Abstract
Tuberculosis in mice is a lung disease. Airborne infection of this host species with Mycobacterium tuberculosis (Mtb) resulted in 20 days of Mtb growth in the lungs before further growth was inhibited and the level of infection stabilized. Inhibition of Mtb growth was associated with the production of interferon-γ (IFN-γ)-producing T cells in the lymph nodes and spleen and with the progressive accumulation of these cells in the lungs. Production of IFN-γ-producing T cells was not discernable until about day 15 of infection, presumably because Mtb did not disseminate from the lungs to the draining lymph nodes and spleen until after an approximate 10-day delay. By contrast, in mice infected via the intravenous (i.v.) route, the spleen became infected almost immediately, resulting in much earlier production of IFN-γ-producing T cells and earlier control of spleen and lung infection. In mice infected concurrently via both routes, earlier generation of immunity to the i.v. infection resulted in earlier accumulation of IFN-γ-producing T cells in the lungs and earlier control of lung infection that was initiated via the airborne route. This protection against airborne infection afforded by an earlier primary immune response is equivalent to that expressed by mice vaccinated with bacillus Calmette–Guérin or certain other vaccines.
Keywords: lung infection, Mycobacterium tuberculosis, T helper type 1 immunity
Introduction
In susceptible humans and laboratory animals tuberculosis is primarily a disease of the lungs. It is known1 that infection of mice, guinea pigs and rabbits with Mycobacterium tuberculosis (Mtb) via the airborne route results in an approximate 3-week period of log linear Mtb growth in the lungs before further growth is inhibited and infection is stabilized at an approximately stationary level by acquired immunity. It is generally agreed that immunity to Mtb infection is predominately mediated by T helper type 1 (Th1) CD4 and CD8 T cells.2–4 The long delay before adaptive Th1-mediated immunity is expressed against this slow-growing pathogen, however, enables infection to reach a relatively high stationary level that induces progressive and, eventually, lethal lung pathology.1 It is important to understand, therefore, why immunity to airborne infection takes so long to be generated. In this connection, an earlier publication from this laboratory5 showed that mice infected with a large number of Mtb bacilli via the intravenous (i.v.) route survive much longer than mice infected with a much smaller number via the airborne route, even though in both cases a similar number of bacilli implanted in the lungs. More importantly, this same publication showed paradoxically, that mice infected via both routes survived considerably longer than mice infected via the airborne route alone. Presumably, increased survival time afforded by a concurrent i.v. infection would require that airborne lung infection be controlled at a lower level, which would require, in turn, that immunity be expressed at an earlier stage of infection. If so, it would mean that immunity to i.v. infection protects against airborne infection because it is generated earlier. The purpose of this paper is to show that this is the case.
Materials and methods
Mice
C57BL/6 male mice were purchased from The Jackson Laboratory (Bar Harbor, ME), and were used in experiments at 12 weeks of age. They were housed under barrier conditions in a Level III Biosafety Animal Facility, according to guidelines formulated by the Trudeau Institute Animal Care and Use Committee. The C57BL/6 strain is considered an Mtb-resistant strain.6
Bacteria and infection
The H37Rv strain of Mtb (Trudeau Mycobacterial Culture Collection no. 102) and a streptomycin-resistant (SR) H37Rv strain (no. 301) were grown in suspension culture in Proskauer and Beck medium and prepared for infection of mice with 105 colony-forming units (CFU) via the i.v. route alone, 102 CFU via the respiratory route alone, or 105 CFU via the i.v. route plus 102 CFU via the respiratory route. Infection via the respiratory route was performed in an aerosol infection chamber as previously described.7,8 Mice infected via both routes were infected via the i.v. route within 1 hr of being removed from the aerosol infection chamber. Progress of infection in the lungs, spleens and draining lymph nodes (LNs) was monitored by plating 10-fold serial dilutions of homogenates of these organs on nutrient agar, and counting CFU after 3 weeks of incubation at 37°, as previously described.7,8 The CFU of SR H37Rv were distinguished from those of wild-type (WT) H37Rv by plating organ homogenates on agar that contained streptomycin.
