Abstract
This study aimed to identify signaling pathways that oppose connective tissue fibrosis in the aortic valve. Using valvular interstitial cells (VICs) isolated from porcine aortic valve leaflets, we show that basic fibroblast growth factor (FGF-2) effectively blocks transforming growth factor-β1 (TGF-β1)-mediated myofibroblast activation. FGF-2 prevents the induction of α-smooth muscle actin (αSMA) expression and the exit of VICs from the cell cycle, both of which are hallmarks of myofibroblast activation. By blocking the activity of the Smad transcription factors that serve as the downstream nuclear effectors of TGF-β1, FGF-2 treatment inhibits fibrosis in VICs. Using an exogenous Smad-responsive transcriptional promoter reporter, we show that Smad activity is repressed by FGF-2, likely an effect of the fact that FGF-2 treatment prevents the nuclear localization of Smads in these cells. This appears to be a direct effect of FGF signaling through mitogen-activated protein kinase (MAPK) cascades as the treatment of VICs with the MAPK/extracellular regulated kinase (MEK) inhibitor U0126 acted to induce fibrosis and blocked the ability of FGF-2 to inhibit TGF-β1 signaling. Furthermore, FGF-2 treatment of VICs blocks the development of pathological contractile and calcifying phenotypes, suggesting that these pathways may be utilized in the engineering of effective treatments for valvular disease.—Cushing, M. C., Mariner, P. D., Liao, J. T., Sims, E. A., Anseth, K. S. Fibroblast growth factor represses Smad-mediated myofibroblast activation in aortic valvular interstitial cells.
Keywords: transforming growth factor-β1, heart disease, α-smooth muscle actin, cell signaling
Valvular interstitial cells (VICs) are the main cell type of the cardiac valve leaflet and constitute a phenotypically dynamic population of cells that can be activated to become myofibroblasts (1). Although myofibroblasts are key participants in wound repair and tissue remodeling, improper regulation of VIC myofibroblast activation can have deleterious effects in heart valves, including excessive scarring and calcific nodule formation (2, 3). For this reason, the life cycle of the VIC is carefully regulated such that activated myofibroblasts undergo apoptosis (4) and/or revert back to a quiescent VIC phenotype (5) when wound healing is completed. Under pathological conditions, prolonged myofibroblast activation leads to an accumulation of compositionally unbalanced extracellular matrix (ECM) that alters the mechanical and hemodynamic properties of the heart valve (6). Similar effects have been observed in other tissues, where the chronic persistence of ECM-producing myofibroblast cells has been linked to deposition of collagenous scar tissue (7,8,9). For this reason, an understanding of the signaling mechanisms that control VIC differentiation in the heart valve is critical to our ability to prevent valvular fibrosis and dysfunction.
Myofibroblast differentiation in the heart can be induced by several mechanisms, including exposure to cytokines (10), growth factors (1), and ECM components (11, 12). Cultured in vitro, VICs isolated from porcine heart valves can be stimulated to become myofibroblasts with transforming growth factor-β1 (TGF-β1). TGF-β is one of the most widely distributed profibrotic cytokines, and disruption of TGF-β homeostasis has been linked to enhanced fibrogenesis in many organs (3, 13, 14), including the aortic valve (3). TGF-β regulates the deposition of ECM in both normal responses to tissue injury and in pathological settings and has been shown to skew the composition of neomatrix toward predominantly collagenous scar tissue (15, 16). The addition of exogenous TGF-β1 to VIC cultures leads to myofibroblast activation, as observed by increased ECM and cytokine secretion, as well as enhanced contractility mediated by the de novo expression of α-smooth muscle actin (αSMA)-containing stress fibers (1, 17). Although much is known about the deleterious effects of chronic TGF-β signaling, little is known about the pathways that work in opposition to TGF-β-mediated pathological fibrosis and keep myofibroblast activation in check.
In several other tissues, basic fibroblast growth factor (FGF-2) has been shown to diminish the activation of myofibroblasts. In retinal pericyte (18), breast gland fibroblasts (7), corneal fibroblasts (19), and synovial fibroblasts (20), FGF-2 appears to play an important role in proper wound- healing responses and may act to prevent the deleterious effects of persistent myofibroblast activation. Recently, it was shown that fibroblasts with reduced FGF receptor (FGF-R) expression spontaneously differentiated to myofibroblasts, presumably as a result of impaired FGF-2 signaling (21). These reports suggest that FGF-2 acts as a general antifibrotic factor that can counteract the profibrotic activity of TGF-β1, leading us to hypothesize that FGF-2 plays a similar role in valvular interstitial cells.
In this study, we show that FGF-2 acts to reduce TGF-β1-mediated myofibroblast activation of VICs by preventing the nuclear translocation of Smad transcription factors. TGF-β1 signals through serine/threonine receptor kinases to activate R-Smads (15). Once activated, these Smads are shuttled to the nucleus where they induce the expression of multiple gene targets. Nucleo-cytoplasmic shuttling of Smads can be blocked, however, by other signaling pathways that act in opposition to Smad-mediated fibrosis. In VICs, we show that FGF-2 treatment blocks the nuclear localization of Smads and leads to a reduction in phenotypic markers that are typical of myofibrotic activation. This effect appears to be mediated by mitogen-activated protein kinases (MAPKs), which have been shown previously to antagonize TGF-β signaling by repressing TGF-β receptor expression and attenuating Smad accumulation in the nucleus (22, 23).
The identification of an FGF-2-mediated pathway that opposes myofibroblast activation in VICs has immediate implications for the prevention and treatment of valvular disease. We demonstrate that FGF-2 not only inhibits TGF-β-mediated myofibroblast activation but that it also prevents calcified nodule formation and matrix contraction in VICs. These phenotypes are both characteristic of end-stage valvular disease, suggesting that the maintenance of FGF-2-mediated pathways is an important aspect to preventing the progression of deleterious fibrosis in heart valves.
