Abstract
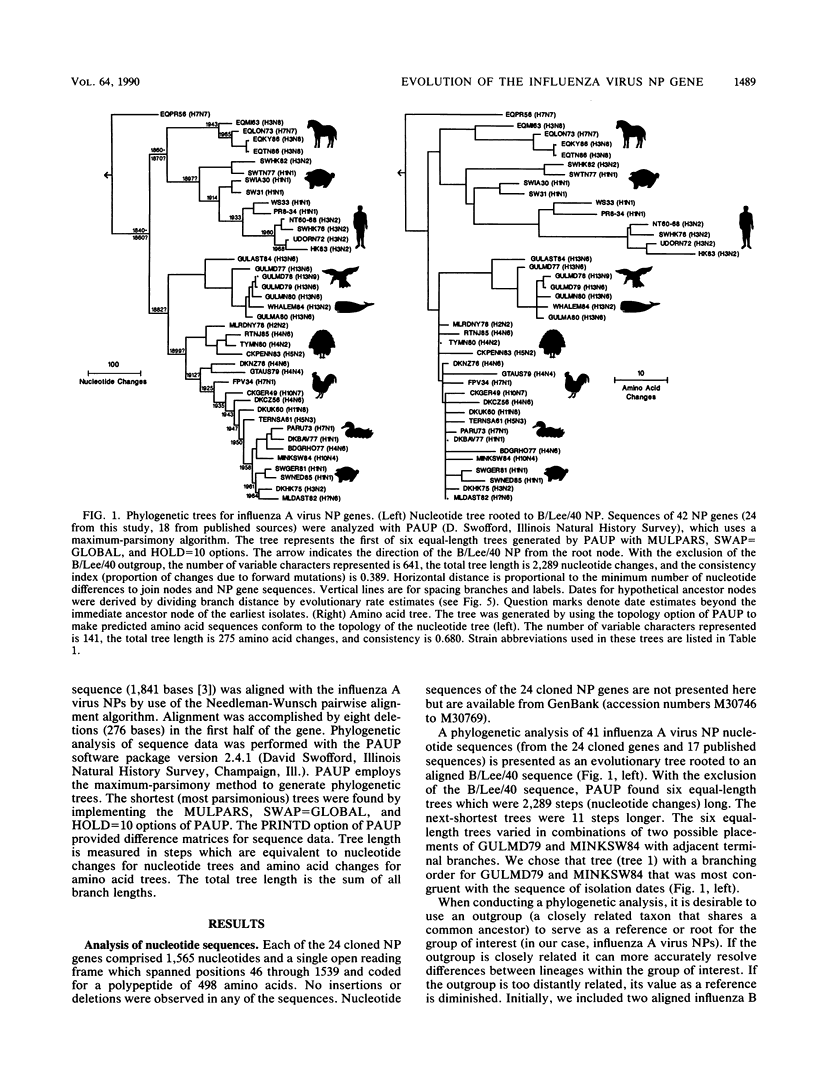
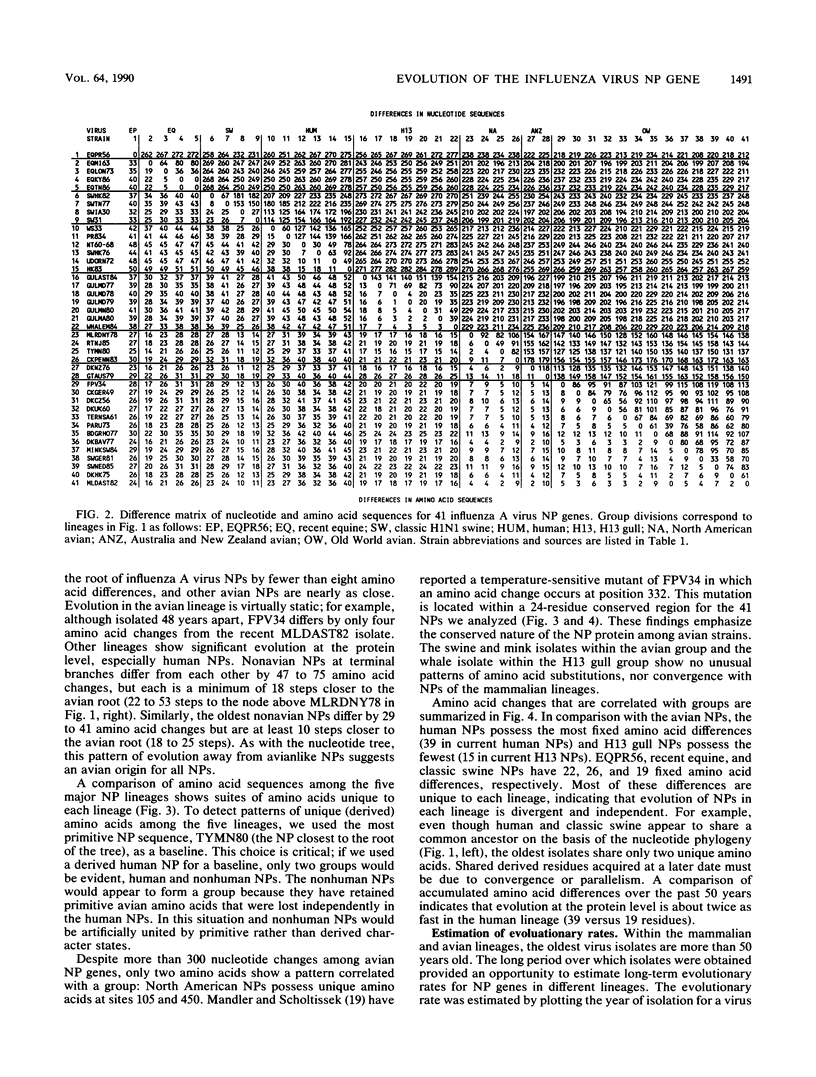
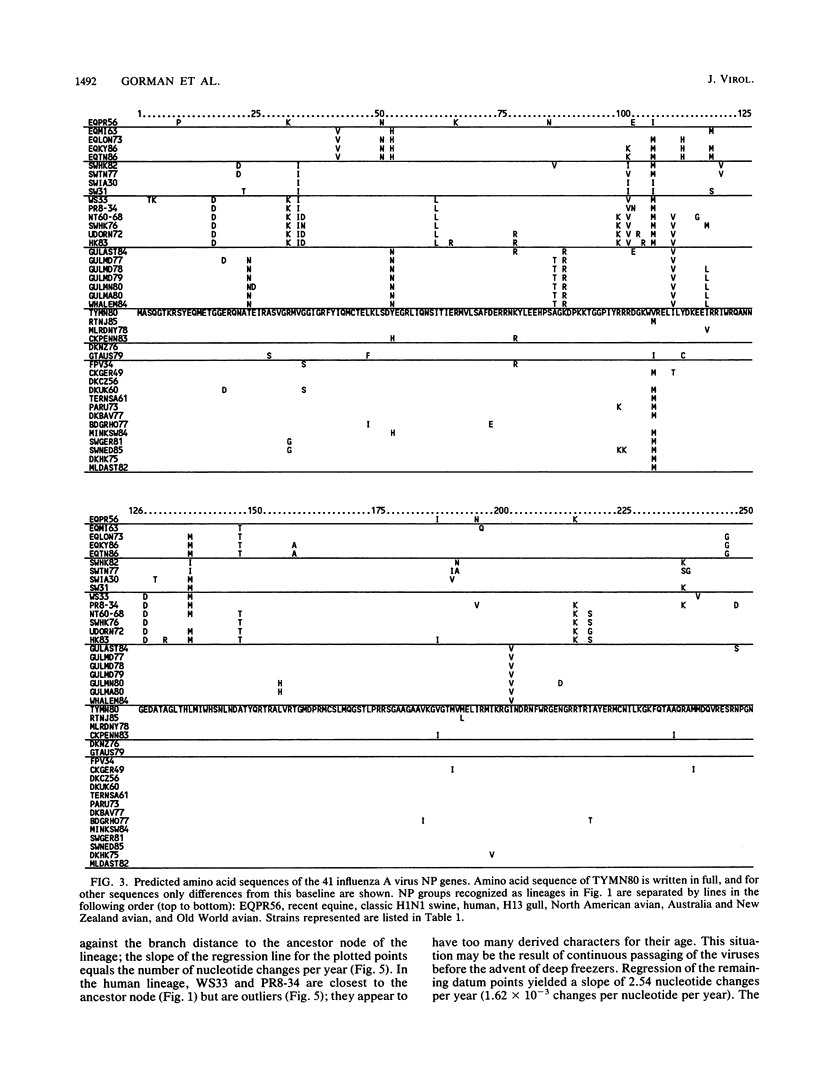
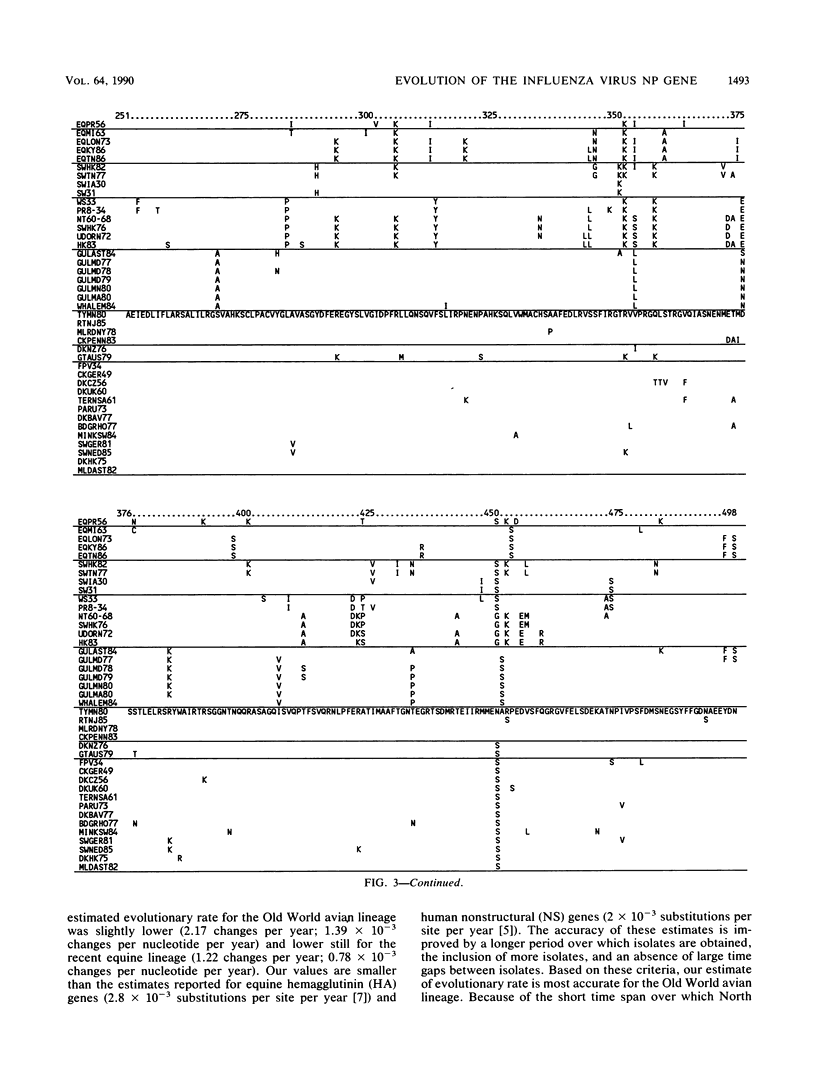
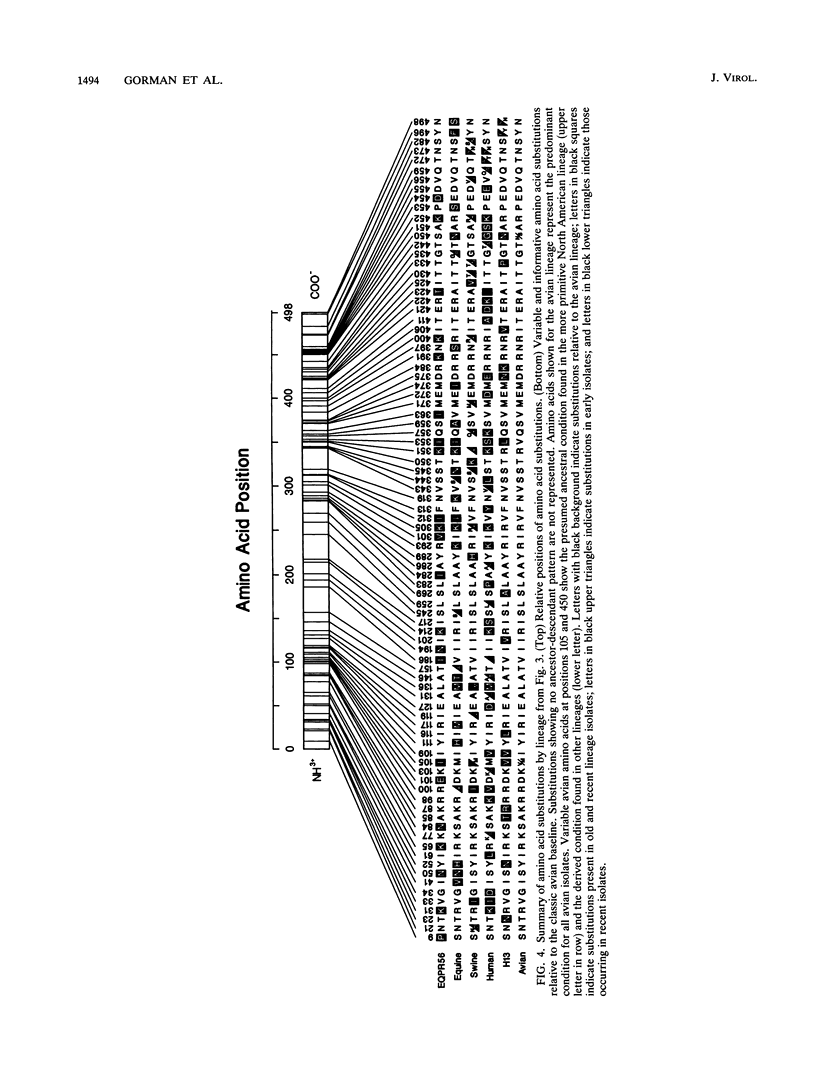
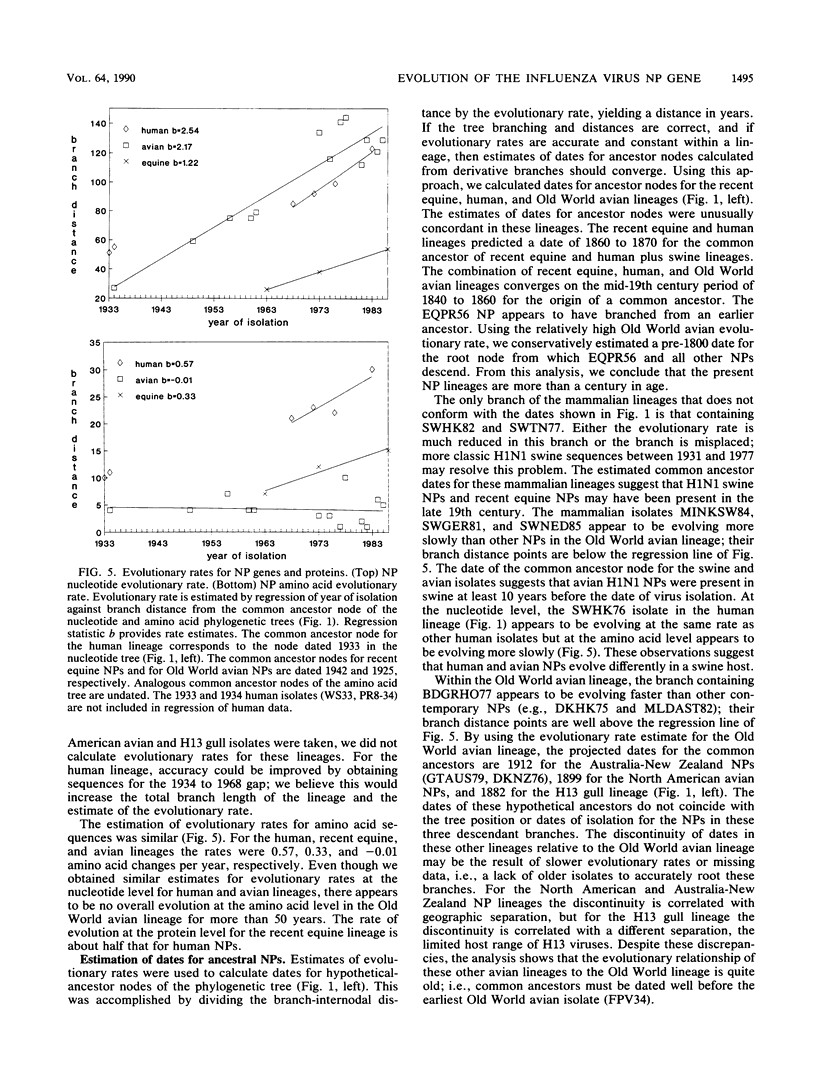
Nucleotide sequences of 24 nucleoprotein (NP) genes isolated from a wide range of hosts, geographic regions, and influenza A virus serotypes and 18 published NP gene sequences were analyzed to determine evolutionary relationships. The phylogeny of NP genes was determined by a maximum-parsimony analysis of nucleotide sequences. Phylogenetic analysis showed that NP genes have evolved into five host-specific lineages, including (i) Equine/Prague/56 (EQPR56), (ii) recent equine strains, (iii) classic swine (H1N1 swine, e.g., A/Swine/Iowa/15/30) and human strains, (iv) gull H13 viruses, and (v) avian strains (including North American, Australian, and Old World subgroups). These NP lineages match the five RNA hybridization groups identified by W. J. Bean (Virology 133:438-442, 1984). Maximum nucleotide differences among the NPs was 18.5%, but maximum amino acid differences reached only 10.8%, reflecting the conservative nature of the NP protein. Evolutionary rates varied among lineages; the human lineage showed the highest rate (2.54 nucleotide changes per year), followed by the Old World avian lineage (2.17 changes per year) and the recent equine lineage (1.22 