Abstract
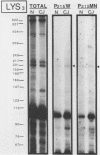
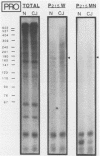
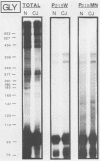
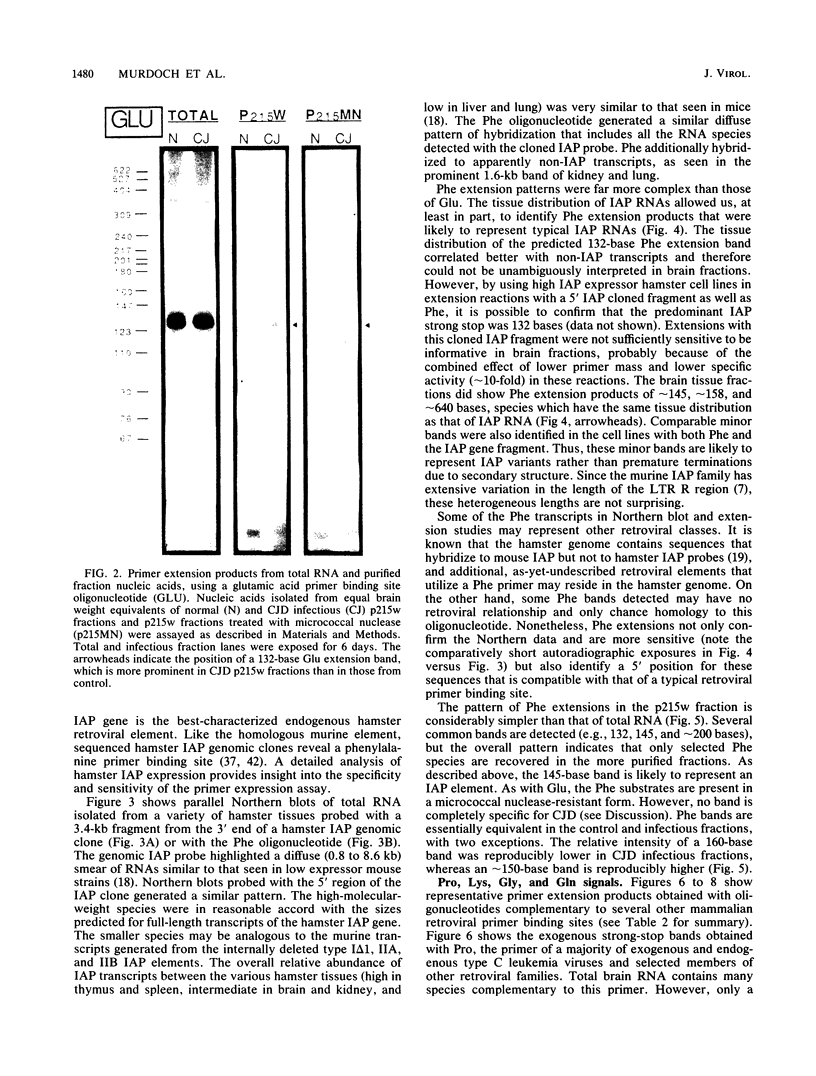
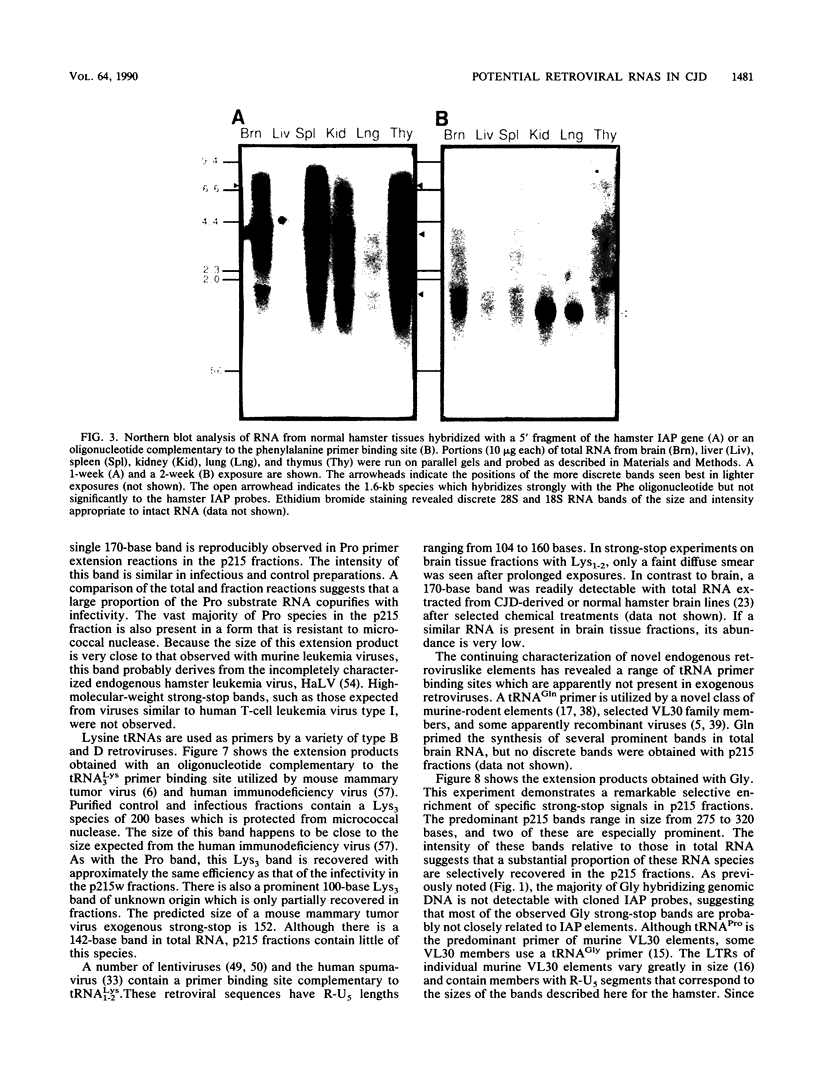
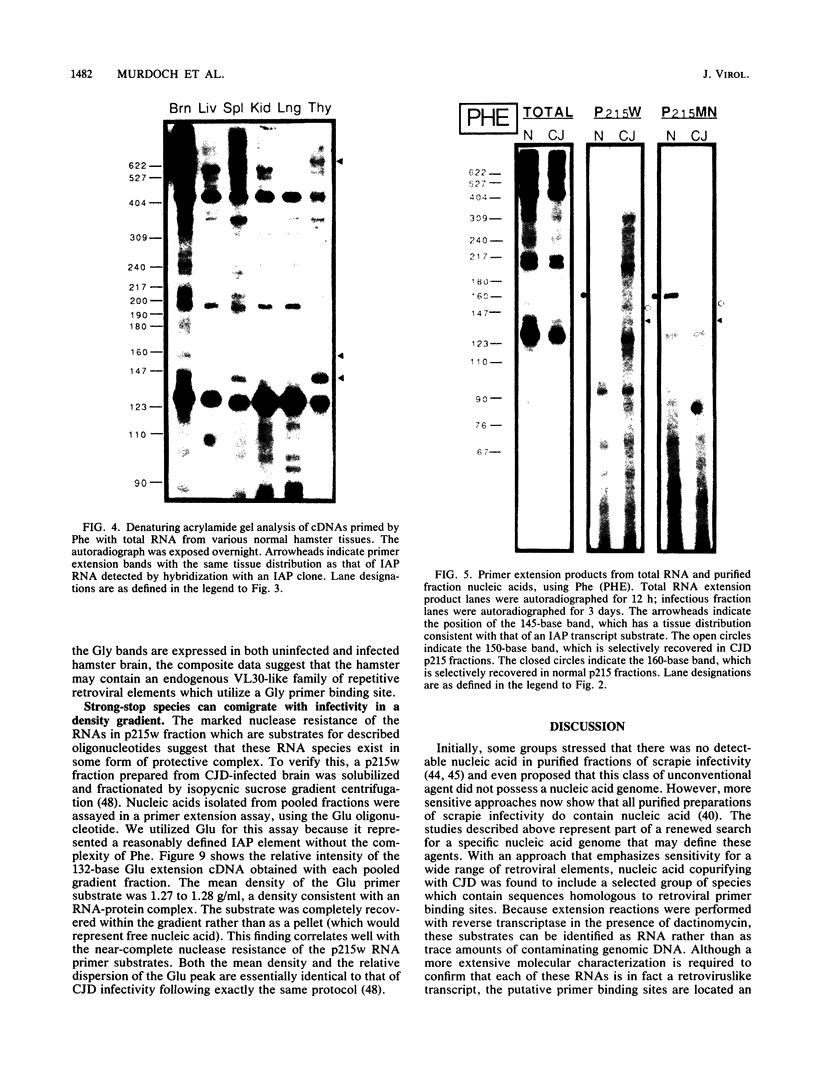
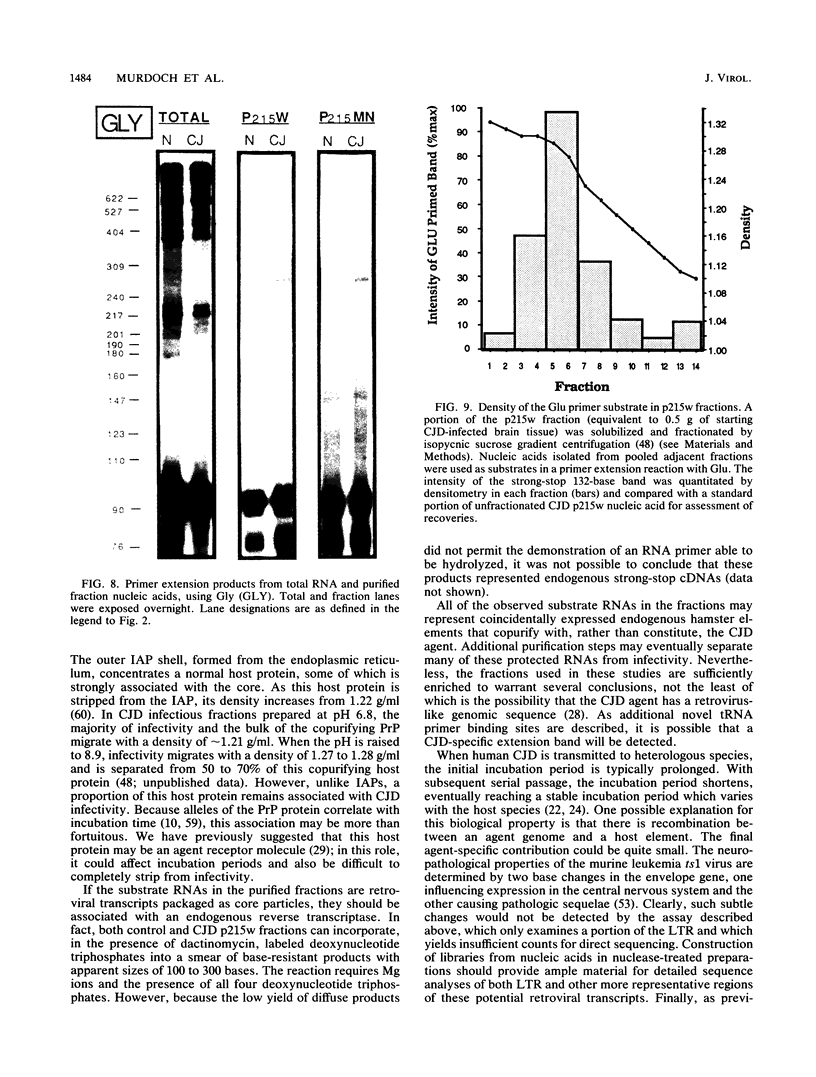
The molecular nature of the related infectious agents that cause Creutzfeldt-Jakob disease (CJD) and scrapie is poorly understood, and an agent-specific nucleic acid genome has not yet been identified. Several biological manifestations of these agents resemble those seen in retrovirus-induced diseases. We therefore attempted to identify an agent-specific retrovirus-like RNA transcript in CJD infectious fractions. A series of synthetic oligonucleotides complementary to known mammalian retroviral primer binding sites were used in a primer extension assay. Substrate nucleic acids isolated from partially purified hamster brain CJD infectious fractions and from parallel normal brain fractions were compared with total starting brain RNA. This sensitive exogenous strong-stop reaction revealed that CJD infectious fractions contained a series of potential retroviral RNAs including apparent transcripts of endogenous hamster IAP genes. Most transcripts selectively recovered in the fractions were substantially protected from micrococcal nuclease digestion, and at least one substrate RNA, consistent with an intracisternal A particle, was packaged in a form that had the same buoyant density as CJD infectivity. Although a completely CJD-specific transcript was not identified, the copurification of potential retroviral transcripts with CJD infectivity suggests that models of disease involving retrovirus-like nucleic acid elements deserve further consideration.
Full text
PDF

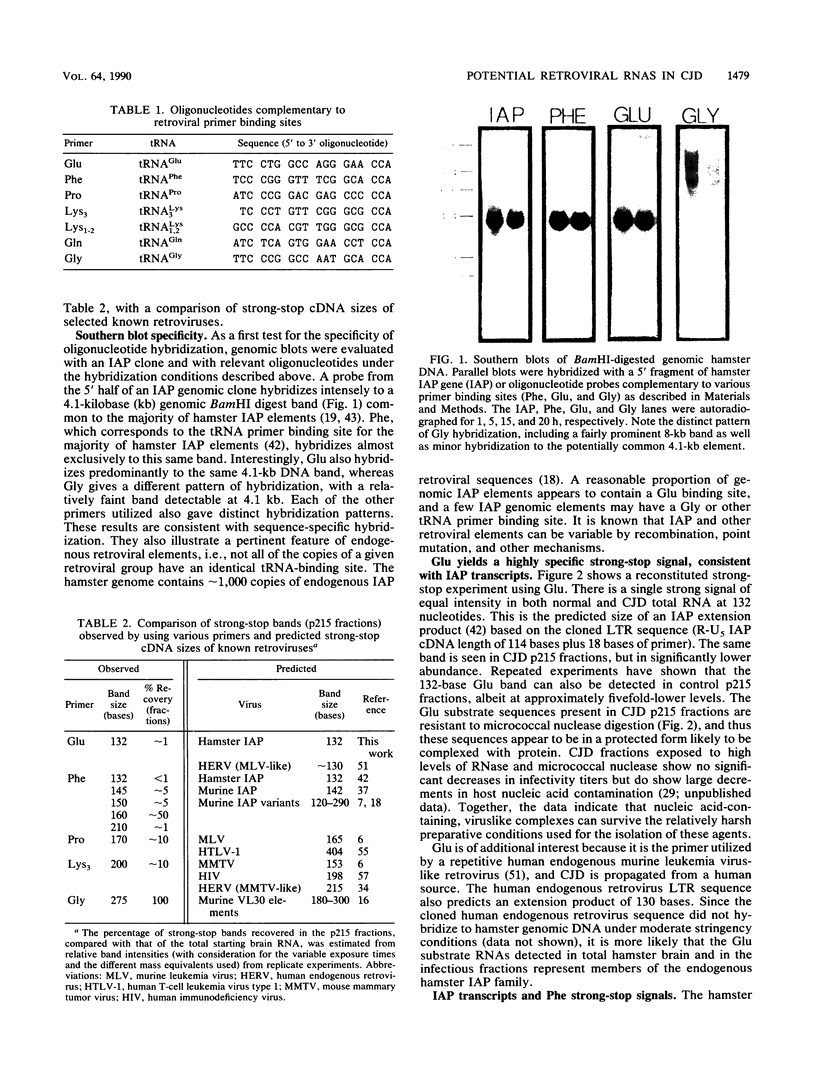



