Abstract
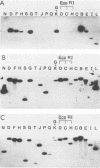
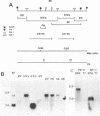
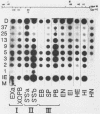
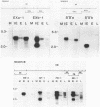
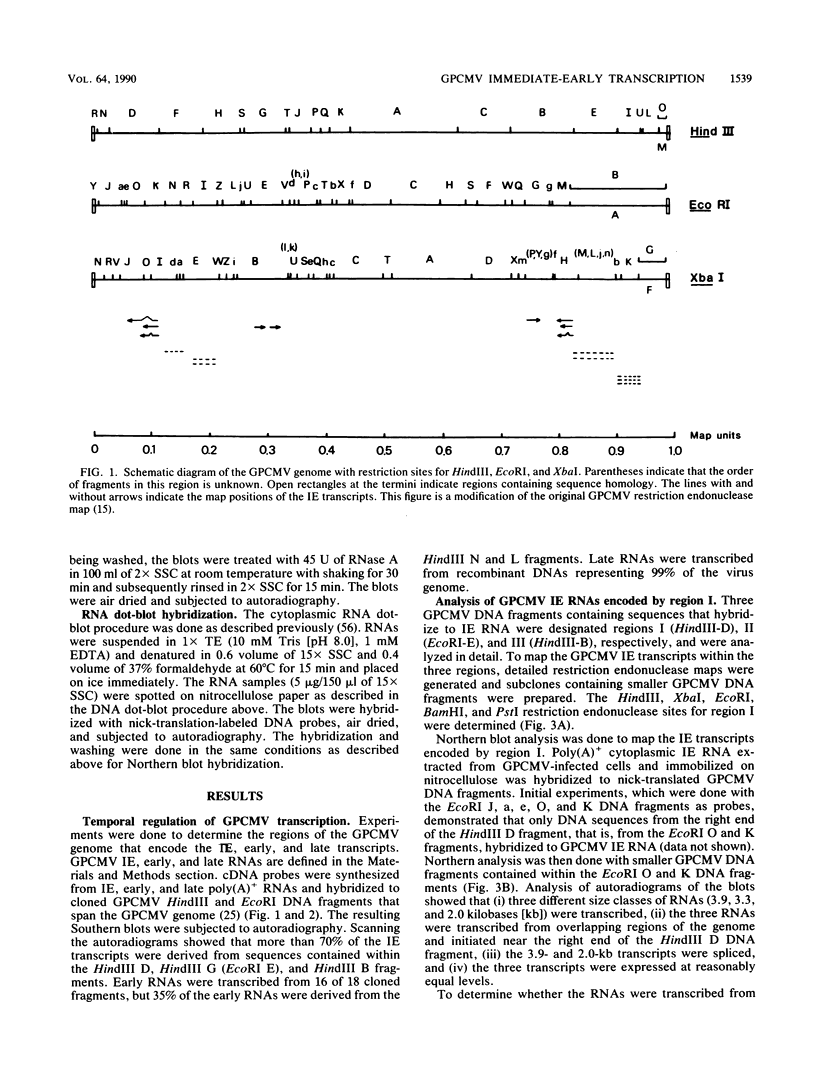
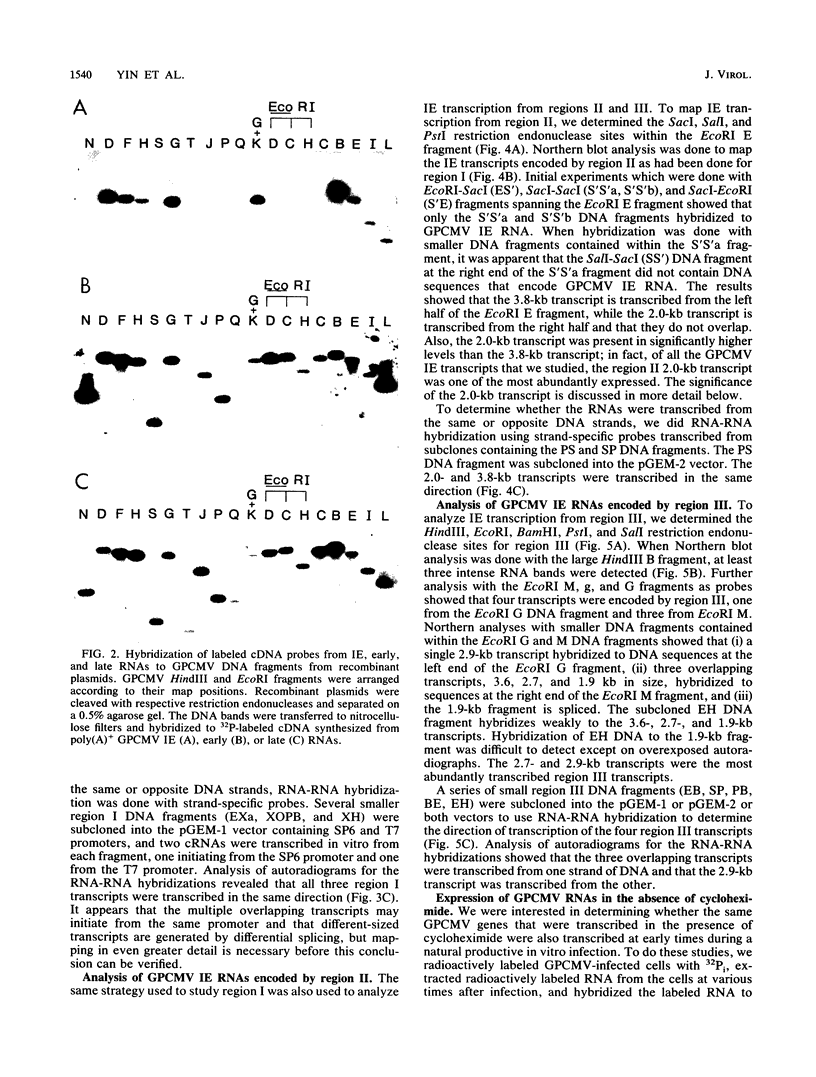
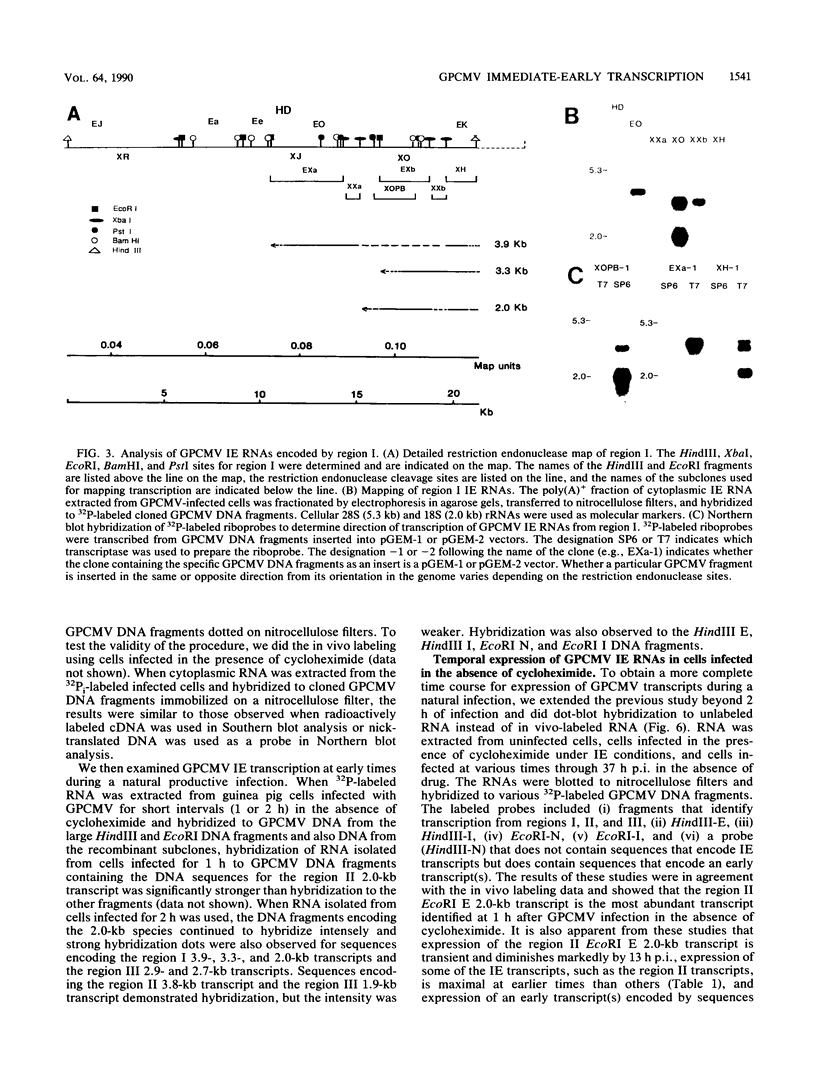
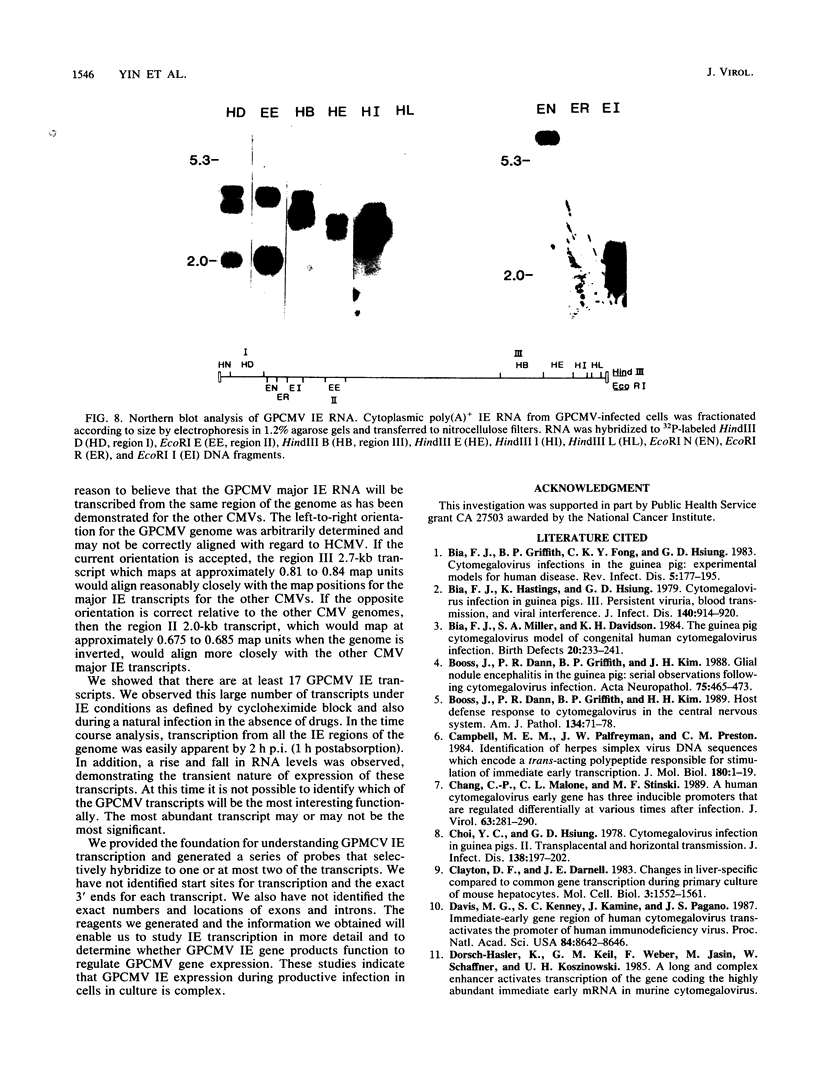
Guinea pig cytomegalovirus (GPCMV) immediate-early (IE) gene expression was analyzed. GPCMV IE RNA was defined as RNA obtained from GPCMV-infected guinea pig cells treated with cycloheximide for 1 h before infection and for 4 h postinfection. Mapping studies showed that GPCMV IE genes are located at several distinct sites on the GPCMV genome. A total of 17 GPCMV IE transcripts were identified, and 9 IE transcripts coded for by three specific regions of the genome (regions I, II, and III) were characterized in detail. A series of recombinant DNA clones were generated to identify the nine IE transcripts. Three of the IE transcripts from region I and three from region III were transcribed in the same direction from overlapping sequences. The 2.0-kilobase (kb) transcript encoded by the EcoRI E DNA fragment (region II) was the most abundant IE GPCMV transcript. The cloned GPCMV DNA subfragment that was used to identify the region II EcoRI E 2.0-kb transcript did not hybridize to GPCMV early or late RNA, indicating that this transcript is expressed only under IE conditions. Expression of RNAs from the IE genes was also measured during a natural GPCMV infection in the absence of cycloheximide. During the natural infection, the transcripts previously identified under IE cycloheximide block conditions were expressed, and the region II EcoRI E 2.0-kb transcript was the most abundant transcript at 1 h postinfection. In addition, a rise and fall in RNA levels was observed during the natural infection, demonstrating the transient nature of expression of these transcripts. We conclude that GPCMV IE gene expression is complex, involving a reasonably large number of genes, and demonstrates some similarities with IE transcription by other CMVs.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bia F. J., Griffith B. P., Fong C. K., Hsiung G. D. Cytomegaloviral infections in the guinea pig: experimental models for human disease. Rev Infect Dis. 1983 Mar-Apr;5(2):177–195. doi: 10.1093/clinids/5.2.177. [DOI] [PubMed] [Google Scholar]