changes per year). The per-nucleotide rates of human and avian NP gene evolution (1.62 x 10(-3) to 1.39 x 10(-3) changes per year) are lower than that reported for human NS genes (2.0 x 10(-3) changes per year; D. A. Buonagurio, S. Nakada, J. D. Parvin, M. Krystal, P. Palese, and W. M. Fitch, Science 232:980-982, 1986). Of the five NP lineages, the human lineage showed the greatest evolution at the amino acid level; over a period of 50 years, human NPs have accumulated 39 amino acid changes. In contrast, the avian lineage showed remarkable conservatism; over the same period, avian NP proteins changed by 0 to 10 amino acids. The specificity of the H13 NP in gulls and its distinct evolutionary separation from the classic avian lineage suggests that H13 NPs may have a large degree of adaptation to gulls. The presence of avian and human NPs in some swine isolates demonstrates the susceptibility of swine to different virus strains and supports the hypothesis that swine may serve as intermediates for the introduction of avian influenza virus genes into the human virus gene pool. EQPR56 is relatively distantly related to all other NP lineages, which suggests that this NP is rooted closest to the ancestor of all contemporary NPs. On the basis of estimation of evolutionary rates from nucleotide branch distances, current NP lineages are at least 100 years old, and the EQPR56 NP is much older.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bean W. J. Correlation of influenza A virus nucleoprotein genes with host species. Virology. 1984 Mar;133(2):438–442. doi: 10.1016/0042-6822(84)90410-0. [DOI] [PubMed] [Google Scholar]