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiken J. M., Williamson J. L., Marsh R. F. Evidence of mitochondrial involvement in scrapie infection. J Virol. 1989 Apr;63(4):1686–1694. doi: 10.1128/jvi.63.4.1686-1694.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker H. F., Ridley R. M., Crow T. J. Experimental transmission of an autosomal dominant spongiform encephalopathy: does the infectious agent originate in the human genome? Br Med J (Clin Res Ed) 1985 Aug 3;291(6491):299–302. doi: 10.1136/bmj.291.6491.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellinger-Kawahara C., Cleaver J. E., Diener T. O., Prusiner S. B. Purified scrapie prions resist inactivation by UV irradiation. J Virol. 1987 Jan;61(1):159–166. doi: 10.1128/jvi.61.1.159-166.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellinger-Kawahara C., Diener T. O., McKinley M. P., Groth D. F., Smith D. R., Prusiner S. B. Purified scrapie prions resist inactivation by procedures that hydrolyze, modify, or shear nucleic acids. Virology. 1987 Sep;160(1):271–274. doi: 10.1016/0042-6822(87)90072-9. [DOI] [PubMed] [Google Scholar]
- Bruce M. E., Dickinson A. G. Biological evidence that scrapie agent has an independent genome. J Gen Virol. 1987 Jan;68(Pt 1):79–89. doi: 10.1099/0022-1317-68-1-79. [DOI] [PubMed] [Google Scholar]
- Chen H. R., Barker W. C. Nucleotide sequences of the retroviral long terminal repeats and their adjacent regions. Nucleic Acids Res. 1984 Feb 24;12(4):1767–1778. doi: 10.1093/nar/12.4.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christy R. J., Brown A. R., Gourlie B. B., Huang R. C. Nucleotide sequences of murine intracisternal A-particle gene LTRs have extensive variability within the R region. Nucleic Acids Res. 1985 Jan 11;13(1):289–302. doi: 10.1093/nar/13.1.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colicelli J., Goff S. P. Isolation of a recombinant murine leukemia virus utilizing a new primer tRNA. J Virol. 1986 Jan;57(1):37–45. doi: 10.1128/jvi.57.1.37-45.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czub M., Braig H. R., Diringer H. Replication of the scrapie agent in hamsters infected intracerebrally confirms the pathogenesis of an amyloid-inducing virosis. J Gen Virol. 1988 Jul;69(Pt 7):1753–1756. doi: 10.1099/0022-1317-69-7-1753. [DOI] [PubMed] [Google Scholar]