Enumeration of interferon-γ (IFN-γ)-producing CD4 T cells by flow cytometry and Elispot
To obtain lung cells, mice were killed by cervical dislocation, and their lungs were perfused via the right ventricle with phosphate-buffered saline containing 10 U/ml heparin to remove intravascular leucocytes. The lungs were then perfused with an enzyme cocktail comprising 150 U/ml collagenase, 0·2 U/ml elastase and 40 μg/ml DNAse in RPMI-1640. The lungs were removed, diced into small fragments and subjected to further enzyme action before being mechanically disrupted to form a single-cell suspension, as described previously.7,8 Lung cells were suspended in RPMI–fetal calf serum in two 5-ml tubes at 1 × 107/ml, and incubated for 5 hr at 37° in the presence of Brefeldin A (Epicenter Technologies, Omaha, NE) and at a concentration of 10 μg/ml. They were then stained for flow cytometry with fluorescein isothiocyanate-conjugated anti-CD3 monoclonal antibody (mAb), R-phycoerythrin-conjugated anti-CD4 mAb, and Peridinin chlorophyll protein-conjugated anti-CD8 mAb. After fixation overnight in 0·5% paraformaldehyde they were stained for intracellular IFN-γ with allophycocyanin-conjugated anti-IFN-γ mAb, as described previously.7 They were then subjected to analysis with a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA).
Spleen cell suspensions were prepared by dicing spleens into small fragments in phosphate-buffered saline containing fetal calf serum and pushing the fragments through a c. 10-mesh/cm2 stainless screen. The resulting suspension was triturated with a pipette to break up aggregates and treated as described for lung cells. Tracheobronchial LN cell suspensions were prepared and treated in the same way as described for spleen cells.
Changes in the numbers of CD4 T cells specific for Mtb antigens in the lungs and spleens with time of infection were determined by enumerating changes in the numbers of cells capable of making IFN-γ in response to Mtb antigens in the enzyme-linked immunosorbent spot-forming cell assay (ELISPOT). This was performed with a commercially available ELISPOT kit (Mouse IFN-γ ELISPOT Set, BD Biosciences) according to the manufacturer’s instructions using cells from four mice, as described previously.7,8 The Mtb antigens used to stimulate IFN-γ production were ESAT-6 (1-20) peptide,9 Ag85B (240-254) peptide10 and an Mtb sonicate prepared as described previously.7 All experiments were performed twice.
Results
Earlier control of lung infection in mice infected via both the airborne and i.v. routes than via the airborne route alone
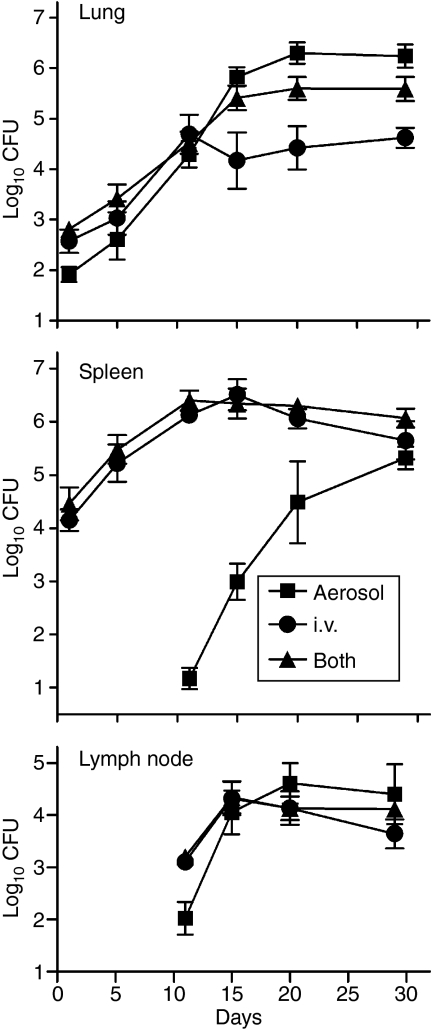
An earlier study5 showed that mice infected via the respiratory route with Mtb paradoxically survive much longer if they are infected at the same time with a much larger number via the i.v. route. To determine whether this was because lung infection is controlled earlier in mice infected via both routes, Mtb growth was followed in the lungs, tracheobronchial LNs and spleens of mice infected via the airborne route, i.v. route, or via both routes. Figure 1 shows that, whereas lung infection initiated via the airborne route progressed for 20 days before being controlled at an approximately stationary level, it was controlled approximately 5 days earlier and at a level that was approximately 1 log lower in mice infected concurrently via both routes. In mice infected via the i.v. route alone, lung infection was controlled at a 1 log lower level still, because it progressed at a slower initial rate than in mice infected via both routes.