- Bean W. J., Jr, Sriram G., Webster R. G. Electrophoretic analysis of iodine-labeled influenza virus RNA segments. Anal Biochem. 1980 Feb;102(1):228–232. doi: 10.1016/0003-2697(80)90343-7. [DOI] [PubMed] [Google Scholar]
- Briedis D. J., Tobin M. Influenza B virus genome: complete nucleotide sequence of the influenza B/lee/40 virus genome RNA segment 5 encoding the nucleoprotein and comparison with the B/Singapore/222/79 nucleoprotein. Virology. 1984 Mar;133(2):448–455. doi: 10.1016/0042-6822(84)90412-4. [DOI] [PubMed] [Google Scholar]
- Buckler-White A. J., Murphy B. R. Nucleotide sequence analysis of the nucleoprotein gene of an avian and a human influenza virus strain identifies two classes of nucleoproteins. Virology. 1986 Dec;155(2):345–355. doi: 10.1016/0042-6822(86)90198-4. [DOI] [PubMed] [Google Scholar]
- Buonagurio D. A., Nakada S., Parvin J. D., Krystal M., Palese P., Fitch W. M. Evolution of human influenza A viruses over 50 years: rapid, uniform rate of change in NS gene. Science. 1986 May 23;232(4753):980–982. doi: 10.1126/science.2939560. [DOI] [PubMed] [Google Scholar]
- Chen E. Y., Seeburg P. H. Supercoil sequencing: a fast and simple method for sequencing plasmid DNA. DNA. 1985 Apr;4(2):165–170. doi: 10.1089/dna.1985.4.165. [DOI] [PubMed] [Google Scholar]
