Abstract
Bacillus subtilis DegS-DegU belongs to a bacterial two-component system that controls many processes, including the production of exocellular proteases and competence development. It was found that when the glutamine synthetase gene glnA, which is involved in nitrogen regulation, was disrupted, the expression of the response regulator degU gene was increased. Deletion analysis and 5′-end mapping of the degU transcripts showed that the increase was caused by induction of a promoter (P2) located before the degU gene. Disruption of tnrA, a global regulator of nitrogen regulation, eliminated the P2 promoter induction by the glnA mutation. The fact that the P2 promoter is under nitrogen regulation was demonstrated by an increase in P2 expression with nitrogen-limited growth. It was also found by primer extension analysis that degU was transcribed by another promoter, P3, that is located downstream of P2. Efficient expression of P3 was dependent on phosphorylated DegU, as inactivation of the sensor kinase gene, degS, resulted in the loss of degU expression, although less efficient stimulation of degU expression was also observed with an enhanced level of DegU in a degS-deficient mutant. The promoter located upstream of the degSU operon, designated the P1 promoter here, was insensitive to glnA and degU mutations. These results suggest that degU expression is controlled by the three promoters under different growth conditions.
The bacterial two-component regulatory system is a device to cope with changing environments around the cell. One such example is the Bacillus subtilis DegS-DegU pair, which controls various cellular processes. These include competence development (6, 10, 11, 25), swarming, flagellum formation (1, 16), biofilm formation (15, 35), osmotic response (29), poly-γ-glutamic acid synthesis (35), salt resistance (19), antibiotic synthesis (17), and the synthesis of extracellular degradative enzymes (21). It has been proposed that some of the events regulated by the DegS-DegU system are manifested by different extents of phosphorylation of the response regulator DegU by the DegS kinase (16, 40). Thus, the wide variety of processes regulated by the DegS-DegU system suggest that the signals activating DegU are transduced to DegS via different mechanisms.
Changes in nitrogen availability lead to the alteration of gene expression in B. subtilis (8). This nitrogen regulation is mediated by two transcriptional regulators, GlnR and TnrA. GlnR is a repressor of the ureABC, glnR-glnA, and tnrA genes, whereas TnrA is a global regulator that exerts positive and negative regulation of many genes (3, 7, 8, 42, 47). In nitrogen-rich environments, glutamine synthetase (GS), the gene product of glnA, is feedback inhibited, resulting in repression of glnA expression via GlnR. On the other hand, feedback-inhibited GS inhibits the global regulator TnrA by protein-protein interaction (44). Since GS is the only means for ammonium assimilation in B. subtilis (2) and inactivating mutations in this enzyme result in high-level expression of glnRA in medium containing excess nitrogen, GS has been proposed to be a monitor for the nitrogen status of the cell (31, 33). Thus, the genes under nitrogen regulation are regulated by either GlnR or TnrA through GS, which senses the nitrogen status in the cell (8, 42).
Production of the extracellular neutral and alkaline proteases, encoded by nprE and aprE, respectively, is subject to regulation by the DegS-DegU two-component system. Since the degradation products of these proteases could supply the cells with a nitrogen source, it may be possible that the expression of the degS and/or degU gene is subject to regulation by nitrogen metabolism. To investigate whether the degS-degU two-component system is influenced by nitrogen availability, we examined the effect of deletion of the glnA gene, whose gene product (glutamine) supplies nitrogen for the synthesis of about 25% of nitrogen-containing compounds in the cell (28).
In this report, we show that there are two promoters before the degU gene, namely, the upstream P2 and the downstream P3 promoters, and that they are under the regulation of GlnA-TnrA and DegS-DegU, respectively.
MATERIALS AND METHODS
Plasmids and plasmid construction.
Plasmid pDLK2, a derivative of pDL2 (9) carrying the kanamycin resistance (Kmr) gene, was provided by K. Kobayashi. pDG148-degU was described previously (27).