Figure 1.
Mice were infected with approximately 102 colony-forming units (CFU) of Mycobacterium tuberculosis (Mtb) via the airborne route, 105 CFU via the intravenous (i.v.) route, or 102 via the airborne plus 105 via the i.v. route. Lung infection in mice infected concurrently via the i.v. and airborne routes was controlled earlier than in mice infected via the respiratory route alone, but was controlled earlier still in mice infected via the i.v. route alone. Spleen infection was initiated immediately and controlled after approximately 10 days in mice infected via the i.v. route, or via both routes. It was not initiated until after about a 12-day delay in mice infected via the respiratory route alone, and was not controlled until after day 20. The draining lymph nodes (LNs) became infected on day 10 in mice infected via the respiratory route alone, and probably earlier in mice infected via the i.v. route, or via both routes. Control of LN infection occurred on day 15 in mice infected via the i.v. route or via both routes, and on day 20 in mice infected via the respiratory route alone. Means ± SD of four mice per group per time-point.
In mice infected via the respiratory route alone the spleen did not become infected until after a 10-day delay, whereas it became infected immediately in mice infected via the i.v. route alone, or via both routes. Moreover, whereas mice infected via the airborne route alone did not begin to control spleen infection until day 20, mice infected via the i.v. route or via both routes began to control spleen infection by day 10. Spleen infection eventually reached roughly the same level in mice of all groups.
The draining LNs of mice of all groups did not show the presence of viable bacilli until day 10. However, whereas there were approximately 102 bacilli on this day in the LNs of mice infected via the respiratory route alone, there were 10 times more in the nodes of mice infected via the i.v. route, or via both routes. This indicates that in the latter two groups the node became infected earlier. Again, whereas in the mice infected by the respiratory route alone the LN infection was not controlled until day 20, it was controlled on day 15 in the nodes of mice of the other groups.
Lung infection initiated via the i.v. route progresses more slowly
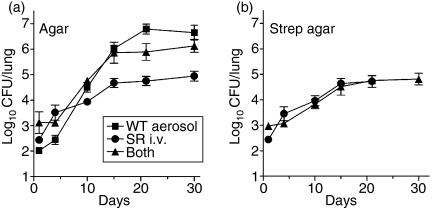
The results in Fig. 1 showing that lung infection initiated via the i.v. route progresses more slowly than when initiated via the airborne route was investigated further by using a SR strain of H37Rv to infect via the i.v. route and the wild-type strain to infect via the airborne route. Growth of the SR strain was distinguished from that of the WT strain by plating lung homogenates on streptomycin-containing nutrient agar, as well on standard nutrient agar. The results obtained by plating on standard agar (Fig. 2a) show that lung infection in mice infected via the i.v. route alone progressed at a slower rate than lung infection initiated via the airborne route alone, or via both routes. Plating on streptomycin-containing agar showed that SR Mtb given via the i.v. route grew at the same slow rate in the lungs both in the presence and absence of WT Mtb given via the airborne route (Fig. 2b). It is obvious from Fig. 2 that Mtb given i.v. contributed very little to the level of lung infection in mice infected via both routes.
Figure 2.
Lung infection initiated via the intravenous (i.v.) route progressed more slowly than when initiated via the airborne route. Mice were infected with approximately 102 colony-forming units (CFU) of wild-type (WT) H37Rv via the airborne route, 105 CFU of SR H37Rv via the i.v. route, or 102 WT H37Rv via the airborne plus 105 SR H37RV via the i.v. route. (a) CFU as determined by plating lung homogenates on standard nutrient agar show that lung infection progressed more slowly in mice infected via the i.v. route. (b) CFU as determined by plating lung homogenates of streptomycin-containing agar show that infection caused by SR H37Rv that reached the lungs after i.v. infection progressed more slowly and was controlled earlier at a lower level in the presence or absence of WT H37Rv delivered via the airborne route. Means ± SD of four mice per group.
