Abstract
Subacute sclerosing panencephalitis (SSPE) is a chronic and usually fatal central nervous system disease caused by a persistent infection with measles virus. The pathogenic mechanisms of the disease are poorly understood, but restricted expression of viral antigens within the infected tissue appears to be involved. We have previously proposed that interferon (IFN) plays a role in the pathogenesis of SSPE by interacting with viral subpopulations that are relatively resistant to IFN-mediated inhibition. Such IFN-resistant viral subpopulations have now been identified in six independent strains of measles virus, two derived from patients with measles and four derived from patients with SSPE. By means of a replicative-plating procedure, these IFN-resistant viruses were found to be heterogeneous with respect to their growth in the presence of high levels of IFN. One viral form replicates fully, with complete destruction of the infected-cell culture, whereas the other form induces a restricted, self-limited form of cytopathic effect, similar to that seen with cell-associated strains of measles virus isolated from SSPE patients. Passage of a virus stock containing both of these viral forms through the central nervous system tissue of newborn hamsters strongly selects for the viral form associated with the self-limiting type of cytopathic effect. The presence of this form of IFN-resistant virus coupled with chronic production of IFN within the central nervous system may account for viral persistence in SSPE patients.
Full text
PDF

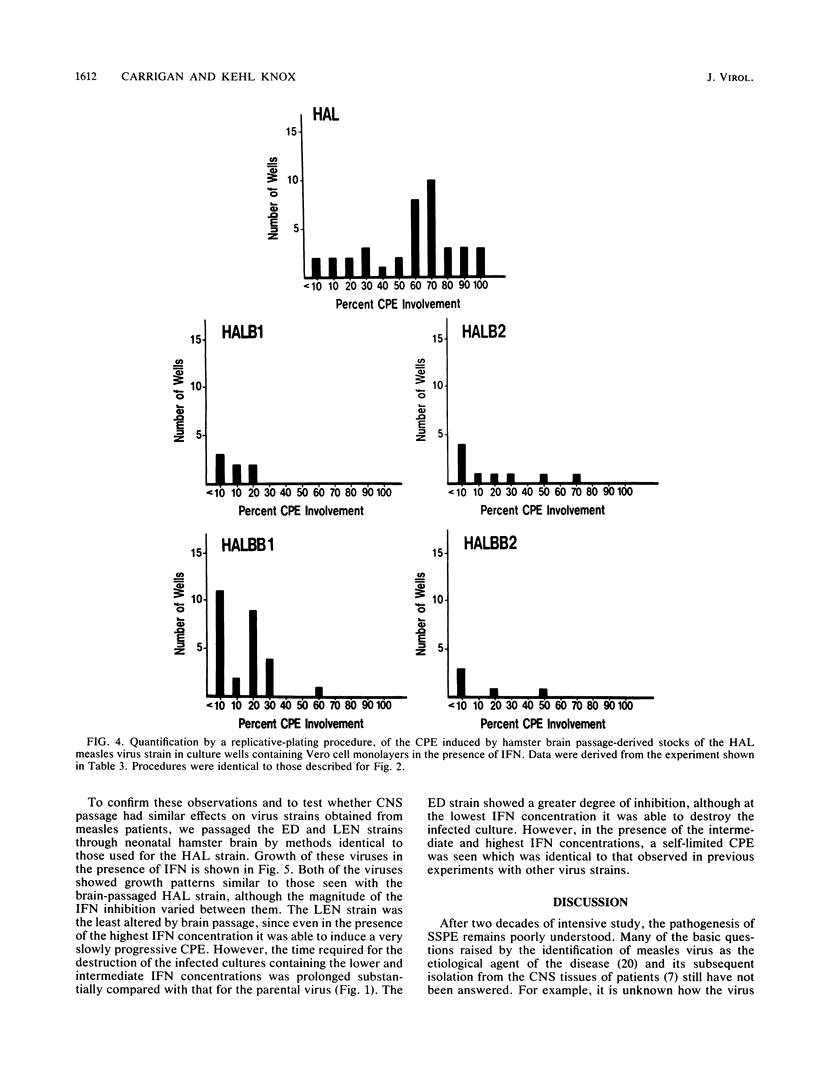


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaby P., Bukh J., Lisse I. M., Smits A. J. Overcrowding and intensive exposure as determinants of measles mortality. Am J Epidemiol. 1984 Jul;120(1):49–63. doi: 10.1093/oxfordjournals.aje.a113874. [DOI] [PubMed] [Google Scholar]
- Aaby P., Bukh J., Lisse I. M., Smits A. J. Risk factors in subacute sclerosing panencephalitis: age- and sex-dependent host reactions or intensive exposure? Rev Infect Dis. 1984 Mar-Apr;6(2):239–250. doi: 10.1093/clinids/6.2.239. [DOI] [PubMed] [Google Scholar]
- Ayata M., Hirano A., Wong T. C. Structural defect linked to nonrandom mutations in the matrix gene of biken strain subacute sclerosing panencephalitis virus defined by cDNA cloning and expression of chimeric genes. J Virol. 1989 Mar;63(3):1162–1173. doi: 10.1128/jvi.63.3.1162-1173.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baczko K., Liebert U. G., Billeter M., Cattaneo R., Budka H., ter Meulen V. Expression of defective measles virus genes in brain tissues of patients with subacute sclerosing panencephalitis. J Virol. 1986 Aug;59(2):472–478. doi: 10.1128/jvi.59.2.472-478.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbanti-Brodano G., Oyanagi S., Katz M., Koprowski H. Presence of 2 different viral agents in brain cells of patients with subacute sclerosing panencephalitis. Proc Soc Exp Biol Med. 1970 May;134(1):230–236. doi: 10.3181/00379727-134-34765. [DOI] [PubMed] [Google Scholar]
- Baublis J. V., Payne F. E. Measles antigen and syncytium formtion in brain cell cultures from subacute sclerosing panencephalitis (SSPE). Proc Soc Exp Biol Med. 1968 Nov;129(2):593–597. doi: 10.3181/00379727-129-33377. [DOI] [PubMed] [Google Scholar]