Plasmid construction was performed with Escherichia coli JM103 (45). Plasmids carrying various upstream regions of degU were constructed by PCR amplification of the regions studied, followed by cleavage of the PCR products with EcoRI and BglII and subsequent cloning into pDLK2 that had been treated with EcoRI and BamHI. The plasmids thus constructed and the PCR primers used were as follows: for pAY4450, DGSU574F and DGSU880R; for pAY4434, DGSU574F and DGSU764R; for pAY4407 and pAY4407M, DGSU574F and DGSU737R; for pAY9707, DGSU627F and DGSU737R; for pAY9734 and pAY9734M, DGSU627F and DGSU764R; and for pAY3934, DGSU669F and DGSU764R. For construction of the plasmids below, the following synthetic DNAs were annealed and cloned into EcoRI- and BamHI-digested pDLK2: 669692F, 693750F, 750723R, and 722669R for pAY3920; 681710F, 711764F, 764737R, and 736681R for pAY5134; 692717F, 718764F, 764744R, and 743692R for pAY6234; and 711764F and 764711R for pAY8134. The cloned DNA regions were confirmed by sequence determination. Plasmid pBEST402, containing the blasticidin S resistance (Bsr) gene, was described previously (13). Plasmid pDegS12 carrying a degS-lacZ fusion was created as follows: a 798-bp EcoRI-BamHI fragment containing the promoter and the N-terminal 22 codons of degS was excised from pHAW3 (22) and ligated to EcoRI- and BamHI-digested pDLK2. pDSNM1 was constructed by insertion of a 1.4-kb SmaI fragment containing the neomycin resistance (Nmr) gene from pBEST509 (M. Itaya, unpublished data) into the AflII site of the degS gene in pOU1 (38) that had been blunted with T4 DNA polymerase. pODELS2 was created by insertion of annealed, synthetic DNAs (DSBSAF and DSBSAR) between the AflII and BstXI sites of pOU1. pHDCS2, pHV33, and pDES200 carrying the degS gene derived from the degS200(Hy) mutant have been described previously (4, 37, 39).
Bacterial strains and media.
The strains used in this study are listed in Table 1. To construct a glnA disruption mutant, the AY741G mutant, by insertion of the Bsr gene, two PCR fragments derived from the N- and C-terminal regions of glnA and a fragment containing the Bsr gene were prepared using the PCR primer pairs GLNA3 plus GLNAR617, GLNAF679 plus GLNA1329, and BSF plus BSR, respectively (Table 2). The templates used were the chromosomal DNA of strain CU741 for the glnA fragments and pBEST402 for the Bsr gene-containing fragment. The three fragments were fused by PCR using the primers GLNA3 and GLNA1329, followed by transformation into CU741. By this procedure, the DNA region between codons 214 and 226 in glnA was replaced by the Bsr gene. Strain CU741T was made by insertion of the tnrA::Cmr (chloramphenicol resistance) DNA provided by K. Kobayashi into CU741. Construction of CU741S was performed in a two-step procedure using the gene conversion method (36). First, strain CU741SN (Table 1) was transformed with pODELS2 and pHDCS2, selecting for Nm-sensitive and Cm-resistant cells. After single-colony isolation, segregationally unstable pHDCS2 was removed from the cell by growth in antibiotic-free LB Lenox medium. Strain AY5134HY was constructed by the same gene conversion method, except that plasmids pDES200 and pHDCS2 were used. The lacZ fusions at the amyE locus were constructed by transformation of linearized plasmids into strain CU741.
TABLE 1.