MATERIALS AND METHODS
Cell Culture
VICs were isolated by sequential collagenase digestion of porcine aortic leaflets as described previously (24). Briefly, aortic leaflets were removed from intact porcine hearts (Hormel, Austin, MN, USA) and subjected to sequential collagenase digestion (250 U/ml, Worthington Biochemical, Lakewood, NJ, USA). Isolated cells were plated and cultured in growth media (1) containing 15% fetal bovine serum (FBS) and allowed to expand until reaching near confluency before freezing and storage at 80°C. As needed, VICs were thawed and taken through two passages before use in these experiments. Cell treatments were added 12 h after cell seeding for a period of 48 h. Recombinant porcine TGF-β1 was purchased from R&D Systems (Minneapolis, MN, USA). Monoclonal antibodies anti-FGF-2, anti-FGFR-2, anti-FGFR-3, as well as the growth factors FGF-2, were purchased from Sigma (St. Louis, MO, USA). U0126 was purchased from Calbiochem (La Jolla, CA, USA). Mouse monoclonal anti-αSMA was purchased from Abcam (Cambridge, MA, USA). Alexa Fluor 488, goat anti-mouse IgG (GAM-488), GAM-horseradish perioxidase (HRP), and GAR-HRP were purchased from Molecular Probes (Eugene, OR, USA). Unless otherwise stated, all other chemicals were purchased from Sigma-Aldrich.
Cell-based fluorometric enzyme-linked immunosorbent assay
Quantification of αSMA expression by enzyme-linked immunosorbent assay (ELISA) was performed as described previously (25). This assay was further modified to allow for semiquantitative labeling of αSMA normalized to DNA in every sample repeat. Seeding was performed in black tissue-culture 96-well flat-bottom plates at 15,000 cells/cm2 for αSMA expression assays. After 48 h of treatment with media containing growth factors or inhibitors, cells were fixed with 10% buffered formalin, washed in phosphate buffered saline containing (PBS) 0.05% Tween-20, quenched of endogenous peroxidases with 0.1 wt % NaN3 and 0.3 mM H2O2, blocked in 3% bovine serum albumin, and incubated with anti-αSMA primary and GAM-HRP secondary antibodies. In corresponding controls, specific staining was confirmed by inverted fluorescent microscopy (GAM-488 secondary) after counterstaining nucleic acids with propidium iodide (500 nmol/L; Eclipse TE300; Nikon, Tokyo, Japan). Fluorogenic HRP-substrate QuantaBlu (Pierce, Rockford, IL, USA) was incubated with HRP-labeled samples, and the development of fluorescence was measured by automated plate reader (Wallac Victor2 Multilabel Counter, PerkinElmer, Waltham, MA, USA) and normalized to a negative control with no primary antibody. The same samples were then digested with papain protease (Worthington Biochemical) for subsequent quantification of DNA content with the PicoGreen dsDNA assay and lambda DNA standard curve (Molecular Probes; ref. 25). For each well, QuantaBlu fluorescence was normalized to DNA content, as determined by PicoGreen dsDNA assay (Invitrogen, Carlsbad, CA, USA), to indicate protein expression per cell with the assumption that that each cell contained equal quantities of DNA.
ECM production
ECM production was evaluated by [3H]proline incorporation over a 96 h period as described previously (26). Briefly, cells were removed from culture after treatment and extensively washed in PBS, and cell-associated proline was removed with a cell lysis step in 25 mM NH4OH to remove cytosolic proteins. Remaining ECM was then digested for 4 h with papain protease at 37°C. Digests were combined with scintillation fluid, and radioactivity was determined by scintigraphy (Beckman LS 6500, Beckman Instruments, Fullerton, CA, USA). DNA from corresponding samples was quantified using the PicoGreen dsDNA assay after cell lysis in GloLysis Buffer (Promega, Madison, WI, USA). Data are presented as the counts per minute normalized to DNA content.
Transfections and Smad-reporter systems
The Smad-3 luciferase reporter PE2.1 was a generous gift of Dr. Xuedong Liu (University of Colorado, Boulder, CO, USA) and contains three Smad binding elements driving the expression of luciferase (27). VICs were transfected using a 1:2 ratio of plasmid:Lipofectamine 2000 in Opti-Mem (Invitrogen) and stimulated with growth factors for 48 h before assay. Luciferase activity was assayed using the Bright-Glo luciferase assay system (Promega) as per the manufacturer’s instructions.
Valve leaflet organ culture and preparation
Porcine aortic leaflets were removed from intact hearts as done for VIC isolation procedures (24). Instead of subjecting the leaflets to collagenase digestion, however, the dissected tissue was cut into constructs measuring 0.7 cm per side with native valve thickness and cultured in 24-well untreated plates with VIC growth media. Plates were placed on an orbital shaker at 65 rpm in a tissue culture incubator. Leaflets were removed from culture, fixed, paraffin-embedded, and sectioned at 4 μm intervals. Sections were deparaffinized using graded ethanol washes and xylene, and immunostained for αSMA using the avidin-biotin amplification complex (immunoperoxidase ABC technique) in conjunction with the NovaRED peroxidase substrate (Vector Labs, Burlingame, CA, USA).
Collagen gel contraction assays
Chilled collagen (PureCol, INAMED, Burlington, Ontario, Canada) was combined with 10× Medium 199 (Invitrogen) and 0.1M NaOH in a ratio of 8:1:1, added to the VIC cell pellet (150,000 cell/gel), and adjusted to a pH of 7.4 ± 0.2 with 0.1 M NaOH. The resulting homogenous solution was immediately aliquoted into 48-well plates (300 μl/well) and incubated at 37°C and 5% CO2 for a minimum of 60 min. After gelation, 500 μl of conditioned media was added to each well. After 24 h, the gels were gently detached from the plates with a thin spatula, and digital images of floating collagen constructs were taken with a high-resolution scanner. The collagen gel area was measured using ImageJ software [National Institutes of Health (NIH), Bethesda, MD, USA], and macroscopic changes in gel area for each condition were determined (28).
Calcific nodule formation
Cells were seeded at 30,000 cells/cm2 in a clear 96-well plate and treated for 72 h. The cells were then fixed overnight in 10% buffered formalin, stained with 2% Alizarin Red (Fisher Scientific, Pittsburgh, PA, USA) solution, and thoroughly washed in PBS. Calcific nodules were visually identified and counted at ×10 (29).