- Dickinson A. G., Outram G. W. Genetic aspects of unconventional virus infections: the basis of the virino hypothesis. Ciba Found Symp. 1988;135:63–83. doi: 10.1002/9780470513613.ch5. [DOI] [PubMed] [Google Scholar]
- Gardner M. B. Retroviral spongiform polioencephalomyelopathy. Rev Infect Dis. 1985 Jan-Feb;7(1):99–110. doi: 10.1093/clinids/7.1.99. [DOI] [PubMed] [Google Scholar]
- Haseltine W. A., Kleid D. G. A method for classification of 5' termini of retroviruses. Nature. 1978 Jun 1;273(5661):358–364. doi: 10.1038/273358a0. [DOI] [PubMed] [Google Scholar]
- Hoffman P. M., Pitts O. M., Rohwer R. G., Gajdusek D. C., Ruscetti S. K. Retrovirus antigens in brains of mice with scrapie- and murine leukemia virus-induced spongiform encephalopathy. Infect Immun. 1982 Oct;38(1):396–398. doi: 10.1128/iai.38.1.396-398.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houghton M., Stewart A. G., Doel S. M., Emtage J. S., Eaton M. A., Smith J. C., Patel T. P., Lewis H. M., Porter A. G., Birch J. R. The amino-terminal sequence of human fibroblast interferon as deduced from reverse transcripts obtained using synthetic oligonucleotide primers. Nucleic Acids Res. 1980 May 10;8(9):1913–1931. doi: 10.1093/nar/8.9.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itin A., Keshet E. A novel retroviruslike family in mouse DNA. J Virol. 1986 Aug;59(2):301–307. doi: 10.1128/jvi.59.2.301-307.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itin A., Keshet E. Diverse long terminal repeats are associated with murine retroviruslike (VL30) elements. Mol Cell Biol. 1986 Apr;6(4):1276–1282. doi: 10.1128/mcb.6.4.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itin A., Keshet E. Primer binding sites corresponding to several tRNA species are present in DNAs of different members of the same retrovirus-like gene family (VL30). J Virol. 