Earlier generation of IFN-γ-producing CD4 T cells in the spleens and their earlier accumulation in the lungs of mice infected via the i.v. or via both routes
The foregoing results show that, whereas Mtb did not reach the draining LNs or spleen until after about a 10-day delay in mice infected via the respiratory route alone, it reached the spleen immediately in mice infected via the i.v. route or via both routes. It was anticipated, therefore, that IFN-γ-producing CD4 T cells would be generated much earlier in the spleens of mice infected via the i.v. route, or via both routes. It was also anticipated that earlier generation of these cells in the spleen would result in their earlier appearance in the lungs. This was investigated by following changes against time of infection in the percentage and total number of IFN-γ-producing CD4 T cells in the spleen and lungs of mice infected via the respiratory route, i.v. route, or both routes.
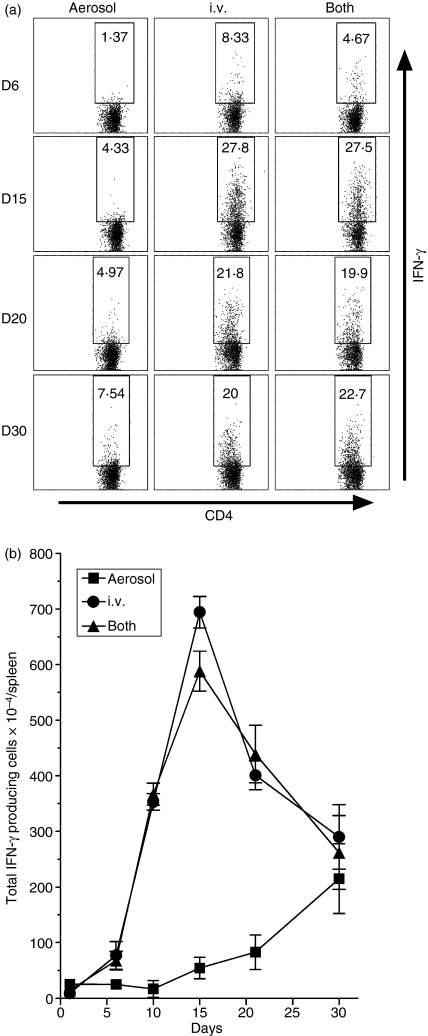
In mice infected via the i.v. route or both routes an increase in the percentage of IFN-γ-producing CD4 cells was evident in the spleen as early as day 6 of infection and increased to reach a high value at day 15 before declining (Fig. 3a). By contrast, an increase in the percentage of these cells was not evident until day 15 in the spleens of mice infected via the airborne route alone, and increased by only a small amount by day 30. As for the total number IFN-γ-producing CD4 cells, an increase was evident on day 6 in the spleens of mice infected via the i.v. route or via both routes (Fig. 3b), after which the number increased progressively to peak on day 15 before undergoing a decline. In mice infected via the airborne route alone, an increase in the total number per spleen was not evident until day 15, and increased by only a small amount by day 30 when the experiment was terminated.
Figure 3.
Kinetics of the splenic T helper type 1 (Th1) response to infection initiated via the airborne, intravenous (i.v.), or both routes as determined by flow cytometry. (a) An increase in the percentage of interferon-γ (IFN-γ)-producing CD4 cells in the spleen was evident as early as day 6 in mice infected via the i.v. route or via both routes, and increased to peak on day 15 before undergoing a decline. In mice infected via the airborne route alone an increase in the percentage of these cells was not evident until day 15–20, and had increased by only a relatively small amount by day 30. (b) An increase in the total number of IFN-γ-producing CD4 cells per spleen was not evident until day15 in mice infected via the airborne route alone, but was evident as early as day 6 in mice infected via the i.v. route or both routes. In mice infected via the i.v. route or both routes, the number increased rapidly to peak on day 15 before declining, whereas it increased relatively slowly after day 10 in mice infected via the airborne route. Means ± SD of four mice per group per time point.