RESULTS
FGF-2 reduces basal markers of myofibroblast activation in VIC cultures
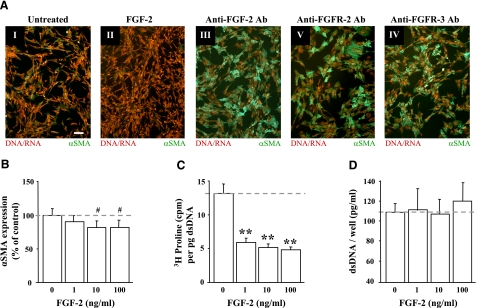
Because FGF-2 has been investigated for its antifibrotic properties in vivo(30), we examined the effects of FGF-2 treatment on αSMA expression in cultured VICs. As presented in Fig. 1AI, II, VICs treated with 10 ng/ml FGF-2 showed reduced staining for αSMA expression (green) when compared with untreated controls. Expression of αSMA is a fundamental feature of activated myofibroblasts and serves as a key marker of fibrotic responses (31). Furthermore, blocking even basal levels of FGF signaling that result from FGF found in FBS, as well as from autocrine-mediated activation of FGF receptors, successfully induced αSMA expression, consistent with a fibrotic response in cultured VICs. As shown in Fig. 1AIII–IV, the addition of antibodies that neutralize FGF-2 (Fig. 1AIII), FGF receptor-2 (FGFR-2; Fig. 1AIV), or FGF receptor-3 (FGFR-3; Fig. 1AV) significantly increased αSMA expression, suggesting that even low levels of FGF signaling are present in VIC cultures and that this acts to keep these cells in an undifferentiated, nonfibrotic state.
Figure 1.
FGF-2 reduced markers of myofibroblast activation in VICs. A) Treatment of VICs with 10 ng/ml FGF-2 (II) reduced αSMA expression when compared with untreated control cell populations (I). Inhibition of FGF signaling with 50 μg/ml of antibodies that neutralize FGF-2 (III) or the FGF-2 receptors, FGFR-2 (IV) and FGFR-3 (V), caused a dramatic induction of de novo αSMA expression (green). Counterstaining of nucleic acid with propidium iodide is shown in red. All images were taken at the same magnification; scale bar = 10 μm. Previously published studies (1) have demonstrated the efficacy of the anti-αSMA antibody to selectively stain stress fibers in myofibroblasts. B) Dose-dependent reduction of αSMA expression by FGF-2 in VICs. VICs were incubated for 48 h with FGF-2 at the concentrations indicated, and expression was detected by cell-based ELISA. Expression is shown as a percentage of the untreated (0 ng/ml) controls. C) Dose-dependent reduction in matrix deposition by FGF-2 in VICs. VICs were incubated for 72 h with FGF-2, and [3H]proline incorporation was measured by scintillation counter. [3H]proline levels were normalized to DNA content of each sample to account for potential differences in cell numbers between samples. D) Cell growth is unaffected by FGF-2. Subconfluent VIC cultures were treated with FGF-2 for 72 h, and the relative number of cells in each sample was determined by measuring total dsDNA content. In all charts, the dashed line indicates the level of untreated controls and represents the level to which treated samples are compared. #P < 0.05; **P < 0.001.
To test the concentration range at which FGF-2 prevents fibrosis in VICs, cell were treated with increasing concentrations of FGF-2 and monitored for specific markers of fibrosis. As shown in Fig. 1B, concentrations of FGF-2 ≥10 ng/ml in the culture media caused a significant reduction in αSMA expression as measured by ELISA. Similarly, FGF-2 reduced the incorporation of [3H]proline into ECM proteins (Fig. 2C), indicating that the cells were not actively producing fibrotic connective tissue (e.g., via the deposition of proline-rich collagen). All concentrations of FGF-2 tested (≥1 ng/ml) caused a significant reduction of matrix production per cell. Furthermore, this decrease in ECM production did not result from reduced cell proliferation, as the quantification of dsDNA suggests that cell numbers remained constant in FGF-2-treated samples when compared with untreated controls (Fig. 1D).
Figure 2.
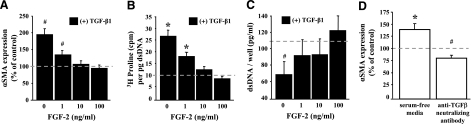
FGF-2 blocks TGF-β1-mediated myofibroblast activation in VICs. A) FGF-2 blocks the TGF-β1-mediated induction of αSMA expression. VICs were incubated for 48 h with 5 ng/ml TGF-β1 in combination with increasing concentrations of FGF-2, and αSMA expression was detected by cell-based ELISA. Expression is shown as a percentage of the untreated controls. B) FGF-2 blocks the TGF-β1-mediated induction of matrix deposition. VICs were incubated for 72 h with 5 ng/ml TGF-β1 in combination with increasing concentrations of FGF-2. [3H]proline incorporation was then measured by scintillation counter. [3H]proline levels were normalized to DNA content of each sample to account for potential differences in cell numbers between samples. C) FGF-2 blocks the TGF-β1-mediated reduction in cell growth. Subconfluent VIC cultures were treated with 5 ng/ml TGF-β1 in combination with increasing concentrations of FGF-2. After 72 h, the relative number of cells in each sample was determined by measuring total dsDNA content. Normal culturing conditions for VICs represses αSMA expression even though TGF-β signaling appears active. VICs were incubated for 48 h in either serum-free media or treated with an anti-TGF-β neutralizing antibody, and αSMA expression was detected by cell-based ELISA. Expression is shown as a percent of the controls cultured in 15% FBS. In all charts, the dashed line indicates the level of untreated controls (0 ng/ml TGF-β1 and FGF-2) and represents the level to which treated samples are compared. #P < 0.05; *P < 0.01.
FGF-2 blocks TGF-β1-mediated myofibroblast activation
Given the ability of FGF-2 to reduce markers of fibrosis under normal culture conditions, we wanted to determine if FGF-2 could block activated myofibroblast differentiation. TGF-β1 has been shown to induce αSMA expression, increase ECM deposition, and block cellular proliferation in VICs (1). As shown in Fig. 2A, treatment of VICs with TGF-β1 (5 ng/ml for 48 h) markedly induced αSMA expression when compared with untreated controls (dashed line). The cotreatment of VICs with TGF-β1 and increasing concentrations of FGF-2, however, prevented increases in αSMA expression, suggesting that myofibroblast activation is blocked by FGF-2. Similarly, treatment of VICs with FGF-2 effectively blocked TGF-β1-mediated increases in matrix production (Fig. 2B). No significant difference was observed in the production of ECM proteins at concentrations ≥10 ng/ml FGF-2, suggesting that these cells had not been activated by TGF-β1 and remained undifferentiated.