- Bia F. J., Hastings K., Hsiung G. D. Cytomegalovirus infection in guinea pigs. III. Persistent viruria, blood transmission, and viral interference. J Infect Dis. 1979 Dec;140(6):914–920. doi: 10.1093/infdis/140.6.914. [DOI] [PubMed] [Google Scholar]
- Bia F. J., Miller S. A., Davidson K. H. The guinea pig cytomegalovirus model of congenital human cytomegalovirus infection. Birth Defects Orig Artic Ser. 1984;20(1):233–241. [PubMed] [Google Scholar]
- Booss J., Dann P. R., Griffith B. P., Kim J. H. Glial nodule encephalitis in the guinea pig: serial observations following cytomegalovirus infection. Acta Neuropathol. 1988;75(5):465–473. doi: 10.1007/BF00687133. [DOI] [PubMed] [Google Scholar]
- Booss J., Dann P. R., Griffith B. P., Kim J. H. Host defense response to cytomegalovirus in the central nervous system. Predominance of the monocyte. Am J Pathol. 1989 Jan;134(1):71–78. [PMC free article] [PubMed] [Google Scholar]
- Campbell M. E., Palfreyman J. W., Preston C. M. Identification of herpes simplex virus DNA sequences which encode a trans-acting polypeptide responsible for stimulation of immediate early transcription. J Mol Biol. 1984 Nov 25;180(1):1–19. doi: 10.1016/0022-2836(84)90427-3. [DOI] [PubMed] [Google Scholar]
- Chang C. P., Malone C. L., Stinski M. F. A human cytomegalovirus early gene has three inducible promoters that are regulated differentially at various times after infection. J Virol. 1989 Jan;63(1):281–290. doi: 10.1128/jvi.63.1.281-290.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y. C., Hsiung G. D. Cytomegalovirus infection in guinea pigs. II. Transplacental and horizontal transmission. J Infect Dis. 1978 Aug;138(2):197–202. doi: 10.1093/infdis/138.2.197. [DOI] [PubMed] [Google Scholar]
- Clayton D. F., Darnell J. E., Jr Changes in liver-specific compared to common gene transcription during primary culture of mouse hepatocytes. Mol Cell Biol. 1983 Sep;3(9):1552–1561. doi: 10.1128/mcb.3.9.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. G., Kenney S. C., Kamine J., Pagano J. S., Huang E. S. Immediate-early gene region of human cytomegalovirus trans-activates the promoter of human immunodeficiency virus. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8642–8646. doi: 10.1073/pnas.84.23.8642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorsch-Häsler K., Keil G. M., Weber F., Jasin M., Schaffner W., Koszinowski U. H. A long and complex enhancer activates transcription of the gene coding for the highly abundant immediate early mRNA in murine cytomegalovirus. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8325–8329. doi: 10.1073/pnas.82.24.8325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett R. D. Activation of cellular promoters during herpes virus infection of biochemically transformed cells. EMBO J. 1985 Aug;4(8):1973–1980. doi: 10.1002/j.1460-2075.1985.tb03880.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felser J. M., Kinchington P. R., Inchauspe G., Straus S. E., Ostrove J. M. Cell lines containing varicella-zoster virus open reading frame 62 and expressing the "IE" 175 protein complement ICP4 mutants of herpes simplex virus type 1. J Virol. 1988 Jun;62(6):2076–2082. doi: 10.1128/jvi.62.6.2076-2082.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong C. K., Lucia H., Bia F. J., Hsiung G. D. Histopathologic and ultrastructural studies of disseminated cytomegalovirus infection in strain 2 guinea pigs. Lab Invest. 1983 Aug;49(2):183–194. [PubMed] [Google Scholar]
- Fukuda S., Keithley E. M., Harris J. P. The development of endolymphatic hydrops following CMV inoculation of the endolymphatic sac. Laryngoscope. 1988 Apr;98(4):439–443. doi: 10.1288/00005537-198804000-00017. [DOI] [PubMed] [Google Scholar]
- Gao M., Isom H. C. Characterization of the guinea pig cytomegalovirus genome by molecular cloning and physical mapping. J Virol. 1984 Nov;52(2):436–447. doi: 10.1128/jvi.52.2.436-447.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelman I. H., Silverstein S. Co-ordinate regulation of herpes simplex virus gene expression is mediated by the functional interaction of two immediate early gene products. J Mol Biol. 1986 Oct 5;191(3):395–409. doi: 10.1016/0022-2836(86)90135-x. [DOI] [PubMed] [Google Scholar]
- Griffith B. P., Lucia H. L., Bia F. J., Hsiung G. D. Cytomegalovirus-induced mononucleosis in guinea pigs. Infect Immun. 1981 May;32(2):857–863. doi: 10.1128/iai.32.2.857-863.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith B. P., Lucia H. L., Hsiung G. D. Brain and visceral involvement during congenital cytomegalovirus infection of guinea pigs. Pediatr Res. 1982 Jun;16(6):455–459. doi: 10.1203/00006450-198206000-00010. [DOI] [PubMed] [Google Scholar]