The earlier production of IFN-γ-producing CD4 T cells in the spleens of mice infected via the i.v. route or via both routes was associated with much earlier accumulation of these cells in the lungs. Figure 4(a) shows that in mice infected via the i.v. route alone the percentage of these cells showed an increase in the lungs as early as day 6 of infection and continued to increase until day 15 before undergoing a decline. In mice infected via both routes an increase in percentage was also evident as early as day 6, but peaked on day 20 before declining. By contrast, in mice infected via the airborne route alone the percentage of IFN-γ-producing CD4 T cells did not show an increase until day 20 and continued to increase until day 30 when the experiment was terminated. As for the total number of IFN-γ-producing cells that accumulated in the lungs (Fig. 4b), an increase was evident in mice infected via the i.v. route or via both routes as early as day 10 of infection. It increased to peak on day 15 before undergoing a decline in mice infected via the i.v. route, and peaked on day 20 before undergoing a decline in mice infected via both routes. By contrast, in mice infected via the airborne route alone, an increase in the total number of IFN-γ-producing CD4 cells in the lungs was not discernable until day 20, after which the number increased until day 30 when the experiment was terminated. It is known6,7 that day 30 is the time that peak numbers of IFN-γ-producing CD4 T cells are present in the lungs of mice infected via the respiratory route.
Figure 4.
Kinetics of accumulation of interferon-γ (IFN-γ)-producing CD4 cells in the lungs of mice infected via the airborne route, intravenous (i.v.) route, or both routes. (a) Mice infected via the i.v. route alone showed a substantial increase in the percentage of CD4 T cells in the lungs between days 6 and 15 of infection. Mice infected via both routes showed a large increase between days 6 and 20. By contrast, mice infected via the airborne route alone did not show a progressive increase until after day 15. (b) As with the increase in percentage, a progressive increase in the total number of IFN-γ-producing CD4 cells per lung was evident between days 6 and 15 in the case of mice infected via the i.v. route alone, between days 6 and 20 in mice infected via both routes, but not until after day 15 in mice infected via the airborne route. Means ± SD of four mice per group per time point.
Earlier generation of CD4 T cells specific for Mtb antigens in mice infected via the i.v. route or via both routes
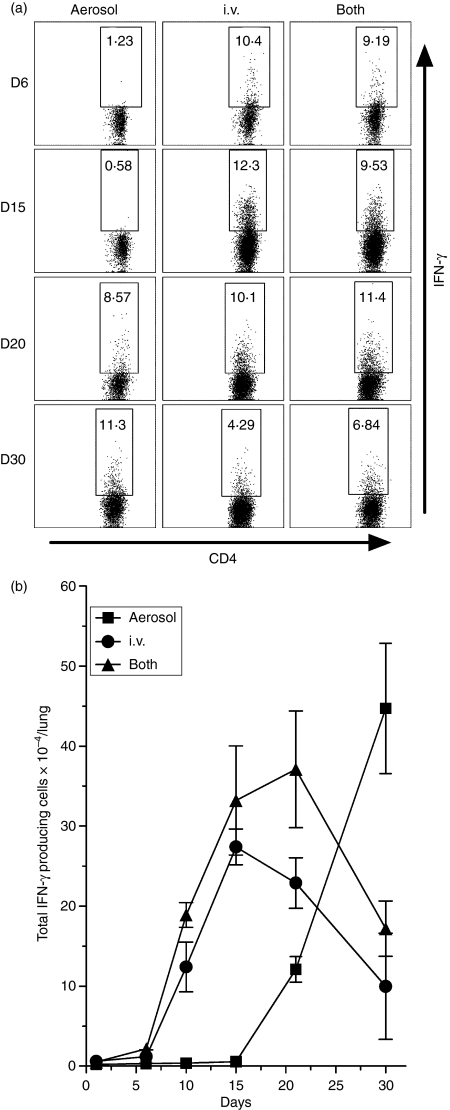
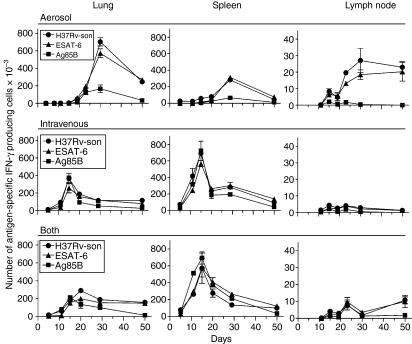
To determine the kinetics of generation of CD4 T cells specific for Mtb antigens, and the kinetics of their accumulation in the lungs, the IFN-γ ELISPOT was employed to enumerate cells in the spleen and lungs capable of making IFN-γ in response to selected antigens at progressive times of infection. The Mtb antigens used in the assay were immunodominant ESAT-6 (1-20)9 and Ag85B (240-54) peptides,10 as well as a sonicate of an Mtb culture.7 It was shown previously7,8 that the cells that respond to these antigens are CD4 T cells. Figure 5 shows that in mice infected via the respiratory route alone Mtb-specific T cells capable of making IFN-γ in response to ESAT-6, Ag85B, or Mtb sonicate began accumulating in the lungs between days 15 and 21 of infection, peaked on day 30, and then underwent a slow decline. By contrast, in the lungs of mice infected via the i.v. route or both routes, Mtb-specific CD4 T cells were present in the lungs as early as day 10 of infection, and peaked in number on day 15 before declining.