1985 Apr;54(1):236–239. doi: 10.1128/jvi.54.1.236-239.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuff E. L., Lueders K. K. The intracisternal A-particle gene family: structure and functional aspects. Adv Cancer Res. 1988;51:183–276. doi: 10.1016/s0065-230x(08)60223-7. [DOI] [PubMed] [Google Scholar]
- Lueders K. K., Kuff E. L. Comparison of the sequence organization of related retrovirus-like multigene families in three evolutionarily distant rodent genomes. Nucleic Acids Res. 1983 Jul 11;11(13):4391–4408. doi: 10.1093/nar/11.13.4391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lueders K. K., Mietz J. A. Structural analysis of type II variants within the mouse intracisternal A-particle sequence family. Nucleic Acids Res. 1986 Feb 11;14(3):1495–1510. doi: 10.1093/nar/14.3.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuelidis E. E., Fritch W. W., Kim J. H., Manuelidis L. Immortality of cell cultures derived from brains of mice and hamsters infected with Creutzfeldt-Jakob disease agent. Proc Natl Acad Sci U S A. 1987 Feb;84(3):871–875. doi: 10.1073/pnas.84.3.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuelidis E. E., Gorgacz E. J., Manuelidis L. Interspecies transmission of Creutzfeldt-Jakob disease to Syrian hamsters with reference to clinical syndromes and strains of agent. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3432–3436. doi: 10.1073/pnas.75.7.3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuelidis E. E., Kim J., Angelo J. N., Manuelidis L. Serial propagation of Creutzfeldt-Jakob disease in guinea pigs. Proc Natl Acad Sci U S A. 1976 Jan;73(1):223–227. doi: 10.1073/pnas.73.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuelidis E. E., Manuelidis L. Suggested links between different types of dementias: Creutzfeldt-Jakob disease, Alzheimer disease, and retroviral CNS infections. Alzheimer Dis Assoc Disord. 1989 Spring-Summer;3(1-2):100–109. doi: 10.1097/00002093-198903010-00009. [DOI] [PubMed] [Google Scholar]