Bacterial strains used in this study
| Strain | Genotype or description | Reference or source |
|---|---|---|
| B. subtilis strains | ||
| CU741 | trpC2 leuC7 | 41 |
| AY741G | trpC2 leuC7 glnA::Bsr | This study |
| CU741T | trpC2 leuC7 tnrA::Cmr | This study |
| TT711 | trpC2 leuC7 degU::Cmr | 38 |
| HJS31 | ΔglnR57 | 32 |
| CU741SN | trpC2 leuC7 degS::Nmr | pDBNM1 × CU741 |
| CU741S | trpC2 leuC7 degS619; carries an in-phase deletion of degS (codons 11 to 170) | This study |
| AY101 | trpC2 leuC7 amyE::lacZ (no promoter) | pDLK2 × CU741 |
| AY4450 | trpC2 leuC7 amyE::degU-lacZ(1044-1350) (Nmr) | pAY4450 × CU741 |
| AY4450G | trpC2 leuC7 amyE::degU-lacZ(1044-1350) (Nmr) glnA::Bsr | AY741G × AY4450 |
| AY4434 | trpC2 leuC7 amyE::degU-lacZ(1044-1234) (Nmr) | pAY4434 × CU741 |
| AY4434G | trpC2 leuC7 amyE::degU-lacZ(1044-1234) (Nmr) glnA::Bsr | AY741G × AY4434 |
| AY4407 | trpC2 leuC7 amyE::degU-lacZ(1044-1207) (Nmr) | pA4407 × CU741 |
| AY4407G | trpC2 leuC7 amyE::degU-lacZ(1044-1207) (Nmr) glnA::Bsr | AY741G × AY4407 |
| AY4407T | trpC2 leuC7 amyE::degU-lacZ(1044-1207) (Nmr) tnrA::Cmr | CU741T × AY4407 |
| AY4407GT | trpC2 leuC7 amyE::degU-lacZ(1044-1207) (Nmr) glnA::BsrtnrA::Cmr | CU741T × AY4407G |
| AY4407U | trpC2 leuC7 amyE::degU-lacZ(1044-1207) (Nmr) degU::Cmr | TT711 × AY4407 |
| AY4407GU | trpC2 leuC7 amyE::degU-lacZ(1044-1207) (Nmr) glnA::BsrdegU::Cmr | TT711 × 4407G |
| AY9707 | trpC2 leuC7 amyE::degU-lacZ(1097-1207) (Nmr) | pAY9707 × CU741 |
| AY9707G | trpC2 leuC7 amyE::degU-lacZ(1097-1207) (Nmr) glnA::Bsr | AY741G × AY9707 |
| AY4407M | Same as AY4407 except for carrying sequence alterations | This study |
| AY4407MG | Same as AY4407G except for carrying sequence alterations | AY741G × AY4407M |
| AY9734 | trpC2 leuC7 amyE::degU-lacZ(1097-1234) (Nmr) | pAY9734 × CU741 |
| AY9734G | trpC2 leuC7 amyE::degU-lacZ(1097-1234) (Nmr) glnA::Bsr | AY741G × AY9734 |
| AY9734M | Same as AY9734 except for a mutation at the P3 promoter | This study |
| AY9734MG | Same as AY9734G except for a mutation at the P3 promoter | AY741G × AY9734M |
| AY3934 | trpC2 leuC7 amyE::degU-lacZ(1139-1234) (Nmr) | pAY3949 × CU741 |
| AY3934G | trpC2 leuC7 amyE::degU-lacZ(1139-1234) (Nmr) glnA::Bsr | AY741G × AY3934 |
| AY3920 | trpC2 leuC7 amyE::degU-lacZ(1139-1220) (Nmr) | pAY3920 × CU741 |
| AY3920G | trpC2 leuC7 amyE::degU-lacZ(1139-1220) (Nmr) glnA::Bsr | AY741G × AY3920 |
| AY5134 | trpC2 leuC7 amyE::degU-lacZ(1151-1234) (Nmr) | pAY5134 × CU741 |
| AY5134G | trpC2 leuC7 amyE::degU-lacZ(1151-1234) (Nmr) glnA::Bsr | AY741G × AY5134 |