- Harris J. P., Woolf N. K., Ryan A. F., Butler D. M., Richman D. D. Immunologic and electrophysiological response to cytomegaloviral inner ear infection in the guinea pig. J Infect Dis. 1984 Oct;150(4):523–530. doi: 10.1093/infdis/150.4.523. [DOI] [PubMed] [Google Scholar]
- Hermiston T. W., Malone C. L., Witte P. R., Stinski M. F. Identification and characterization of the human cytomegalovirus immediate-early region 2 gene that stimulates gene expression from an inducible promoter. J Virol. 1987 Oct;61(10):3214–3221. doi: 10.1128/jvi.61.10.3214-3221.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer E., Darnell J. E., Jr The primary transcription unit of the mouse beta-major globin gene. Cell. 1981 Feb;23(2):585–593. doi: 10.1016/0092-8674(81)90154-9. [DOI] [PubMed] [Google Scholar]
- Hsiung G. D., Bia F. J., Fong C. K. Viruses of guinea pigs: considerations for biomedical research. Microbiol Rev. 1980 Sep;44(3):468–490. doi: 10.1128/mr.44.3.468-490.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiung G. D., Choi Y. C., Bia F. Cytomegalovirus infection in guinea pigs. I. Viremia during acute primary and chronic persistent infection. J Infect Dis. 1978 Aug;138(2):191–196. doi: 10.1093/infdis/138.2.191. [DOI] [PubMed] [Google Scholar]
- Isom H. C., Gao M., Wigdahl B. Characterization of guinea pig cytomegalovirus DNA. J Virol. 1984 Feb;49(2):426–436. doi: 10.1128/jvi.49.2.426-436.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn G., Knust E., Schmolla H., Sarre T., Nelson J. A., McDougall J. K., Fleckenstein B. Predominant immediate-early transcripts of human cytomegalovirus AD 169. J Virol. 1984 Feb;49(2):363–370. doi: 10.1128/jvi.49.2.363-370.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeang K. T., Cho M. S., Hayward G. S. Abundant constitutive expression of the immediate-early 94K protein from cytomegalovirus (Colburn) in a DNA-transfected mouse cell line. Mol Cell Biol. 1984 Oct;4(10):2214–2223. doi: 10.1128/mcb.4.10.2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K. P., Connor W. S. Guinea pig cytomegalovirus: transplacental transmission. Brief report. Arch Virol. 1979;59(3):263–267. doi: 10.1007/BF01317422. [DOI] [PubMed] [Google Scholar]