Additionally, cellular proliferation was restored in VICs that were treated with FGF-2. As shown in Fig. 2C, the treatment of VICs with TGF-β1 resulted in a marked decrease in the quantity of dsDNA per well, suggesting that DNA synthesis and cellular proliferation were reduced compared with untreated cells (dashed line). TGF-β1 removed cells from the cell cycle and effectively decreased cell numbers when compared with untreated controls. In contrast, cells that were also treated with FGF-2 had DNA quantities similar to untreated cells, suggesting that these cells were not induced to differentiate and that their normal cellular proliferation rates were maintained.
These data suggest that the fine balance between FGF-mediated pathways and TGF-β-mediated pathways is tipped in favor of FGF under normal culturing conditions with 15% FBS. Indeed, these conditions act to promote proliferation and are used to expand cell numbers. The presence of FGF signaling under normal culturing conditions is also demonstrated in previous experiments in which blocking FGF signaling with neutralizing antibodies resulted in increased αSMA expression (Fig. 1). Given this observation, we hypothesized that the removal of serum from these cells would reduce FGF signaling and result in myofibroblast differentiation. As shown in Fig. 2D, culturing VICs in serum-free conditions caused an increase in αSMA expression, consistent with a fibrotic response. Together, these data suggest that FGF is present in FBS and likely maintains VICs in an undifferentiated state. The cells remain responsive to FGF supplementation (Fig. 1), however, indicating that the level of FGF in FBS is not causing a maximal response.
FGF signaling does not appear to be acting unopposed under these conditions, however, and TGF-β-mediated pathways also appear to be active. As shown in Fig. 2D, treatment of VICs with an anti-TGF-β neutralizing antibody caused a decrease in αSMA expression, suggesting that TGF-β signaling occurs under these conditions and acts to maintain a certain level of differentiation. The cells remain responsive to TGF-β supplementation (Fig. 2A–C), however, indicating that the level of TGF-β in FBS is not causing a maximal response. Together, these data suggest that the FGF- and TGF-β-mediated pathways are both activated when VICs are cultured with 15% FBS but that FGF-mediated effects predominate.
FGF-2 prevents TGF-β1-mediated Smad activation
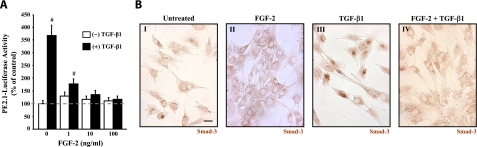
To identify the mechanism by which FGF-2 blocks TGF-β1-mediated activation of VICs, we investigated the activity of Smad transcription factors in these cells. Both Smad-2 and Smad-3 are phosphorylated by TGF-β type I receptors, causing them to become activated. Once activated, these Smads are translocated to the nucleus where they increase the expression of a variety of fibrosis-related genes (32). By transfecting VICs with the PE2.1 luciferase reporter plasmid (27), we tested the ability of FGF-2 to inhibit TGF-β1-mediated Smad transcriptional activation. As shown in Fig. 3A, stimulation of transfected VICs with TGF-β1 caused an induction of the Smad-binding luciferase reporter. This increase was blocked, however, when FGF-2 was codelivered with TGF-β1, indicating that FGF-2 effectively blocked the transcriptional activity of Smads that are the downstream targets of TGF-β1 signaling in these cells.
Figure 3.
TGF-β1-stimulated Smad activity is modulated by FGF-2. A) FGF-2 blocks TGF-β1-mediated transcriptional induction of Smad-responsive promoter. VICs transfected with the Smad-responsive luciferase reporter plasmid PE2.1 were treated with increasing concentrations of FGF-2 and luciferase activity was measured after 48 h. TGF-β1 (5 ng/ml) caused a 3.5-fold increase in luciferase activity when compared with untreated controls. This increase is markedly attenuated when FGF-2 (1–100 ng/ml) is codelivered with TGF-β1. Dashed line indicates level of untreated controls (0 ng/ml TGF-β1 and FGF-2). B) FGF-2 blocks TGF-β1-mediated Smad-3 nuclear accumulation in VICs. VICs were treated with FGF-2 (10 ng/ml; II), TGF-β1 (5 ng/ml; III), or both (IV). Representative micrographs show cellular localization of Smad-3 by indirect immunoperoxidase staining. VICs treated with TGF-β1 alone showed increased nuclear staining, indicating that Smad-3 had been translocated to the nucleus in these cells. FGF-2 treated cells have cytoplasmic staining with obvious occlusion of Smad-3 from the nucleus. All images were taken at the same magnification. Scale bar = 2.5 μm. #P < 0.05.
Activated Smads require partnering with DNA-binding cofactors to regulate selective transcriptional activity of target genes in the nucleus, suggesting that FGF-2 either prevents Smad translocation to the nucleus or blocks the interaction of Smads with their binding partners in the nucleus. As shown in Fig. 3B, TGF-β1-mediated translocation of Smad-3 to the nucleus was blocked by FGF-2. Smad-3 was clearly localized to the nucleus in cells treated with TGF-β1 (45 min, 5 ng/ml), whereas Smad-3 was excluded from the nuclei of cells that were also treated with FGF-2. These results suggest that FGF-2 prevents fibrosis in VICs by interfering with TGF-β1 signaling by blocking Smads from entering the nucleus to activate genes involved in myofibroblast differentiation.
