang L (2004) Identification of multivesicular bodies as prevacuolar compartments in Nicotiana tabacum BY-2 cells. Plant Cell 16 672–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda T, Uemura T, Sato MH, Nakano A (2004) Functional differentiation of endosomes in Arabidopsis cells. Plant J 40 783–789 [DOI] [PubMed] [Google Scholar]
- Vermeer JE, van Leeuwen W, Tobena-Santamaria R, Laxalt AM, Jones DR, Divecha N, Gadella TW Jr, Munnik T (2006) Visualization of PtdIns3P dynamics in living plant cells. Plant J 47 687–700 [DOI] [PubMed] [Google Scholar]
- Waizenegger I, Lukowitz W, Assaad F, Schwarz H, Jurgens G, Mayer U (2000) The Arabidopsis KNOLLE and KEULE genes interact to promote vesicle fusion during cytokinesis. Curr Biol 10 1371–1374 [DOI] [PubMed] [Google Scholar]
- Walker KL, Muller S, Moss D, Ehrhardt DW, Smith LG (2007) Arabidopsis TANGLED identifies the division plane throughout mitosis and cytokinesis. Curr Biol 17 1827–1836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee EG, Sherrier DJ, Prime TA, Dupree P (1998) Targeting of active sialyltransferase to the plant Golgi apparatus. Plant Cell 10 1759–1768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuhara H, Sonobe S, Shibaoka H (1995) Effects of brefeldin A on the formation of the cell plate in tobacco BY-2 cells. Eur J Cell Biol 66 274–281 [PubMed] [Google Scholar]
- Yu Y, Wang HY, Liu LN, Chen ZL, Xia GX (2007) Functional identification of cytokinesis-related genes from tobacco BY-2 cells. Plant Cell Rep 26 889–894 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.