- Behan P. O., Behan W. M. Immunological abnormalities and immunotherapeutic attempts in subacute sclerosing panencephalitis. Prog Brain Res. 1983;59:149–162. doi: 10.1016/S0079-6123(08)63860-1. [DOI] [PubMed] [Google Scholar]
- Burnstein T., Jacobsen L. B., Zeman W., Chen T. T. Persistent infection of BSC-1 cells by defective measles virus derived from subacute sclerosing panencephalitis. Infect Immun. 1974 Dec;10(6):1378–1382. doi: 10.1128/iai.10.6.1378-1382.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bye A., Balkwill F., Brigden D., Wilson J. Use of interferon in the management of patients with subacute sclerosing panencephalitis. Dev Med Child Neurol. 1985 Apr;27(2):170–175. doi: 10.1111/j.1469-8749.1985.tb03766.x. [DOI] [PubMed] [Google Scholar]
- Byington D. P., Castro A. E., Burnstein T. Adaptation to hamsters of neurotropic measles virus from subacute sclerosing panencephalitis. Nature. 1970 Feb 7;225(5232):554–555. doi: 10.1038/225554b0. [DOI] [PubMed] [Google Scholar]
- Cape C. A., Martinez A. J., Robertson J. T., Hamilton R., Jabbour J. T. Adult onset of subacute sclerosing panencephalitis. Arch Neurol. 1973 Feb;28(2):124–127. doi: 10.1001/archneur.1973.00490200072010. [DOI] [PubMed] [Google Scholar]
- Carrigan D. R., Kabacoff C. M. Identification of a nonproductive, cell-associated form of measles virus by its resistance to inhibition by recombinant human interferon. J Virol. 1987 Jun;61(6):1919–1926. doi: 10.1128/jvi.61.6.1919-1926.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrigan D. R., Kabacoff C. M. Nonproductive, cell-associated virus exists before the appearance of antiviral antibodies in experimental measles encephalitis. Virology. 1987 Jan;156(1):185–188. doi: 10.1016/0042-6822(87)90452-1. [DOI] [PubMed] [Google Scholar]
- Carrigan D. R. Round cell variant of measles virus: mechanisms involved in the establishment of defective viral infection of the central nervous system. Virology. 1986 Dec;155(2):614–624. doi: 10.1016/0042-6822(86)90221-7. [DOI] [PubMed] [Google Scholar]
- Carrigan D. R. Round cell variant of measles virus: neurovirulence and pathogenesis of acute encephalitis in newborn hamsters. Virology. 1986 Jan 30;148(2):349–359. doi: 10.1016/0042-6822(86)90331-4. [DOI] [PubMed] [Google Scholar]
- Carrigan D. R. Round cell variant of measles virus: spontaneous conversion from productive to cell-associated state of infection. Virology. 1985 Jul 30;144(2):337–350. doi: 10.1016/0042-6822(85)90276-4. [DOI] [PubMed] [Google Scholar]
- Cattaneo R., Schmid A., Billeter M. A., Sheppard R. D., Udem S. A. Multiple viral mutations rather than host factors cause defective measles virus gene expression in a subacute sclerosing panencephalitis cell line. J Virol. 1988 Apr;62(4):1388–1397. doi: 10.1128/jvi.62.4.1388-1397.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo R., Schmid A., Rebmann G., Baczko K., Ter Meulen V., Bellini W. J., Rozenblatt S., Billeter M. A. Accumulated measles virus mutations in a case of subacute sclerosing panencephalitis: interrupted matrix protein reading frame and transcription alteration. Virology. 1986 Oct 15;154(1):97–107. doi: 10.1016/0042-6822(86)90433-2. [DOI] [PubMed] [Google Scholar]
- Connolly J. H., Allen I. V., Hurwitz L. J., Millar J. H. Measles-virus antibody and antigen in subacute sclerosing panencephalitis. Lancet. 1967 Mar 11;1(7489):542–544. doi: 10.1016/s0140-6736(67)92117-4. [DOI] [PubMed] [Google Scholar]