- Manuelidis E. E., Rorke L. B. Transmission of Alpers' disease (chronic progressive encephalopathy) produces experimental Creutzfeldt-Jakob disease in hamsters. Neurology. 1989 May;39(5):615–621. doi: 10.1212/wnl.39.5.615. [DOI] [PubMed] [Google Scholar]
- Manuelidis L., Manuelidis E. E. Recent developments in scrapie and Creutzfeldt-Jakob disease. Prog Med Virol. 1986;33:78–98. [PubMed] [Google Scholar]
- Manuelidis L., Murdoch G., Manuelidis E. E. Potential involvement of retroviral elements in human dementias. Ciba Found Symp. 1988;135:117–134. doi: 10.1002/9780470513613.ch8. [DOI] [PubMed] [Google Scholar]
- Manuelidis L., Sklaviadis T., Manuelidis E. E. Evidence suggesting that PrP is not the infectious agent in Creutzfeldt-Jakob disease. EMBO J. 1987 Feb;6(2):341–347. doi: 10.1002/j.1460-2075.1987.tb04760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuelidis L., Tesin D. M., Sklaviadis T., Manuelidis E. E. Astrocyte gene expression in Creutzfeldt-Jakob disease. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5937–5941. doi: 10.1073/pnas.84.16.5937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuelidis L., Valley S., Manuelidis E. E. Specific proteins associated with Creutzfeldt-Jakob disease and scrapie share antigenic and carbohydrate determinants. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4263–4267. doi: 10.1073/pnas.82.12.4263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marciani D. J., Kuff E. L. Isolation and partial characterization of the internal structural proteins from murine intracisternal A particles. Biochemistry. 1973 Dec 4;12(25):5075–5083. doi: 10.1021/bi00749a008. [DOI] [PubMed] [Google Scholar]
