Abstract
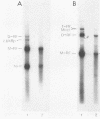
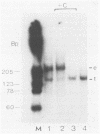
We have observed the binding of viral and cellular proteins to the Aleutian disease virus (ADV) 3' terminus of replicative-form DNA. Gel retardation assays showed specific band shifts produced by whole-cell extracts from either ADV-infected or uninfected cells, as well as band reduction produced by ADV capsids. In all cases, binding was confined to the turnaround, T-shaped terminal form; no binding to the extended conformation of replicative-form DNA was detected. This indicates the importance of the T-shaped secondary structure in protein recognition. We have previously reported the binding of a 3'-terminal ADV DNA restriction fragment to the ADV capsid protein VP1 (K. Willwand and O.-R. Kaaden, Virology 166:52-57, 1988). Here we show that the region between nucleotides 14 and 102 on the ADV genome is required for binding. It is suggested that the VP1-DNA interaction mediates the binding of ADV DNA to empty viral capsids and that this is followed by displacement synthesis and packaging of ADV progeny DNA. A scheme for the possible mechanism of this process is presented.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexandersen S., Bloom M. E., Perryman S. Detailed transcription map of Aleutian mink disease parvovirus. J Virol. 1988 Oct;62(10):3684–3694. doi: 10.1128/jvi.62.10.3684-3694.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astell C. R., Chow M. B., Ward D. C. Sequence analysis of the termini of virion and replicative forms of minute virus of mice DNA suggests a modified rolling hairpin model for autonomous parvovirus DNA replication. J Virol. 1985 Apr;54(1):171–177. doi: 10.1128/jvi.54.1.171-177.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates R. C., Snyder C. E., Banerjee P. T., Mitra S. Autonomous parvovirus LuIII encapsidates equal amounts of plus and minus DNA strands. J Virol. 1984 Feb;49(2):319–324. doi: 10.1128/jvi.49.2.319-324.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom M. E., Alexandersen S., Perryman S., Lechner D., Wolfinbarger J. B. Nucleotide sequence and genomic organization of Aleutian mink disease parvovirus (ADV): sequence comparisons between a nonpathogenic and a pathogenic strain of ADV. J Virol. 1988 Aug;62(8):2903–2915. doi: 10.1128/jvi.62.8.2903-2915.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom M. E., Mayer L. W., Garon C. F. Characterization of the Aleutian disease virus genome and its intracellular forms. J Virol. 1983 Mar;45(3):977–984. doi: 10.1128/jvi.45.3.977-984.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom M. E., Race R. E., Wolfinbarger J. B. Characterization of Aleutian disease virus as a parvovirus. J Virol. 1980 Sep;35(3):836–843. doi: 10.1128/jvi.35.3.836-843.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom M. E., Race R. E., Wolfinbarger J. B. Identification of a nonvirion protein of Aleutian disease virus: mink with Aleutian disease have antibody to both virion and nonvirion proteins. J Virol. 1982 Aug;43(2):608–616. doi: 10.1128/jvi.43.2.608-616.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotmore S. F., Gunther M., Tattersall P. Evidence for a ligation step in the DNA replication of the autonomous parvovirus minute virus of mice. J Virol. 1989 Feb;63(2):1002–1006. doi: 10.1128/jvi.63.2.1002-1006.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotmore S. F., Tattersall P. The autonomously replicating parvoviruses of vertebrates. Adv Virus Res. 1987;33:91–174. doi: 10.1016/s0065-3527(08)60317-6. [DOI] [PubMed] [Google Scholar]
- Faust E. A., Gloor G. Characterization of a metastable, partially replicated dimeric intermediate of minute virus of mice. J Virol. 1984 Feb;49(2):621–625. doi: 10.1128/jvi.49.2.621-625.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermonat P. L., Labow M. A., Wright R., Berns K. I., Muzyczka N. Genetics of adeno-associated virus: isolation and preliminary characterization of adeno-associated virus type 2 mutants. J Virol. 1984 Aug;51(2):329–339. doi: 10.1128/jvi.51.2.329-339.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Im D. S., Muzyczka N. Factors that bind to adeno-associated virus terminal repeats. J Virol. 1989 Jul;63(7):3095–3104. doi: 10.1128/jvi.63.7.3095-3104.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaaden O. R., Haas L., Löchelt M., Roth S. Replication of Aleutian disease virus in mink lymphocytes infected in vitro. Intervirology. 1986;25(4):201–209. doi: 10.1159/000149676. [DOI] [PubMed] [Google Scholar]
- Kierek-Jaszczuk D., Moennig W., Stolze B., Neth R., Tan S., Greiser de Wilke I., Kaaden O. R. Epitopic mapping of structural and nonstructural Aleutian disease virus proteins. Intervirology. 1986;26(1-2):74–84. doi: 10.1159/000149684. [DOI] [PubMed] [Google Scholar]
- Kyhse-Andersen J. Electroblotting of multiple gels: a simple apparatus without buffer tank for rapid transfer of proteins from polyacrylamide to nitrocellulose. J Biochem Biophys Methods. 1984 Dec;10(3-4):203–209. doi: 10.1016/0165-022x(84)90040-x. [DOI] [PubMed] [Google Scholar]
- Lederman M., Shull B. C., Stout E. R., Bates R. C. Bovine parvovirus DNA-binding proteins: identification by a combined DNA hybridization and immunodetection assay. J Gen Virol. 1987 Jan;68(Pt 1):147–157. doi: 10.1099/0022-1317-68-1-147. [DOI] [PubMed] [Google Scholar]