| AY5134T | trpC2 leuC7 amyE::degU-lacZ(1151-1234) (Nmr) tnrA::Cmr | CU741T × AY5134 |
| AY5134GT | trpC2 leuC7 amyE::degU-lacZ(1151-1234) (Nmr) glnA::BsrtnrA::Cmr | CU741T × AY5134G |
| AY5134U | trpC2 leuC7 amyE::degU-lacZ(1151-1234) (Nmr) degU::Cmr | TT711 × AY5134 |
| AY5134GU | trpC2 leuC7 amyE::degU-lacZ(1151-1234) (Nmr) glnA::BsrdegU::Cmr | TT711 × AY5134G |
| AY5134S | trpC2 leuC7 amyE::degU-lacZ(1151-1234) (Nmr) degS619 | AY5134 × CU741S |
| AY5134GS | trpC2 leuC7 amyE::degU-lacZ(1151-1234) (Nmr) glnA::BsrdegS619 | AY741G × AY5134S |
| AY5134HY | trpC2 leuC7 amyE::degU-lacZ(1151-1234) (Nmr) degS200(Hy) | This study |
| AY6234 | trpC2 leuC7 amyE::degU-lacZ(1162-1234) (Nmr) | pAY6234 × CU741 |
| AY6234G | trpC2 leuC7 amyE::degU-lacZ(1162-1234) (Nmr) glnA::Bsr | AY741G × AY6234 |
| AY6234U | trpC2 leuC7 amyE::degU-lacZ(1162-1234) (Nmr) degU::Cmr | TT711 × AY6234 |
| AY8134 | trpC2 leuC7 amyE::degU-lacZ(1181-1234) (Nmr) | pAY8134 × CU741 |
| AY8134G | trpC2 leuC7 amyE::degU-lacZ(1181-1234) (Nmr) glnA::Bsr | AY741G × AY8134 |
| AYDS11 | trpC2 leuC7 amyE::degS-lacZ | pDegS12 × CU741 |
| AYDS11G | trpC2 leuC7 amyE::degS-lacZ glnA::Bsr | AY741G × AYDS11 |
| AYDS11U | trpC2 leuC7 amyE::degS-lacZ degU::Cmr | TT711 × AYDS11 |
| E. coli strain | ||
| JM103 | Δlac-pro thi rpsL supE sbcB hsdR4 F′ [traD36 proAB+lacIqlacZΔM15] | 45 |
TABLE 2.
Primers used in this study
| Primer | Nucleotide sequence (5′-3′)a |
|---|---|
| GLNA3 | GGCAAAGTACACTAGAGAAG |
| GLNAR617 | CACTTCATAACCGATATTAGGGTTTGGATGTCATCACAAGAGC |
| GLNAF679 | TACCCGAAATTAAAAGTTTTACCACTGCATGCGACATTTATGCC |
| GLNA1329 | CTGAGACATATACRGTTCGC |
| BSF | CCTAATATCGGTTATGAAGTG |
| BSR | GGTAAAACTTTTAATTTCGGGTA |
| DegS618 | CGAGCGGATTTTCCGTG |
| DegUF | GTAAAGCTTGACCGAATGCTAGAGTATATAG |
| DegUR | GTAGTCGACTAGTAAAAGGCAAGTCTCC |
| DGSU574F | AGTTGAATTCGGCTTGCTGGGCATG |
| DGSU880R | AGTTAGATCTTCATCACCTTCGGCTACCAC |
| DGSU764R | AGTTAGATCTCGCCTCCTTGTATTATTGTTC |
| DGSU737R | AGTTAGATCTCTAGCATTCGGTCAATATG |
| DGSU627F | AGTTGAATTCAATAGATTCGAAAATAGGTCTTGG |
| DGSU669F | AGTTGAATTCAGGTTCCGTTATCTCTTTGACT |
| MU14F | GAGTAGATTTATTGGACGGAACGACGGTTATAGATTCGAAAATAGGTCTTG |
| MU14R | CAAGACCTATTTTCGAATCTATAACCGTCGTTCCGTCCAATAAATCTACTC |
| 669692F | AATTCAGGTTCCGTTATCTCTTTGACTAT |
| 693750F | GATTTGTAAAATAGAGCCAAAAGGCATATTGACCGAATGCTAGAGTATATAGAACAAT |
| 750723R | GATCATTGTTCTATATACTCTAGCATTCGGTC |
| 722669R | AATATGCCTTTTGGCTCTATTTTACAAATCATAGTCAAAGAGATAACGGAACCTG |
| 681710F | AATTCTCTTTGACTATGATTTGTAAAATAGAGCC |
| 711764F | AAAAGGCATATTGACCGAATGCTAGAGTATATAGAACAATAATACAAGGAGGCGA |
| 764737R | GATCTCGCCTCCTTGTATTATTGTTCTATATAC |