- Maurer B., Bannert H., Darai G., Flügel R. M. Analysis of the primary structure of the long terminal repeat and the gag and pol genes of the human spumaretrovirus. J Virol. 1988 May;62(5):1590–1597. doi: 10.1128/jvi.62.5.1590-1597.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May F. E., Westley B. R. Structure of a human retroviral sequence related to mouse mammary tumor virus. J Virol. 1986 Nov;60(2):743–749. doi: 10.1128/jvi.60.2.743-749.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merz P. A., Somerville R. A., Wisniewski H. M., Iqbal K. Abnormal fibrils from scrapie-infected brain. Acta Neuropathol. 1981;54(1):63–74. doi: 10.1007/BF00691333. [DOI] [PubMed] [Google Scholar]
- Merz P. A., Somerville R. A., Wisniewski H. M., Manuelidis L., Manuelidis E. E. Scrapie-associated fibrils in Creutzfeldt-Jakob disease. Nature. 1983 Dec 1;306(5942):474–476. doi: 10.1038/306474a0. [DOI] [PubMed] [Google Scholar]
- Mietz J. A., Grossman Z., Lueders K. K., Kuff E. L. Nucleotide sequence of a complete mouse intracisternal A-particle genome: relationship to known aspects of particle assembly and function. J Virol. 1987 Oct;61(10):3020–3029. doi: 10.1128/jvi.61.10.3020-3029.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikbakht K. N., Ou C. Y., Boone L. R., Glover P. L., Yang W. K. Nucleotide sequence analysis of endogenous murine leukemia virus-related proviral clones reveals primer-binding sites for glutamine tRNA. J Virol. 1985 Jun;54(3):889–893. doi: 10.1128/jvi.54.3.889-893.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesch B., Groth D. F., Prusiner S. B., Weissmann C. Search for a scrapie-specific nucleic acid: a progress report. Ciba Found Symp. 1988;135:209–223. doi: 10.1002/9780470513613.ch14. [DOI] [PubMed] [Google Scholar]
- Oleszak E. L., Manuelidis L., Manuelidis E. E. In vitro transformation elicited by Creutzfeldt-Jakob-infected brain material. J Neuropathol Exp Neurol. 1986 Sep;45(5):489–502. doi: 10.1097/00005072-198609000-00001. [DOI] [PubMed] [Google Scholar]