- Daniels R. S., Skehel J. J., Wiley D. C. Amino acid sequences of haemagglutinins of influenza viruses of the H3 subtype isolated from horses. J Gen Virol. 1985 Mar;66(Pt 3):457–464. doi: 10.1099/0022-1317-66-3-457. [DOI] [PubMed] [Google Scholar]
- Donis R. O., Bean W. J., Kawaoka Y., Webster R. G. Distinct lineages of influenza virus H4 hemagglutinin genes in different regions of the world. Virology. 1989 Apr;169(2):408–417. doi: 10.1016/0042-6822(89)90166-9. [DOI] [PubMed] [Google Scholar]
- Gammelin M., Mandler J., Scholtissek C. Two subtypes of nucleoproteins (NP) of influenza A viruses. Virology. 1989 May;170(1):71–80. doi: 10.1016/0042-6822(89)90353-x. [DOI] [PubMed] [Google Scholar]
- Hinshaw V. S., Bean W. J., Geraci J., Fiorelli P., Early G., Webster R. G. Characterization of two influenza A viruses from a pilot whale. J Virol. 1986 May;58(2):655–656. doi: 10.1128/jvi.58.2.655-656.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huddleston J. A., Brownlee G. G. The sequence of the nucleoprotein gene of human influenza A virus, strain A/NT/60/68. Nucleic Acids Res. 1982 Feb 11;10(3):1029–1038. doi: 10.1093/nar/10.3.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones K. L., Huddleston J. A., Brownlee G. G. The sequence of RNA segment 1 of influenza virus A/NT/60/68 and its comparison with the corresponding segment of strains A/PR/8/34 and A/WSN/33. Nucleic Acids Res. 1983 Mar 11;11(5):1555–1566. doi: 10.1093/nar/11.5.