Figure 5.
Changes in the total number of CD4 cells capable of making interferon-γ (IFN-γ) in response to ESAT-6 (1-20) peptide, Ag85B (240-54) peptide, or a Mycobacterium tuberculosis (Mtb) sonicate, according to the IFN-γ enzyme-linked immunosorbent spot-forming cell assay. In the lungs of mice infected via the respiratory route alone Mtb-specific CD4 began to increase after about day 20 of infection to peak on day 30 before undergoing a decline. By contrast, in the lungs of mice infected via the i.v. route or via both routes Mtb-specific cells began to increase in number much earlier to peak on day 15 and day 20, respectively. In the spleens of mice infected via the i.v. route or via both routes the production of antigen-specific IFN-γ-producing cells began after about day 5 to peak on day 15, whereas in mice infected via the airborne route alone production did not begin until after day 20 and did not to peak until day 30. In the draining lymph nodes (LNs) of mice infected via the airborne route day 30 was again the time of peak production of IFN-γ-producing cells. The number in the LNs of mice infected via the i.v. route or both routes was too small to make comparisons. Means ± SD of results obtained with cells of four mice per group per time-point.
The earlier appearance of Mtb-specific T cells in the lungs of mice infected via the i.v. route or both routes, was in keeping with the earlier generation of these cells in the spleen. It can be seen in Fig. 5 that in mice of both of these groups appreciable numbers of Mtb-specific T cells were present in the spleen as early as day 10, and that their numbers increased to peak on day 15 before undergoing a decline. This was in contrast to the kinetics of production of these cells in the spleens of mice infected via the respiratory route alone where an increase in number was not discernable until day 20 and did not peak until day 30.
The numbers of Mtb-specific Th1 cells present in the draining tracheobronchial LNs was only a very small fraction of the numbers present in the spleen and lungs. The kinetics of production of these cells in the nodes of mice infected via the respiratory route was in keeping with the kinetics of their production in the lungs. Too few Mtb-specific T cells were present in the nodes of mice of the other two groups to assess their production kinetics.
Discussion
Tuberculosis in mice is a progressive disease exclusively of the lungs, regardless of whether infection is initiated via the airborne route or the i.v. route.1 In either case, Mtb infection is eventually controlled at a stationary level in all major organs, although at a higher stationary level in the lungs. It is known1–4 that control of infection depends on the acquisition of Th1 immunity, which is mediated predominately by CD4 and CD8 T cells. It is generally agreed, however, that the expression of immunity is performed by macrophages in which Mtb resides at sites of infection. The role of Th1 cells is to activate infected macrophages to a mycobacteriastatic state via the secretion of Th1 cytokines. It is known1 that in mice infected via the respiratory route, lung infection is not controlled at a stationary level until after 20 days of Mtb growth, which allows the pathogen to increase in number by more than 4 logs. According to the results presented here this delay is a consequence of the long period of time required for the immune response to airborne infection to be generated. This is a consequence, in turn, of the long period of time required for Mtb or its antigens to disseminate from the lungs to the draining LNs and spleen where immunity needs to be generated. In mice infected via the airborne route it took about 10 days for the LNs and spleen to become infected, a finding that matches other reports.11,12 By contrast, in mice infected via the i.v. route the spleen became infected almost immediately, resulting in much earlier generation of immunity and in stabilization of infection in this organ and also in the lungs. The kinetics of the splenic immune response to i.v. infection as reported here is in agreement with other reports.13 The key finding from this study is that an earlier splenic immune response to i.v. infection provided protection against a concurrent lung infection initiated via the airborne route by enabling the earlier accumulation of IFN-γ-producing T cells in the lungs. Control of airborne lung infection 5 days earlier in mice infected via both routes translates into almost a 1 log lower level of infection. This undoubtedly represents the reason for the previously reported paradoxical finding5that mice infected via both the i.v. and airborne routes survive much longer than mice infected via the airborne route alone.