- Keil G. M., Ebeling-Keil A., Koszinowski U. H. Immediate-early genes of murine cytomegalovirus: location, transcripts, and translation products. J Virol. 1987 Feb;61(2):526–533. doi: 10.1128/jvi.61.2.526-533.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keil G. M., Ebeling-Keil A., Koszinowski U. H. Sequence and structural organization of murine cytomegalovirus immediate-early gene 1. J Virol. 1987 Jun;61(6):1901–1908. doi: 10.1128/jvi.61.6.1901-1908.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keil G. M., Ebeling-Keil A., Koszinowski U. H. Temporal regulation of murine cytomegalovirus transcription and mapping of viral RNA synthesized at immediate early times after infection. J Virol. 1984 Jun;50(3):784–795. doi: 10.1128/jvi.50.3.784-795.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keil G. M., Fibi M. R., Koszinowski U. H. Characterization of the major immediate-early polypeptides encoded by murine cytomegalovirus. J Virol. 1985 May;54(2):422–428. doi: 10.1128/jvi.54.2.422-428.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keithley E. M., Sharp P., Woolf N. K., Harris J. P. Temporal sequence of viral antigen expression in the cochlea induced by cytomegalovirus. Acta Otolaryngol. 1988 Jul-Aug;106(1-2):46–54. doi: 10.3109/00016488809107370. [DOI] [PubMed] [Google Scholar]
- Kouzarides T., Bankier A. T., Satchwell S. C., Preddy E., Barrell B. G. An immediate early gene of human cytomegalovirus encodes a potential membrane glycoprotein. Virology. 1988 Jul;165(1):151–164. doi: 10.1016/0042-6822(88)90668-x. [DOI] [PubMed] [Google Scholar]
- Latchman D. S., Estridge J. K., Kemp L. M. Transcriptional induction of the ubiquitin gene during herpes simplex virus infection is dependent upon the viral immediate-early protein ICP4. Nucleic Acids Res. 1987 Sep 25;15(18):7283–7293. doi: 10.1093/nar/15.18.7283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucia H. L., Griffith B. P., Hsiung G. D. Lymphadenopathy during cytomegalovirus-induced mononucleosis in guinea pigs. Arch Pathol Lab Med. 1985 Nov;109(11):1019–1023. [PubMed] [Google Scholar]
- Manning W. C., Mocarski E. S. Insertional mutagenesis of the murine cytomegalovirus genome: one prominent alpha gene (ie2) is dispensable for growth. Virology. 1988 Dec;167(2):477–484. [PubMed] [Google Scholar]