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaoka Y., Chambers T. M., Sladen W. L., Webster R. G. Is the gene pool of influenza viruses in shorebirds and gulls different from that in wild ducks? Virology. 1988 Mar;163(1):247–250. doi: 10.1016/0042-6822(88)90260-7. [DOI] [PubMed] [Google Scholar]
- Kawaoka Y., Krauss S., Webster R. G. Avian-to-human transmission of the PB1 gene of influenza A viruses in the 1957 and 1968 pandemics. J Virol. 1989 Nov;63(11):4603–4608. doi: 10.1128/jvi.63.11.4603-4608.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kida H., Shortridge K. F., Webster R. G. Origin of the hemagglutinin gene of H3N2 influenza viruses from pigs in China. Virology. 1988 Jan;162(1):160–166. doi: 10.1016/0042-6822(88)90405-9. [DOI] [PubMed] [Google Scholar]
- Krystal M., Elliott R. M., Benz E. W., Jr, Young J. F., Palese P. Evolution of influenza A and B viruses: conservation of structural features in the hemagglutinin genes. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4800–4804. doi: 10.1073/pnas.79.15.4800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Londo D. R., Davis A. R., Nayak D. P. Complete nucleotide sequence of the nucleoprotein gene of influenza B virus. J Virol. 1983 Sep;47(3):642–648. doi: 10.1128/jvi.47.3.642-648.