| 736681R | TCTAGCATTCGGTCAATATGCCTTTTGGCTCTATTTTACAAATCATAGTCAAAGAG |
| 692717F | AATTGATTTGTAAAATAGAGCCAAAAGGC |
| 718764F | ATATTGACCGAATGCTAGAGTATATAGAACAATAATACAAGGAGGCGA |
| 764744R | GATCTCGCCTCCTTGTATTATTGTTC |
| 743692R | TATATACTCTAGCATTCGGTCAATATGCCTTTTGGCTCTATTTTACAAATC |
| 711764F | AATTAAAAGGCATATTGACCGAATGCTAGAGTATATAGAACAATAATACAAGGAGGCGA |
| 764711R | GATCTCGCCTCCTTGTATTATTGTTCTATATACTCTAGCATTCGGTCAATATGCCTTTT |
| DSBSAF | TTAACTTCCCGGGAAGGTACCTTAGATCCACT |
| DSBSAR | GATCTAAGGTACCTTCCCGGGAAG |
| DUBio866 | XTACCACTTCAAAGGTAGGTTC |
| DUBio1 | XCTGATGGTCGTCGATAATAAC |
X, biotin attached to the nucleotide at the 5′ end.
LB Lenox medium and antibiotic medium III were obtained from Difco Co. Modified competence medium and Shaffer's sporulation medium were prepared as described previously (18, 30). These media contained glutamine at a final concentration of 0.2%. BSS medium was made by the procedure of Chasin and Magasanik (5).
Northern analysis.
Cells grown in Schaeffer's medium were collected from 40-ml cultures at the indicated times, and total RNAs were isolated as previously described (46). The RNA samples (20 μg) were denatured with formamide and electrophoresed in a 1.2% agarose gel, and degU mRNA was detected as described previously, using a DIG luminescent detection kit (Boehringer Mannheim) (24). A digoxigenin-labeled DNA probe for the entire degU gene was prepared by PCR with a DIG probe synthesis kit (Boehringer Mannheim) and the primer pair DegUF plus DegUR. The RNA size marker set used was RNA molecular weight marker III, obtained from Roche Diagnostics.
Primer extension analysis.
Primer extension was performed with an avian myeloblastosis virus reverse transcriptase cDNA synthesis kit obtained from Life Sciences, Inc. The reaction mixture contained 10 μg of RNA and the biotinylated primer DUBio866 or DUBio1. The reaction products were run in sequencing gels together with sequencing ladders prepared by using the same primers and a PCR fragment prepared with primers DegS618 and DegUR as a template.
Site-directed mutagenesis.
Introduction of nucleotide changes into the putative TnrA recognition site was performed as follows. Two PCR fragments, amplified using primer pairs DGSU574F plus MU14R and MU14F plus DGSU737R, were prepared with CU741 DNA as a template, and after purification, they were mixed and subjected to a second PCR with primers DGSU574F and DGSU737R. The resultant PCR fragment was purified, digested with EcoRI and BamHI, and cloned between the EcoRI and BanHI sites of pDLK2.