- Marks J. R., Mercer J. A., Spector D. H. Transcription in mouse embryo cells permissively infected by murine cytomegalovirus. Virology. 1983 Nov;131(1):247–254. doi: 10.1016/0042-6822(83)90550-0. [DOI] [PubMed] [Google Scholar]
- Mosca J. D., Bednarik D. P., Raj N. B., Rosen C. A., Sodroski J. G., Haseltine W. A., Hayward G. S., Pitha P. M. Activation of human immunodeficiency virus by herpesvirus infection: identification of a region within the long terminal repeat that responds to a trans-acting factor encoded by herpes simplex virus 1. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7408–7412. doi: 10.1073/pnas.84.21.7408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson J. A., Fleckenstein B., Jahn G., Galloway D. A., McDougall J. K. Structure of the transforming region of human cytomegalovirus AD169. J Virol. 1984 Jan;49(1):109–115. doi: 10.1128/jvi.49.1.109-115.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzorno M. C., O'Hare P., Sha L., LaFemina R. L., Hayward G. S. trans-activation and autoregulation of gene expression by the immediate-early region 2 gene products of human cytomegalovirus. J Virol. 1988 Apr;62(4):1167–1179. doi: 10.1128/jvi.62.4.1167-1179.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sha M., Griffith B. P., Raveh D., Isom H. C., Ward D. C., Hsiung G. D. Detection of guinea pig cytomegalovirus nucleic acids in cultured cells with biotin-labelled hybridization probes. Virus Res. 1987 Jan;6(4):317–329. doi: 10.1016/0168-1702(87)90064-5. [DOI] [PubMed] [Google Scholar]
- Stenberg R. M., Depto A. S., Fortney J., Nelson J. A. Regulated expression of early and late RNAs and proteins from the human cytomegalovirus immediate-early gene region. J Virol. 1989 Jun;63(6):2699–2708. doi: 10.1128/jvi.63.6.2699-2708.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenberg R. M., Stinski M. F. Autoregulation of the human cytomegalovirus major immediate-early gene. J Virol. 1985 Dec;56(3):676–682. doi: 10.1128/jvi.56.3.676-682.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenberg R. M., Thomsen D. R., Stinski M. F. Structural analysis of the major immediate early gene of human cytomegalovirus. J Virol. 1984 Jan;49(1):190–199. doi: 10.1128/jvi.49.1.190-199.