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandler J., Scholtissek C. Localisation of the temperature-sensitive defect in the nucleoprotein of an influenza A/FPV/Rostock/34 virus. Virus Res. 1989 Feb;12(2):113–121. doi: 10.1016/0168-1702(89)90058-0. [DOI] [PubMed] [Google Scholar]
- Nakada S., Creager R. S., Krystal M., Palese P. Complete nucleotide sequence of the influenza C/California/78 virus nucleoprotein gene. Virus Res. 1984 Sep;1(6):433–441. doi: 10.1016/0168-1702(84)90001-7. [DOI] [PubMed] [Google Scholar]
- Nakajima K., Nobusawa E., Nakajima S. Genetic relatedness between A/Swine/Iowa/15/30(H1N1) and human influenza viruses. Virology. 1984 Nov;139(1):194–198. doi: 10.1016/0042-6822(84)90341-6. [DOI] [PubMed] [Google Scholar]
- Reinhardt U., Scholtissek C. Comparison of the nucleoprotein genes of a chicken and a mink influenza A H 10 virus. Arch Virol. 1988;103(1-2):139–145. doi: 10.1007/BF01319816. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholtissek C., Bürger H., Kistner O., Shortridge K. F. The nucleoprotein as a possible major factor in determining host specificity of influenza H3N2 viruses. Virology. 1985 Dec;147(2):287–294. doi: 10.1016/0042-6822(85)90131-x. [DOI] [PubMed] [Google Scholar]
- Scholtissek C., Bürger H., Kistner O., Shortridge K. F. The nucleoprotein as a possible major factor in determining host specificity of influenza H3N2 viruses. Virology. 1985 Dec;147(2):287–294. doi: 10.1016/0042-6822(85)90131-x. [DOI] [PubMed] [Google Scholar]