β-Galactosidase assays.
Cells from frozen stock cultures were spread on glutamine-supplemented LB plates containing appropriate antibiotics 1 day before experiments, and the colonies formed were transferred to glutamine-containing LB medium and incubated overnight. The cultures were then inoculated into glutamine-containing Schaeffer's medium at a concentration of 1%. For experiments with synthetic medium, cells were grown overnight in BSS medium without NH4Cl and then inoculated at 1% into fresh BSS medium with or without NH4Cl. Samples were withdrawn at hourly intervals, and β-galactosidase activities (in Miller units) were determined as described previously (24). The enzyme levels in all experiments were determined for the samples taken from T−1 (1 hour before the end of exponential growth phase) to T5 (5 hours after the end of exponential growth phase). The experiments were repeated at least twice, and the variations of the enzyme levels determined were within 20%. The data shown in this work are those from a typical experiment.
Transformation.
Cells were made competent in MC medium by a previously described method (26).
RESULTS
Transcriptional activation of degU by glnA deletion.
In an attempt to examine whether degU expression is under nitrogen regulation, we introduced a glnA disruption mutation into a strain carrying a transcriptional degU-lacZ fusion at the amyE locus (strain AY4450) (Fig. 1) and determined the β-galactosidase activity. The DNA region that we examined spans nucleotides (nt) 1,044 to 1,350; we define nt 1 as the first nucleotide of the degS coding sequence, and by this definition, the degU gene starts at nt 1,241 (Fig. 1). It was shown that β-galactosidase activities in the glnA mutant (AY4450G) were increased 12- to 7-fold compared with those in the wild-type strain before and around the end of exponential growth (T−1 to T1), and the expression level remained 2- to 3-fold higher thereafter (Fig. 2A).
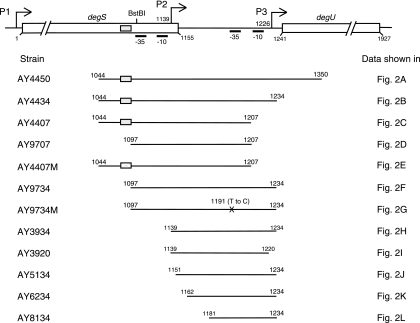
FIG. 1.
Search for degU promoters by deletion analysis. The numbers indicate the nucleotide positions in the degS-degU region, with nt 1 being the first nucleotide of the degS coding sequence. Bent arrows depict the transcriptional initiation sites, which are shown by nucleotide numbers. The gray and crossed boxes depict the wild-type and mutant TnrA boxes, respectively. The map is not drawn to scale.
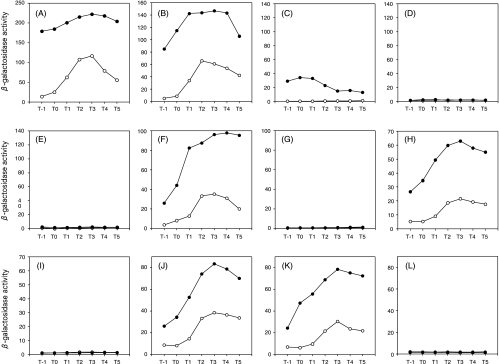
FIG. 2.
Effects of glnA disruption on expression of lacZ fused to various regions upstream of degU. Cells grown overnight in glutamine-containing LB medium were inoculated into glutamine-containing Schaeffer's sporulation medium, and β-galactosidase activities were determined as described in Materials and Methods. Open circles, wild type; solid circles, glnA mutant. (A) Strains AY4450 and AY4450G; (B) AY4434 and AY4434G; (C) AY4407 and AY4407G; (D) AY9707 and AY9707G; (E) AY4407M and AY4407MG; (F) AY9734 and AY9734G; (G) AY9734M and AY9734MG; (H) AY3934 and AY3934G; (I) AY3920 and AY3920G; (J) AY5134 and AY5134G; (K) AY6234 and AY6234G; (L) AY8134 and AY8134G.