Inhibition of MAPK signaling prevents FGF-2 activity
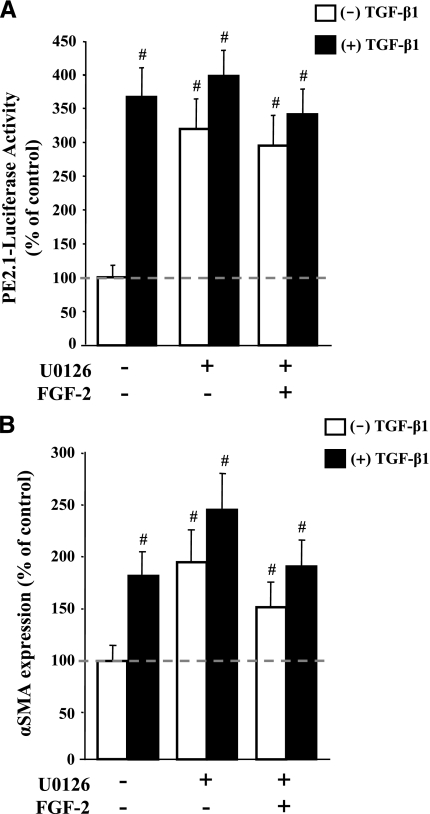
Since FGF-2 is known to activate MAPK pathways (33), we hypothesized that blocking MAK kinase cascades would attenuate the antifibrotic activity of FGF-2 in VICs. By using the pharmacological MAPK/extracellular regulated kinase (ERK) (MEK)-1/2 inhibitor U0126 to block downstream FGF-2 signaling in VIC cultures, we successfully abrogated the ability of FGF-2 to interfere with TGF-β1-mediated fibrosis. As shown in Fig. 4A, the addition of U0126 to the culture media caused an increase in the activity of the PE2.1 luciferase reporter, indicating that Smad-mediated signaling was increased as a result of MAPK inhibition. This effect is likely a result of an increase in the nuclear localization of Smad-3 in U0126-treated cells (data not shown). Furthermore, U0126 did not significantly alter TGF-β1 activation of the PE2.1 reporter; instead, it prevented FGF-2 from interfering with Smad activation. The use of U0126 in combination with TGF-β1 had little effect on the PE2.1 reporter activity in that luciferase activity under these conditions was not significantly different from the luciferase activity of cells treated with TGF-β1 alone. As demonstrated earlier, FGF-2 effectively blocked this TGF-β1-induced increase in PE2.1 activity (Fig. 3A); however, FGF-2 was unable to prevent the activation of the Smad reporter when U0126 was present (Fig. 4A), indicating that MEK-1/2 is involved in the FGF-2-mediated repression of Smad activity. These results are consistent with endogenous gene markers of fibrosis in that U0126 also significantly increased αSMA expression in VIC cultures, even when FGF-2 is added to the media (Fig. 4B). Together, these data suggest that FGF-2 acts to inhibit myofibroblast activation of VICs via MAPK-mediated inhibition of Smad signaling.
Figure 4.
MAPK inhibitor blocks myofibroblast activation in VICs. A) The inhibition of ERK-1/2 prevents FGF-2 from blocking TGF-β1-medaited Smad activation. VICs were transfected with the Smad-responsive luciferase reporter plasmid PE2.1 and treated with a combination of the MEK-1/2 inhibitor U0126 (15 μmol/L), FGF-2 (10 ng/ml), and/or TGF-β1 (5 ng/ml). Luciferase activity was measured after 48 h after treatment. The MEK-1/2 inhibitor significantly increases Smad activity when compared with untreated controls. Moreover, U0126 prevented FGF-2 from reducing the TGF-β1-mediated activation of the Smad-responsive promoter that was observed in a previous experiment (Fig. 3A). B) The inhibition of ERK-1/2 prevents FGF-2 from blocking the TGF-β1-medaited induction of αSMA expression. VICs were treated with a combination of the MEK-1/2 inhibitor U0126 (15 mol/L), FGF-2 (10ng/ml), and/or TGF-β1 (5 ng/ml) for 48 h before αSMA detection by ELISA. The MEK-1/2 inhibitor U0126 significantly increased αSMA expression when compared with untreated controls. In addition, U0126 prevented FGF-2 from reducing the TGF-β1-mediated induction of αSMA that was observed in a previous experiment (Fig. 2A). In each chart, the dashed line indicates the level of untreated controls (0 ng/ml TGF-β1 and FGF-2) and represents the level to which treated samples are compared. #P < 0.05.
FGF-2/MAPK signaling prevents fibrosis in intact aortic leaflets
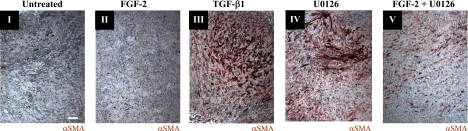
To validate our culture system and evaluate the effects of FGF-2 and MAPK blockade on VICs within their native ECM architecture, valve leaflet organ culture was performed. Aortic leaflets were surgically isolated from porcine hearts and cultured in vitro with TGF-β1, FGF-2, U0126, or FGF-2 combined with U0126. αSMA expression was monitored after 2 wk of treatment by histological sectioning, and representative leaflets are shown in Fig. 5. In control constructs, treated only with serum-containing growth media, there was evidence of small clusters of αSMA-positive cells, particularly near the leaflet free edge (Fig. 5I). FGF-2-treated cultures also had low levels of diffuse positive staining uniformly throughout the leaflet interior (Fig. 5II). In contrast, leaflets treated with TGF-β1 (Fig. 5III) or U0126 (Fig. 5IV) had enhanced foci of positive staining, where treatment formed bands or clusters of activated cells. Additionally, αSMA staining remained elevated when FGF-2 and U0126 were codelivered (Fig. 5V). These clusters may represent the early stages of nodule formation and demonstrate the importance of FGF-2 and MAPK signaling in preventing fibrosis in the aortic valve.
Figure 5.
FGF-2 reduces fibrosis in intact aortic leaflets. αSMA expression in cultured porcine aortic valve leaflets is indicated by red immunoperoxidase staining. Intact valve leaflets were treated for 2 wk with control media (I), FGF-2 (10 ng/ml; II), TGF-β1 (5 ng/ml; III), U0126 (15 μmol/L; (IV), or U0126 (15 μmol/L) + FGF-2 (10 ng/ml; V). All images were taken at the same magnification, and the scale bar is equal to 20 μm. Previously published studies (1) have demonstrated the efficacy of the anti-αSMA antibody to selectively stain stress fibers in myofibroblasts.