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenberg R. M., Witte P. R., Stinski M. F. Multiple spliced and unspliced transcripts from human cytomegalovirus immediate-early region 2 and evidence for a common initiation site within immediate-early region 1. J Virol. 1985 Dec;56(3):665–675. doi: 10.1128/jvi.56.3.665-675.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinski M. F., Thomsen D. R., Stenberg R. M., Goldstein L. C. Organization and expression of the immediate early genes of human cytomegalovirus. J Virol. 1983 Apr;46(1):1–14. doi: 10.1128/jvi.46.1.1-14.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tevethia M. J., Spector D. J., Leisure K. M., Stinski M. F. Participation of two human cytomegalovirus immediate early gene regions in transcriptional activation of adenovirus promoters. Virology. 1987 Dec;161(2):276–285. doi: 10.1016/0042-6822(87)90119-x. [DOI] [PubMed] [Google Scholar]
- Tremblay M. L., Yee S. P., Persson R. H., Bacchetti S., Smiley J. R., Branton P. E. Activation and inhibition of expression of the 72,000-Da early protein of adenovirus type 5 in mouse cells constitutively expressing an immediate early protein of herpes simplex virus type 1. Virology. 1985 Jul 15;144(1):35–45. doi: 10.1016/0042-6822(85)90302-2. [DOI] [PubMed] [Google Scholar]
- Weston K. An enhancer element in the short unique region of human cytomegalovirus regulates the production of a group of abundant immediate early transcripts. Virology. 1988 Feb;162(2):406–416. doi: 10.1016/0042-6822(88)90481-3. [DOI] [PubMed] [Google Scholar]
- White B. A., Bancroft F. C. Cytoplasmic dot hybridization. Simple analysis of relative mRNA levels in multiple small cell or tissue samples. J Biol Chem. 1982 Aug 10;257(15):8569–8572. [PubMed] [Google Scholar]
- Wilkinson G. W., Akrigg A., Greenaway P. J. Transcription of the immediate early genes of human cytomegalovirus strain AD169. Virus Res. 1984;1(2):101–106. doi: 10.1016/0168-1702(84)90067-4. [DOI] [PubMed] [Google Scholar]
- Woolf N. K., Ochi J. W., Silva E. J., Sharp P. A., Harris J. P., Richman D. D. Ganciclovir prophylaxis for cochlear pathophysiology during experimental guinea pig cytomegalovirus labyrinthitis. Antimicrob Agents Chemother. 1988 Jun;32(6):865–872. doi: 10.1128/aac.32.6.865. [DOI] [PMC free article] [PubMed] [Google Scholar]