- Snyder M. H., Buckler-White A. J., London W. T., Tierney E. L., Murphy B. R. The avian influenza virus nucleoprotein gene and a specific constellation of avian and human virus polymerase genes each specify attenuation of avian-human influenza A/Pintail/79 reassortant viruses for monkeys. J Virol. 1987 Sep;61(9):2857–2863. doi: 10.1128/jvi.61.9.2857-2863.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steuler H., Schröder B., Bürger H., Scholtissek C. Sequence of the nucleoprotein gene of influenza A/parrot/Ulster/73. Virus Res. 1985 Jul;3(1):35–40. doi: 10.1016/0168-1702(85)90039-5. [DOI] [PubMed] [Google Scholar]
- Webster R. G., Hinshaw V. S., Laver W. G. Selection and analysis of antigenic variants of the neuraminidase of N2 influenza viruses with monoclonal antibodies. Virology. 1982 Feb;117(1):93–104. doi: 10.1016/0042-6822(82)90510-4. [DOI] [PubMed] [Google Scholar]
- Winter G., Fields S., Gait M. J., Brownlee G. G. The use of synthetic oligodeoxynucleotide primers in cloning and sequencing segment of 8 influenza virus (A/PR/8/34). Nucleic Acids Res. 1981 Jan 24;9(2):237–245. doi: 10.1093/nar/9.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter G., Fields S. The structure of the gene encoding the nucleoprotein of human influenza virus A/PR/8/34. Virology. 1981 Oct 30;114(2):423–428. doi: 10.1016/0042-6822(81)90223-3. [DOI] [PubMed] [Google Scholar]