- Lefebvre R. B., Riva S., Berns K. I. Conformation takes precedence over sequence in adeno-associated virus DNA replication. Mol Cell Biol. 1984 Jul;4(7):1416–1419. doi: 10.1128/mcb.4.7.1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusby E., Fife K. H., Berns K. I. Nucleotide sequence of the inverted terminal repetition in adeno-associated virus DNA. J Virol. 1980 May;34(2):402–409. doi: 10.1128/jvi.34.2.402-409.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löchelt M., Delius H., Kaaden O. R. A novel replicative form DNA of Aleutian disease virus: the covalently closed linear DNA of the parvoviruses. J Gen Virol. 1989 May;70(Pt 5):1105–1116. doi: 10.1099/0022-1317-70-5-1105. [DOI] [PubMed] [Google Scholar]
- Löchelt M., Kaaden O. R. Lymphotropic strain SL3 of Aleutian disease virus: identification of replicative form DNA, molecular cloning and expression of capsid-specific proteins. J Gen Virol. 1987 Apr;68(Pt 4):1041–1048. doi: 10.1099/0022-1317-68-4-1041. [DOI] [PubMed] [Google Scholar]
- Miskimins W. K., Roberts M. P., McClelland A., Ruddle F. H. Use of a protein-blotting procedure and a specific DNA probe to identify nuclear proteins that recognize the promoter region of the transferrin receptor gene. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6741–6744. doi: 10.1073/pnas.82.20.6741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller D. E., Siegl G. Maturation of parvovirus LuIII in a subcellular system. I. Optimal conditions for in vitro synthesis and encapsidation of viral DNA. J Gen Virol. 1983 May;64(Pt 5):1043–1054. doi: 10.1099/0022-1317-64-5-1043. [DOI] [PubMed] [Google Scholar]
- Rhode S. L., 3rd, Klaassen B. DNA sequence of the 5' terminus containing the replication origin of parvovirus replicative form DNA. J Virol. 1982 Mar;41(3):990–999. doi: 10.1128/jvi.41.3.990-999.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhode S. L., 3rd Replication process of the parvovirus H-1 V. Isolation and characterization of temperature-sensitive H-1 mutants. J Virol. 1976 Feb;17(2):659–667. doi: 10.1128/jvi.17.2.659-667.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards R., Linser P., Armentrout R. W. Kinetics of assembly of a parvovirus, minute virus of mice, in synchronized rat brain cells. J Virol. 1977 Jun;22(3):778–793. doi: 10.1128/jvi.22.3.778-793.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senapathy P., Tratschin J. D., Carter B. J. Replication of adeno-associated virus DNA. Complementation of naturally occurring rep- mutants by a wild-type genome or an ori- mutant and correction of terminal palindrome deletions. J Mol Biol. 1984 Oct 15;179(1):1–20. doi: 10.1016/0022-2836(84)90303-6. [DOI] [PubMed] [Google Scholar]
- Shade R. O., Blundell M. C., Cotmore S. F., Tattersall P., Astell C. R. Nucleotide sequence and genome organization of human parvovirus B19 isolated from the serum of a child during aplastic crisis. J Virol. 1986 Jun;58(3):921–936. doi: 10.1128/jvi.58.3.921-936.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegl G., Bates R. C., Berns K. I., Carter B. J., Kelly D. C., Kurstak E., Tattersall P. Characteristics and taxonomy of Parvoviridae. Intervirology. 1985;23(2):61–73. doi: 10.1159/000149587. [DOI] [PubMed] [Google Scholar]
- Sliski A. H., Essex M., Meyer C., Todaro G. Feline oncornavirus-associated cell membrane antigen: expression in transformed nonproducer mink cells. Science. 1977 Jun 17;196(4296):1336–1339. doi: 10.1126/science.194310. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Straus S. E., Sebring E. D., Rose J. A. Concatemers of alternating plus and minus strands are intermediates in adenovirus-associated virus DNA synthesis. Proc Natl Acad Sci U S A. 1976 Mar;73(3):742–746. doi: 10.1073/pnas.73.3.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugden B., Keller W. Mammalian deoxyribonucleic acid-dependent ribonucleic acid polymerases. I. Purification and properties of an -amanitin-sensitive ribonucleic acid polymerase and stimulatory factors from HeLa and KB cells. J Biol Chem. 1973 Jun 10;248(11):3777–3788. [PubMed] [Google Scholar]
- Tattersall P., Ward D. C. Rolling hairpin model for replication of parvovirus and linear chromosomal DNA. Nature. 1976 Sep 9;263(5573):106–109. doi: 10.1038/263106a0. [DOI] [PubMed] [Google Scholar]
- Tratschin J. D., Miller I. L., Carter B. J. Genetic analysis of adeno-associated virus: properties of deletion mutants constructed in vitro and evidence for an adeno-associated virus replication function. J Virol. 1984 Sep;51(3):611–619. doi: 10.1128/jvi.51.3.611-619.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tullis G. E., Labieniec-Pintel L., Clemens K. E., Pintel D. Generation and characterization of a temperature-sensitive mutation in the NS-1 gene of the autonomous parvovirus minute virus of mice. J Virol. 1988 Aug;62(8):2736–2744. doi: 10.1128/jvi.62.8.2736-2744.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willwand K., Kaaden O. R. Capsid protein VP1 (p85) of Aleutian disease virus is a major DNA-binding protein. Virology. 1988 Sep;166(1):52–57. doi: 10.1016/0042-6822(88)90145-6. [DOI] [PubMed] [Google Scholar]
- van Dawen S., Kaaden O. R., Roth S. Propagation of Aleutian disease parvovirus in cell line CCC clone 81. Arch Virol. 1983;77(1):39–50. doi: 10.1007/BF01314862. [DOI] [PubMed] [Google Scholar]