FGF-2 prevents TGF-β1-mediated contraction and pathological node formation
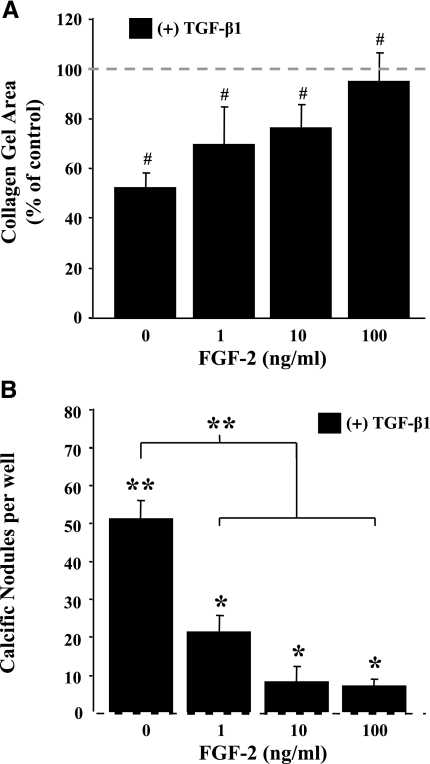
TGF-β accumulation in valves has been linked to progression of valve fibrosis and subsequent calcification (3). In vitro, TGF-β1 has been shown to up-regulate the contractile cytoskeleton of valve myofibroblasts and induce the formation of calcific nodules (1). These deleterious effects are attenuated by the addition of FGF-2, however. As shown in Fig. 6A, FGF-2 prevented, in a dose-dependent manner, TGF-β1-mediated compaction (reduction of gel diameter) of collagen gels seeded with VICs. Moreover, fibrotic contraction was completely prevented when FGF-2 was supplied at a concentration of 100 ng/ml. Similarly, FGF-2 reduced TGF-β1-mediated formation of calcific nodules. As shown in Fig. 6B, untreated control cultures formed no calcific nodules at the cell seeding densities tested; however, delivery of TGF-β1 induced the formation of numerous calcium-containing nodules (50±5 nodules/well). Codelivery of FGF-2 (1–100 ng/ml) with TGF-β1 significantly reduced the number of nodules that formed when compared with cultures that had been treated with TGF-β1 alone.
Figure 6.
FGF-2 inhibits pathological phenotypes observed in TGF-β1-treated VICs. A) FGF-2 prevents fibrotic contraction of VICs treated with TGF-β1. VICs were encapsulated into 3-dimensional collagen matrices in 48-well tissue culture plates and treated with TGF-β1 (5 ng/ml) and FGF-2 (0–100 ng/ml) for 24 h. Matrices were then detached from the plate surface, and gel surface area was determined using ImageJ software. Collagen gel areas are plotted as a percentage of untreated control (0 ng/ml TGF-β1 and FGF-2). B) Dose-dependent reduction in TGF-β1-mediated calcific nodule formation by FGF-2 in VICs. VICs were seeded into 96-well plates and treated with TGF-β1 (5 ng/ml) and FGF-2 (0–100 ng/ml) for 72 h. The cells were then stained with Alizarin red to identify calcium-based mineralization, and the number of calcific nodules was counted in each well. No calcific nodules were observed in the untreated controls (0 ng/ml TGF-β1 and FGF-2), which are represented as a dashed line on the x axis. However, VICs treated with TGF-β1 showed a dramatic increase in nodule formation (>50 nodules/well), which is significantly attenuated by FGF-2 at concentrations ≥1 ng/ml. In each chart, the dashed line indicates the level of untreated controls (0 ng/ml TGF-β1 and FGF-2) and represents the level to which treated samples are compared, unless indicated by brackets. #P < 0.05; *P < 0.01; **P < 0.001.
DISCUSSION
In this study, we show that FGF-2 serves as a potent inhibitor of TGF-β1-mediated myofibroblast activation. Treatment of VIC cultures with FGF-2 inhibited activated αSMA expression, deposition of collagen matrix, fibrotic contractility, and calcified node formation. Although it has been reported that the actions of TGF-β on myofibroblast development require the presence of mechanical tension (31), VICs have been recently shown to interact naturally with their matrix to significantly alter leaflet stiffness (34). While further investigation into the responses of VICs to these pharmacological stimuli under externally applied mechanical stress is warranted, our experiments with intact aortic leaflets (Fig. 5) suggest that FGF-2 signaling prevents myofibroblast differentiation in native, three-dimensional valve environments.
Our study implicates a distinct signaling pathway whereby FGF-2 acts to block Smad-mediated fibroblast activation through a MEK-ERK-dependent mechanism in valvular interstitial cells. By blocking the nuclear localization of Smads in response to TGF-β1, FGF-2 effectively prevents Smad-dependent transcriptional activation of genes involved in the transition of VICs to a fibrotic phenotype. Many studies have demonstrated that there is significant intracellular crosstalk between MEK-ERK and TGF-β1; however, modulation of Smad activity by MAPKs is cell-type dependent (35). In our work with VICs, however, we see that blocking MEK activity with the pharmacological inhibitor U0126 abrogates the ability of FGF-2 to block TGF-β1-mediated fibrosis, suggesting that the MAPKs are involved in regulating Smad activity in these cells. Several reports indicate that Smads are phosphorylated by MAPKs (22, 36,37,38,39), such as phosphorylation of the linker region of R-Smads by ERK (37, 38), leading us to believe that MAPKs alter Smad activity and localization via direct interactions in the cytoplasm.
The antagonistic function of FGF-2 and TGF-β1 on Smad activity and nuclear localization in valve myofibroblasts reported here demonstrates that Smad activity is controlled by a delicate balance of signaling emanating from receptor tyrosine kinases and serine/threonine receptor kinases. These data can be assimilated into a working model of myofibroblast differentiation resulting from competing inputs of TGF-β and FGF signaling. In this model, basal activation of MAPK by FGF-2 is an important antagonistic checkpoint for regulation of TGF-β signals. Furthermore, inhibition of FGF/MAPK signaling creates a permissive environment for enhanced TGF-β activity. Collectively, this model suggests that reduction of MAPK activation induces myofibroblast activation and progression of myofibroblast-related fibrotic pathologies in an analogous manner to TGF-β1 over-expression. Although our data indicate that MEK activity is important for FGF-2 to repress TGF-β1-mediated fibrosis, other reports (40) have shown that activation of ERK activity by hepatocyte growth factor and epidermal growth factor also suppresses myofibroblast differentiation, suggesting that multiple stimuli work through this central MAPK-Smad pathway.
The identification of critical regulatory pathways that control fibrosis is of profound importance to the fields of tissue engineering and medicine. By identifying a signaling pathway that acts to oppose myofibrotic differentiation, the work presented here extends our understanding of the role of FGF-2 in controlling cell fate decisions and provides insight into the management of the myofibroblast phenotype. Controlling fibrotic differentiation is crucial to engineering applications that hope to create tissue replacements of damaged heart valves. Armed with an improved understanding of the mechanisms that control cell growth and differentiation, engineers will be better prepared to design and manage the cellular environments that promote the development of normal tissues. Moreover, the identification of pathways that regulate myofibrotic differentiation will be essential to the development of improved therapeutic strategies for combating fibrotic lesions in the heart valve, as well as other tissues known to be affected by the enduring presence of matrix-synthesizing myofibroblasts.