The results as a whole represent a demonstration of increased protection against Mtb lung infection by an earlier primary immune response. The amount of protection afforded was almost as much as that reported for a secondary response to airborne infection in mice vaccinated with bacillus Calmette–Guérin or other vaccines.14 According to a previous study7 protection afforded by a secondary response to Mtb lung infection is a consequence of an earlier Th1 response that results in earlier accumulation of Th1 cells in the lungs. However, given that there is no reason to expect that Mtb can disseminate from the lungs to the LNs and spleen any sooner in immunized than in naive mice, and that a secondary response, like a primary response, needs to be generated in draining lymphoid tissue,15 it is likely that it is initiated no earlier than a primary response. If so, the observed earlier production of Th1 cells in vaccinated mice7 was probably more apparent than real, in that it was a consequence of being based on stimulation of memory Th1 cells that were present at much higher frequency than the Mtb-specific naive cells in unvaccinated mice. According to the results of the present study, one way to generate an earlier secondary Th1 response to Mtb challenge in vaccinated mice would be to challenge via the i.v. route.
The results generated by this study of mouse tuberculosis might be viewed by some to be of limited relevance to vaccination against tuberculosis in humans. However, it was recently pointed out in defence of the mouse model1 that, in spite of differences in lung histopathology, tuberculosis in mice, like the disease in more than 85% of susceptible humans, is exclusively a disease of the lungs, and the type of immunity generated to combat infection is essentially the same in both host species. Immunity to mouse tuberculosis is a model of immunity to the disease in susceptible hosts. It has been argued,1 therefore, that it can serve as a model of immunity to the disease in the 5–10% of humans who are susceptible. The long period of time before Mtb can be carried from sites of lung infection to draining LNs via afferent lymphatics, and then to the blood vasculature via efferent lymphatics, as illustrated here with the mouse model, is to a large extent a function of physiological processes common to all mammalian hosts. The results draw attention to peculiarities of lung immunology, which Mtb has evolved to take advantage of. In a vaccinated, genetically susceptible host the delay before immunity can be generated against airborne lung infection is likely to be long enough to allow infection to become established enough to induce pathology.
An additional finding generated by this study that is deserving of mention is that lung infection initiated via the i.v. route progresses more slowly than lung infection initiated via the airborne route. In other words, Mtb that enters the lungs from the blood multiplies with a longer doubling time than Mtb that enters by inhalation. For this reason, immunity generated in response to i.v. infection is not only expressed earlier, but is expressed against a lower level of lung infection. This is undoubtedly the reason why Mtb appears much less virulent when given via the i.v. route.5 It needs to be kept in mind that for Mtb given via the i.v. route to enter the lung alveoli it needs to be carried first across vascular endothelium and then across respiratory epithelium by blood phagocytes. By contrast, bacilli that are inhaled are ingested directly by resident alveolar macrophages. Presumably, the cellular and humoral events that follow ingestion of Mtb by alveolar macrophages are different from those that follow entry of Mtb into the lungs inside blood phagocytes.
Acknowledgments
This study was supported by U.S. National Institutes of Health grants AI-37844 and AI-069161.