- Ono M., Ohishi H. Long terminal repeat sequences of intracisternal A particle genes in the Syrian hamster genome: identification of tRNAPhe as a putative primer tRNA. Nucleic Acids Res. 1983 Oct 25;11(20):7169–7179. doi: 10.1093/nar/11.20.7169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono M., Toh H., Miyata T., Awaya T. Nucleotide sequence of the Syrian hamster intracisternal A-particle gene: close evolutionary relationship of type A particle gene to types B and D oncovirus genes. J Virol. 1985 Aug;55(2):387–394. doi: 10.1128/jvi.55.2.387-394.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusiner S. B. Novel proteinaceous infectious particles cause scrapie. Science. 1982 Apr 9;216(4542):136–144. doi: 10.1126/science.6801762. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B. Prions causing degenerative neurological diseases. Annu Rev Med. 1987;38:381–398. doi: 10.1146/annurev.me.38.020187.002121. [DOI] [PubMed] [Google Scholar]
- Quigley J. P., Rifkin D. B., Compans R. W. Isolation and characterization of ribonucleoprotein substructures from Rous sarcoma virus. Virology. 1972 Oct;50(1):65–75. doi: 10.1016/0042-6822(72)90346-7. [DOI] [PubMed] [Google Scholar]
- Repaske R., Steele P. E., O'Neill R. R., Rabson A. B., Martin M. A. Nucleotide sequence of a full-length human endogenous retroviral segment. J Virol. 1985 Jun;54(3):764–772. doi: 10.1128/jvi.54.3.764-772.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sklaviadis T. K., Manuelidis L., Manuelidis E. E. Physical properties of the Creutzfeldt-Jakob disease agent. J Virol. 1989 Mar;63(3):1212–1222. doi: 10.1128/jvi.63.3.1212-1222.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonigo P., Alizon M., Staskus K., Klatzmann D., Cole S., Danos O., Retzel E., Tiollais P., Haase A., Wain-Hobson S. Nucleotide sequence of the visna lentivirus: relationship to the AIDS virus. Cell. 1985 Aug;42(1):369–382. doi: 10.1016/s0092-8674(85)80132-x. [DOI] [PubMed] [Google Scholar]
- Sonigo P., Barker C., Hunter E., Wain-Hobson S. Nucleotide sequence of Mason-Pfizer monkey virus: an immunosuppressive D-type retrovirus. Cell. 1986 May 9;45(3):375–385. doi: 10.1016/0092-8674(86)90323-5. [DOI] [PubMed] [Google Scholar]