Acknowledgments
Funding for this project was provided by the Howard Hughes Medical Institute and a grant from the American Heart Association (0355488Z), as well as fellowships to M.C.C. from the Department of Education Graduate Assistance in Areas of National Need (GAANN) and NIH Leadership Training in Pharmaceutical Biotechnology to J.T.L. and to E.A.S. from the University of Colorado’s Undergraduate Research Opportunity Program, Bioscience Undergraduate Research Skills and Training Biological Sciences Initiative, and Discovery Learning Center Apprenticeship.
References
- Walker G. A., Masters K. S., Shah D. N., Anseth K. S., Leinwand L. A. Valvular myofibroblast activation by transforming growth factor-beta. Circ Res. 2004;95:253–260. doi: 10.1161/01.RES.0000136520.07995.aa. [DOI] [PubMed] [Google Scholar]
- Kaden J. J., Kilic R., Sarikoc A., Hagl S., Lang S., Hoffmann U., Brueckmann M., Borggrefe M. Tumor necrosis factor alpha promotes an osteoblast-like phenotype in human aortic valve myofibroblasts: a potential regulatory mechanism of valvular calcification. Int J Mol Med. 2005;16:869–872. [PubMed] [Google Scholar]
- Jian B., Narula N., Li Q. Y., Mohler E. R., 3rd, Levy R. J. Progression of aortic valve stenosis: TGF-beta1 is present in calcified aortic valve cusps and promotes aortic valve interstitial cell calcification via apoptosis. Ann Thorac Surg. 2003;75:457–465. doi: 10.1016/s0003-4975(02)04312-6. [DOI] [PubMed] [Google Scholar]
- Desmouliere A., Redard M., Darby I., Gabbiani G. Apoptosis mediates the decrease in cellularity during the transition between granulation tissue and scar. Am J Pathol. 1995;146:56–66. [PMC free article] [PubMed] [Google Scholar]
- Rabkin-Aikawa E., Farber M., Aikawa M., Schoen F. J. Dynamic and reversible changes of interstitial cell phenotype during remodeling of cardiac valves. J Heart Valve Dis. 2004;13:841–847. [PubMed] [Google Scholar]
- Rabkin E., Aikawa M., Stone J. R., Fukumoto Y., Libby P., Schoen F. J. Activated interstitial myofibroblasts express catabolic enzymes and mediate matrix remodeling in myxomatous heart valves. Circulation. 2001;104:2525–2532. doi: 10.1161/hc4601.099489. [DOI] [PubMed] [Google Scholar]
- Ronnov-Jessen L., Petersen O. W. Induction of alpha-smooth muscle actin by transforming growth factor-beta 1 in quiescent human breast gland fibroblasts Implications for myofibroblast generation in breast neoplasia. Lab Invest. 1993;68:696–707. [PubMed] [Google Scholar]
- Sato M., Suzuki S., Senoo H. Hepatic stellate cells: unique characteristics in cell biology and phenotype. Cell Struct Funct. 2003;28:105–112. doi: 10.1247/csf.28.105. [DOI] [PubMed] [Google Scholar]
- Badid C., Mounier N., Costa A. M., Desmouliere A. Role of myofibroblasts during normal tissue repair and excessive scarring: interest of their assessment in nephropathies. Histol Histopathol. 2000;15:269–280. doi: 10.14670/HH-15.269. [DOI] [PubMed] [Google Scholar]
- Kaden J. J., Dempfle C.-E., Grobholz R., Fischer C. S., Vocke D. C., Kilic R., Sarikoc A., Pinol R., Hagl S., Lang S. Inflammatory regulation of extracellular matrix remodeling in calcific aortic valve stenosis. Cardiovasc Path. 2005;14:80–87. doi: 10.1016/j.carpath.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Tamaoki M., Imanaka-Yoshida K., Yokoyama K., Nishioka T., Inada H., Hiroe M., Sakakura T., Yoshida T. Tenascin-C regulates recruitment of myofibroblasts during tissue repair after myocardial injury. Am J Pathol. 2005;167:71–80. doi: 10.1016/S0002-9440(10)62954-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serini G., Bochaton-Piallat M.-L., Ropraz P., Geinoz A., Borsi L., Zardi L., Gabbiani G. The fibronectin domain ED-A is crucial for myofibroblastic phenotype induction by transforming growth factor-beta 1. J Cell Biol. 1998;142:873–881. doi: 10.1083/jcb.142.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yata Y., Gotwals P., Koteliansky V., Rockey D. C. Dose-dependent inhibition of hepatic fibrosis in mice by a TGF-beta soluble receptor: implications for antifibrotic therapy. Hepatology. 2002;35:1022–1030. doi: 10.1053/jhep.2002.32673. [DOI] [PubMed] [Google Scholar]
- Yu L., Noble N. A., Border W. A. Therapeutic strategies to halt renal fibrosis. Curr Opin Pharmacol. 2002;2:177–181. doi: 10.1016/s1471-4892(02)00144-3. [DOI] [PubMed] [Google Scholar]
- Wells R. G. Fibrogenesis:V TGF-beta signaling pathways. Am J Physiol Gastrointest Liver Physiol. 2000;279:G845–850. doi: 10.1152/ajpgi.2000.279.5.G845. [DOI] [PubMed] [Google Scholar]
- Leask A., Abraham D. J. TGF-beta signaling and the fibrotic response. FASEB J. 2004;18:816–827. doi: 10.1096/fj.03-1273rev. [DOI] [PubMed] [Google Scholar]
- Hinz B., Celetta G., Tomasek J. J., Gabbiani G., Chaponnier C. Alpha-smooth muscle actin expression upregulates fibroblast contractile activity. Mol Biol Cell. 2001;12:2730–2741. doi: 10.1091/mbc.12.9.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papetti M., Shujath J., Riley K. N., Herman I. M. FGF-2 antagonizes the TGF-beta1-mediated induction of pericyte alpha-smooth muscle actin expression: a role for myf-5 and Smad-mediated signaling pathways. Invest Ophthalmol Vis Sci. 2003;44:4994–5005. doi: 10.1167/iovs.03-0291. [DOI] [PubMed] [Google Scholar]