Abbreviations
- Ag85B
antigen 85B
- CFU
colony-forming unit
- ELISPOT
enzyme-linked immunosorbent spot-forming cell assay
- ESAT-6
early secretory antigen-6
- Mtb
Mycobacterium tuberculosis
- SR
streptomycin-resistant
- Th1
T helper type 1
- WT
wild-type
References
- 1.North RJ, Jung YJ. Immunity to tuberculosis. Annu Rev Immunol. 2004;22:599–623. doi: 10.1146/annurev.immunol.22.012703.104635. [DOI] [PubMed] [Google Scholar]
- 2.Boom WH. The role of T cell subsets in Mycobacterium tuberculosis infection. Infect Agents Dis. 1996;5:3–81. [PubMed] [Google Scholar]
- 3.Flynn JL, Chan J. Immunobiology of tuberculosis. Annu Rev Immunol. 2001;19:93–129. doi: 10.1146/annurev.immunol.19.1.93. [DOI] [PubMed] [Google Scholar]
- 4.Kaufmann SH. How can immunology contribute to the control of tuberculosis? Nat Rev Immunol. 2001;1:20–30. doi: 10.1038/35095558. [DOI] [PubMed] [Google Scholar]
- 5.North RJ. Mycobacterium tuberculosis is strikingly more virulent for mice when given via the respiratory than via the intravenous route. J Infect Dis. 1995;172:1550. doi: 10.1093/infdis/172.6.1550. [DOI] [PubMed] [Google Scholar]
- 6.Medina E, North RJ. Resistance ranking of some common inbred mouse strains to Mycobacterium tuberculosis and relationship to major histocompatibility complex haplotype and Nramp1 genotype. Immunology. 1998;93:270–4. doi: 10.1046/j.1365-2567.1998.00419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jung YJ, Ryan L, LaCourse R, North RJ. Properties and protective value of the secondary versus primary T helper type 1 response to airborne Mycobacterium tuberculosis infection in mice. J Exp Med. 2005;201:1915–24. doi: 10.1084/jem.20050265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rogerson BJ, Jung YJ, LaCourse R, Ryan L, Enright N, North RJ. Expression levels of Mycobacterium tuberculosis antigen-encoding genes versus production levels of antigen-specific T cells during stationary level lung infection in mice. Immunology. 2006;118:195–201. doi: 10.1111/j.1365-2567.2006.02355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brandt L, Oettinger T, Holm A, Andersen AB, Andersen P. Key epitopes on the ESAT-6 Ag recognized in mice during the recall of protective immunity to Mycobacterium tuberculosis. J Immunol. 1996;157:3527–33. [PubMed] [Google Scholar]
- 10.D’Souza S, Rosseels V, Romano M, et al. Mapping of murine Th1 helper T-cell epitopes of mycolyl transferases Ag85A, Ag85B, and Ag85C from Mycobacterium tuberculosis. Infect Immun. 2003;71:483–93. doi: 10.1128/IAI.71.1.483-493.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chackerian AA, Alt JM, Perera TV, Dascher CC, Behar SM. Dissemination of Mycobacterium tuberculosis is influenced by host factors and precedes the initiation of T-cell immunity. Infect Immun. 2002;70:4501–9. doi: 10.1128/IAI.70.8.4501-4509.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garcia-Romo GS, Pedroza-Gonzalez A, Aguilar-Leon D, Orozco-Estevez H, Lambrecht BN, Estrada-Garcia I, Flores-Romo L, Hernandez-Pando R. Airways infection with virulent Mycobacterium tuberculosis delays the influx of dendritic cells and the expression of costimulatory molecules in mediastinal lymph nodes. Immunology. 2004;112:661–8. doi: 10.1046/j.1365-2567.2004.01904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tian T, Woodworth J, Skold M, Behar SM. In vivo depletion of CD11c+ cells delays the CD4+ T cell response to Mycobacterium tuberculosis and exacerbates the outcome of infection. J Immunol. 2005;175:3268–72. doi: 10.4049/jimmunol.175.5.3268. [DOI] [PubMed] [Google Scholar]
- 14.Britton WJ, Palendira U. Improving vaccines against tuberculosis. Immunol Cell Biol. 2003;81:34–45. doi: 10.1046/j.0818-9641.2002.01143.x. [DOI] [PubMed] [Google Scholar]
- 15.Zinkernagel RM. Localization dose and time of antigens determine immune reactivity. Semin Immunol. 2000;12:163–71. doi: 10.1006/smim.2000.0253. [DOI] [PubMed] [Google Scholar]