- Crespi M., Chiu M. N., Struthers J. K., Schoub B. D., Lyons S. F. Effect of interferon on Vero cells persistently infected with Sendai virus compared to Vero cells persistently infected with SSPE virus. Arch Virol. 1988;98(3-4):235–251. doi: 10.1007/BF01322172. [DOI] [PubMed] [Google Scholar]
- Detels R., Brody J. A., McNew J., Edgar A. H. Further epidemiological studies of subacute sclerosing panencephalitis. Lancet. 1973 Jul 7;2(7819):11–14. doi: 10.1016/s0140-6736(73)91946-6. [DOI] [PubMed] [Google Scholar]
- Dhib-Jalbut S., Jacobson S., McFarlin D. E., McFarland H. F. Impaired human leukocyte antigen-restricted measles virus-specific cytotoxic T-cell response in subacute sclerosing panencephalitis. Ann Neurol. 1989 Mar;25(3):272–280. doi: 10.1002/ana.410250311. [DOI] [PubMed] [Google Scholar]
- Doi Y., Sanpe T., Nakajima M., Okawa S., Koto T. Properties of a cytopathic agent isolated from a patient with subacute sclerosing panencephalitis in Japan. Jpn J Med Sci Biol. 1972 Oct;25(5):321–333. doi: 10.7883/yoken1952.25.321. [DOI] [PubMed] [Google Scholar]
- ENDERS J. F., PEEBLES T. C. Propagation in tissue cultures of cytopathogenic agents from patients with measles. Proc Soc Exp Biol Med. 1954 Jun;86(2):277–286. doi: 10.3181/00379727-86-21073. [DOI] [PubMed] [Google Scholar]
- Friis B., Bloch B., Faber V., Salmi A., Ziola B. Coincidental fluctuations of humoral immunity and clinical progression in a patient with subacute sclerosing panencephalitis. J Neurol. 1985;232(1):52–54. doi: 10.1007/BF00314042. [DOI] [PubMed] [Google Scholar]
- Haase A. T., Gantz D., Eble B., Walker D., Stowring L., Ventura P., Blum H., Wietgrefe S., Zupancic M., Tourtellotte W. Natural history of restricted synthesis and expression of measles virus genes in subacute sclerosing panencephalitis. Proc Natl Acad Sci U S A. 1985 May;82(9):3020–3024. doi: 10.1073/pnas.82.9.3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heymann D. L., Smith E. L., Nakano J. H., Jato J. G., Martin G. E., Maben G. K. Further field testing of the more heat-stable measles vaccines in Cameroon. Br Med J (Clin Res Ed) 1982 Aug 21;285(6341):531–533. doi: 10.1136/bmj.285.6341.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland J., Spindler K., Horodyski F., Grabau E., Nichol S., VandePol S. Rapid evolution of RNA genomes. Science. 1982 Mar 26;215(4540):1577–1585. doi: 10.1126/science.7041255. [DOI] [PubMed] [Google Scholar]
- Horta-Barbosa L., Hamilton R., Wittig B., Fuccillo D. A., Sever J. L., Vernon M. L. Subacute sclerosing panencephalitis: isolation of suppressed measles virus from lymph node biopsies. Science. 1971 Aug 27;173(3999):840–841. doi: 10.1126/science.173.3999.840. [DOI] [PubMed] [Google Scholar]
- Huttenlocher P. R., Picchietti D. L., Roos R. P., Cashman N. R., Horowitz B., Horowitz M. S. Intrathecal interferon in subacute sclerosing panencephalitis. Ann Neurol. 1986 Mar;19(3):303–305. doi: 10.1002/ana.410190317. [DOI] [PubMed] [Google Scholar]
- Hänninen P., Arstila P., Lang H., Salmi A., Panelius M. Involvement of the central nervous system in acute, uncomplicated measles virus infection. J Clin Microbiol. 1980 Jun;11(6):610–613. doi: 10.1128/jcm.11.6.610-613.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson S., McFarland H. F. Measles virus persistence in human lymphocytes: a role for virus-induced interferon. J Gen Virol. 1982 Dec;63(2):351–357. doi: 10.1099/0022-1317-63-2-351. [DOI] [PubMed] [Google Scholar]