- Maltseva O., Folger P., Zekaria D., Petridou S., Masur S. K. Fibroblast growth factor reversal of the corneal myofibroblast phenotype. Invest Ophthalmol Vis Sci. 2001;42:2490–2495. [PubMed] [Google Scholar]
- Mattey D. L., Dawes P. T., Nixon N. B., Slater H. Transforming growth factor beta 1 and interleukin 4 induced alpha smooth muscle actin expression and myofibroblast-like differentiation in human synovial fibroblasts in vitro: modulation by basic fibroblast growth factor. Ann Rheum Dis. 1997;56:426–431. doi: 10.1136/ard.56.7.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg R. S., Bernstein A. M., Benezra M., Gelman I. H., Taliana L., Masur S. K. FAK-dependent regulation of myofibroblast differentiation. FASEB J. 2006;20:1006–1008. doi: 10.1096/fj.05-4838fje. [DOI] [PubMed] [Google Scholar]
- Kretzschmar M., Doody J., Timokhina I., Massague J. A mechanism of repression of TGFbeta/Smad signaling by oncogenic Ras. Genes Dev. 1999;13:804–816. doi: 10.1101/gad.13.7.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massague J. How cells read TGF-beta signals. Nat Rev Mol Cell Biol. 2000;1:169–178. doi: 10.1038/35043051. [DOI] [PubMed] [Google Scholar]
- Johnson C., Hanson M., Helgeson S. Porcine cardiac valvular subendothelial cells in culture: cell isolation and growth characteristics. J Mol Cell Cardiol. 1987;19:1185–1193. doi: 10.1016/s0022-2828(87)80529-1. [DOI] [PubMed] [Google Scholar]
- Cushing M. C., Liao J.-T., Anseth K. S. Activation of valvular interstitial cells is mediated by transforming growth factor-beta1 interactions with matrix molecules. Matrix Biol. 2005;24:428–437. doi: 10.1016/j.matbio.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Scott-Burden T., Resink T. J., Burgin M., Buhler F. R. Extracellular matrix: differential influence on growth and biosynthesis patterns of vascular smooth muscle cells from SHR and WKY rats. J Cell Physiol. 1989;141:267–274. doi: 10.1002/jcp.1041410206. [DOI] [PubMed] [Google Scholar]
- Hua X., Miller Z. A., Wu G., Shi Y., Lodish H. F. Specificity in transforming growth factor beta-induced transcription of the plasminogen activator inhibitor-1 gene: interactions of promoter DNA, transcription factor muE3, and Smad proteins. Proc Natl Acad Sci U S A. 1999;96:13130–13135. doi: 10.1073/pnas.96.23.13130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abramoff M. D., Magelhaes P. J., Ram S.J. Image Processing with ImageJ. Biophoton Int. 2004;11:36–42. [Google Scholar]
- Mohler E. R., 3rd, Chawla M. K., Chang A. W., Vyavahare N., Levy R. J., Graham L., Gannon F. H. Identification and characterization of calcifying valve cells from human and canine aortic valves. J Heart Valve Dis. 1999;8:254–260. [PubMed] [Google Scholar]
- Doukas J., Blease K., Craig D., Ma C., Chandler L. A., Sosnowski B. A., Pierce G. F. Delivery of FGF genes to wound repair cells enhances arteriogenesis and myogenesis in skeletal muscle. Mol Ther. 2002;5:517–527. doi: 10.1006/mthe.2002.0579. [DOI] [PubMed] [Google Scholar]
- Tomasek J. J., Gabbiani G., Hinz B., Chaponnier C., Brown R. A. Myofibroblasts and mechano-regulation of connective tissue temodelling. Nat Rev Mol Cell Biol. 2002;3:349–363. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- Schiller M., Javelaud D., Mauviel A. TGF-beta-induced SMAD signaling and gene regulation: consequences for extracellular matrix remodeling and wound healing. J Dermatol Sci. 2004;35:83–92. doi: 10.1016/j.jdermsci.2003.12.006. [DOI] [PubMed] [Google Scholar]
- Eswarakumar V. P., Lax I., Schlessinger J. Cellular signaling by fibroblast growth factor receptors. Cytokine Growth Factor Rev. 2005;16:139–149. doi: 10.1016/j.cytogfr.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Merryman W. D., Huang H.-Y., Schoen F. J., Sacks M. S. The effects of cellular contraction on aortic valve leaflet flexural stiffness. J Biomech. 2006;39:88–96. doi: 10.1016/j.jbiomech.2004.11.008. [DOI] [PubMed] [Google Scholar]
- Javelaud D., Mauviel A. Crosstalk mechanisms between the mitogen-activated protein kinase pathways and Smad signaling downstream of TGF-beta: implications for carcinogenesis. Oncogene. 2005;24:5742–5750. doi: 10.1038/sj.onc.1208928. [DOI] [PubMed] [Google Scholar]
- Matsuura I., Wang G., He D., Liu F. Identification and characterization of ERK MAP kinase phosphorylation sites in Smad3. Biochemistry. 2005;44:12546–12553. doi: 10.1021/bi050560g. [DOI] [PubMed] [Google Scholar]
- Massague J. Integration of Smad and MAPK pathways: a link and a linker revisited. Genes Dev. 2003;17:2993–2997. doi: 10.1101/gad.1167003. [DOI] [PubMed] [Google Scholar]
- Kretzschmar M., Doody J., Massagu J. Opposing BMP and EGF signalling pathways converge on the TGF-β family mediator Smad1. Nature. 1997;389:618–622. doi: 10.1038/39348. [DOI] [PubMed] [Google Scholar]
- De Caestecker M. P., Parks W. T., Frank C. J., Castagnino P., Bottaro D. P., Roberts A. B., Lechleider R. J. Smad2 transduces common signals from receptor serine-threonine and tyrosine kinases. Genes Dev. 1998;12:1587–1592. doi: 10.1101/gad.12.11.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Dai C., Liu Y. Hepatocyte Growth Factor Suppresses Renal Interstitial Myofibroblast Activation and Intercepts Smad Signal Transduction. Am J Pathol. 2003;163:621–632. doi: 10.1016/S0002-9440(10)63689-9. [DOI] [PMC free article] [PubMed] [Google Scholar]