- Steele P. E., Rabson A. B., Bryan T., Martin M. A. Distinctive termini characterize two families of human endogenous retroviral sequences. Science. 1984 Aug 31;225(4665):943–947. doi: 10.1126/science.6089336. [DOI] [PubMed] [Google Scholar]
- Stromberg K. Surface-active agents for isolation of the core component of avian myeloblastosis virus. J Virol. 1972 Apr;9(4):684–697. doi: 10.1128/jvi.9.4.684-697.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szurek P. F., Yuen P. H., Jerzy R., Wong P. K. Identification of point mutations in the envelope gene of Moloney murine leukemia virus TB temperature-sensitive paralytogenic mutant ts1: molecular determinants for neurovirulence. J Virol. 1988 Jan;62(1):357–360. doi: 10.1128/jvi.62.1.357-360.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wain-Hobson S., Sonigo P., Danos O., Cole S., Alizon M. Nucleotide sequence of the AIDS virus, LAV. Cell. 1985 Jan;40(1):9–17. doi: 10.1016/0092-8674(85)90303-4. [DOI] [PubMed] [Google Scholar]
- Wallace R. B., Miyada C. G. Oligonucleotide probes for the screening of recombinant DNA libraries. Methods Enzymol. 1987;152:432–442. doi: 10.1016/0076-6879(87)52050-x. [DOI] [PubMed] [Google Scholar]
- Westaway D., Goodman P. A., Mirenda C. A., McKinley M. P., Carlson G. A., Prusiner S. B. Distinct prion proteins in short and long scrapie incubation period mice. Cell. 1987 Nov 20;51(4):651–662. doi: 10.1016/0092-8674(87)90134-6. [DOI] [PubMed] [Google Scholar]
- Wivel N. A., Lueders K. K., Kuff E. L. Structural organization of murine intracisternal A particles. J Virol. 1973 Feb;11(2):329–334. doi: 10.1128/jvi.11.2.329-334.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachary J. F., Knupp C. J., Wong P. K. Noninflammatory spongiform polioencephalomyelopathy caused by a neurotropic temperature-sensitive mutant of Moloney murine leukemia virus TB. Am J Pathol. 1986 Sep;124(3):457–468. [PMC free article] [PubMed] [Google Scholar]