- Johnson K. P., Norrby E., Swoveland P., Carrigan D. R. Expression of five viral antigens in cells infected with wild-type and SSPE strains of measles virus: correlation with cytopathic effects and productivity of infections. Arch Virol. 1982;73(3-4):255–262. doi: 10.1007/BF01318079. [DOI] [PubMed] [Google Scholar]
- Joncas J. H., Robillard L. R., Boudreault A., Leyritz M., McLaughlin B. J. Letter: Interferon in serum and cerebrospinal fluid in subacute sclerosing panencephalitis. Can Med Assoc J. 1976 Aug 21;115(4):309–309. [PMC free article] [PubMed] [Google Scholar]
- Liebert U. G., Baczko K., Budka H., ter Meulen V. Restricted expression of measles virus proteins in brains from cases of subacute sclerosing panencephalitis. J Gen Virol. 1986 Nov;67(Pt 11):2435–2444. doi: 10.1099/0022-1317-67-11-2435. [DOI] [PubMed] [Google Scholar]
- Panitch H. S., Gomez-Plascencia J., Norris F. H., Cantell K., Smith R. A. Subacute sclerosing panencephalitis: remission after treatment with intraventricular interferon. Neurology. 1986 Apr;36(4):562–566. doi: 10.1212/wnl.36.4.562. [DOI] [PubMed] [Google Scholar]
- Rager-Zisman B., Egan J. E., Kress Y., Bloom B. R. Isolation of cold-sensitive mutants of measles virus from persistently infected murine neuroblastoma cells. J Virol. 1984 Sep;51(3):845–855. doi: 10.1128/jvi.51.3.845-855.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharova O. K., Rozina E. E., Gordienko N. M., Shteinberg L. S. Effect of immunosuppression on morphological changes in CNS of monkeys infected with different measles virus vaccine strains. Acta Virol. 1984 Mar;28(2):144–147. [PubMed] [Google Scholar]
- Shteinberg L. S., Gordienko N. M. Genetic characteristics of clones derived from measles virus strain L-16. Acta Virol. 1977 Sep;21(5):383–390. [PubMed] [Google Scholar]
- Thormar H., Jervis G. A., Karl S. C., Brown H. R. Passage in ferrets of encephalitogenic cell-associated measles virus isolated from brain of a patient with subacute sclerosing panencephalitis. J Infect Dis. 1973 Jun;127(6):678–685. doi: 10.1093/infdis/127.6.678. [DOI] [PubMed] [Google Scholar]
- Traugott U., Lebon P. Multiple sclerosis: involvement of interferons in lesion pathogenesis. Ann Neurol. 1988 Aug;24(2):243–251. doi: 10.1002/ana.410240211. [DOI] [PubMed] [Google Scholar]
- Tsai S. C., Summers B. A., Appel M. J. Interferon in cerebrospinal fluid. A marker for viral persistence of canine distemper encephalomyelitis. Arch Virol. 1982;72(4):257–265. doi: 10.1007/BF01315222. [DOI] [PubMed] [Google Scholar]
- Ueda S., Okuno Y., Hamamoto Y., Oya H. Subacute sclerosing panencephalitis (SSPE): isolation of a defective variant of measles virus from brain obtained at autopsy. Biken J. 1975 Jun;18(2):113–122. [PubMed] [Google Scholar]
- Vydelingum S., Ilonen J., Salonen R., Marusyk R., Salmi A. Infection of human peripheral blood mononuclear cells with a temperature-sensitive mutant of measles virus. J Virol. 1989 Feb;63(2):689–695. doi: 10.1128/jvi.63.2.689-695.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler S. L., Meissner H. C. Measles and SSPE viruses: similarities and differences. Prog Med Virol. 1982;28:65–95. [PubMed] [Google Scholar]
- Wirguin I., Steiner I., Kidron D., Brenner T., Udem S., Rager B., Abramsky O. Fulminant subacute sclerosing panencephalitis in association with pregnancy. Arch Neurol. 1988 Dec;45(12):1324–1325. doi: 10.1001/archneur.1988.00520360042009. [DOI] [PubMed] [Google Scholar]