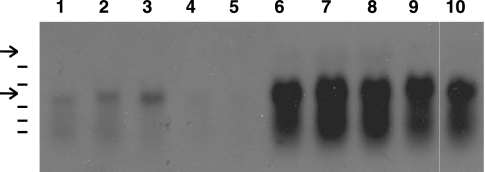
To confirm that the enhanced expression of the lacZ fusion was caused at the transcriptional level, we performed Northern analysis, using RNAs obtained at different culture times and a digoxigenin-labeled degU probe. An RNA band with a size of 0.7 to 0.8 kb was detected in the samples from the wild-type cells (Fig. 3, bottom arrow). In contrast, the RNA band from samples of the glnA mutant was much more intense and accompanied by possible degradation products. Since the RNA band covers the entire degU coding sequence (690 bp), we concluded that the transcription of degU was enhanced by the deletion of glnA. It should be noted that Kobayashi, using an undomesticated strain (16), found RNA bands with sizes larger than the 0.7-kb degU band at the end of exponential growth, which were probably the degradation products of the degSU operon mRNA originating from the promoter upstream of degS, suggesting that transcription from the degS promoter extends into the degU region. No such bands, however, were detectable at the positions expected for the sizes of the degSU mRNA (Fig. 3, top arrow) and its degradation products in our hands, probably reflecting the low-level synthesis and degradation of the degSU mRNA.
FIG. 3.
Stimulation of degU mRNA synthesis by glnA disruption. RNAs were isolated from CU741 (lanes 1 through 5) and CU741G (glnA mutant) (lanes 6 through 10) cells at different time points and used for Northern analysis as described in Materials and Methods. The upper and lower arrows indicate the positions of the expected transcript sizes of the degSU operon and the degU gene, respectively. Lanes 1 and 6, T0; lanes 2 and 7, T1; lanes 3 and 8, T2; lanes 4 and 9, T3; lanes 5 and 10, T4. The lines on the left are RNA size markers, with the following sizes (bases), from the top: 1,517, 1,049, 575, 438, and 310.
Search for promoters of degU expression.
In order to locate the site of stimulation by the glnA mutation, we introduced deletions into the upstream region of degU and estimated β-galactosidase activities derived from the degU-lacZ fusions in the presence and absence of the glnA disruption. The DNA regions tested are depicted in Fig. 1. There was essentially no change of the overall profile of β-galactosidase activities when the deletion extended from the 3′ end (nt 1,350) to nt 1,234 (strains AY4434 and AY4434G) (Fig. 1 and 2B). However, for strain AY4407, carrying a further deletion to nt 1,207 (Fig. 1), the expression of the lacZ fusion was observed only in the glnA disruptant (AY4407G), and the expression level was higher around the end of exponential growth phase (Fig. 2C). On the other hand, for strain AY9707, in which a region spanning nt 1,097 to 1,207 was fused to lacZ, there was no detectable β-galactosidase activity irrespective of the glnA disruption (Fig. 2D). These results suggested that there are at least two promoters between nt 1,044 and nt 1,234, with the upstream and downstream promoters being completely dependent and partially dependent on glnA deletion for expression, respectively.
We then successively deleted nucleotides from the 5′ end (nt 1,097) to nt 1,181 (Fig. 1). It was found that similar profiles and levels of β-galactosidase activities were exhibited in strains AY9734, AY3934, AY5134, and AY6234 and their glnA derivatives (Fig. 2F, H, J, and K, respectively), but a further deletion to nt 1,180 (AY8134) resulted in a total loss of lacZ fusion expression (Fig. 2L), irrespective of glnA deletion. It was also shown that strain AY3920, in which the 14 nucleotides at the 3′ end of the sequence in AY3934 were deleted (Fig. 1), showed no detectable β-galactosidase activity (Fig. 2I).
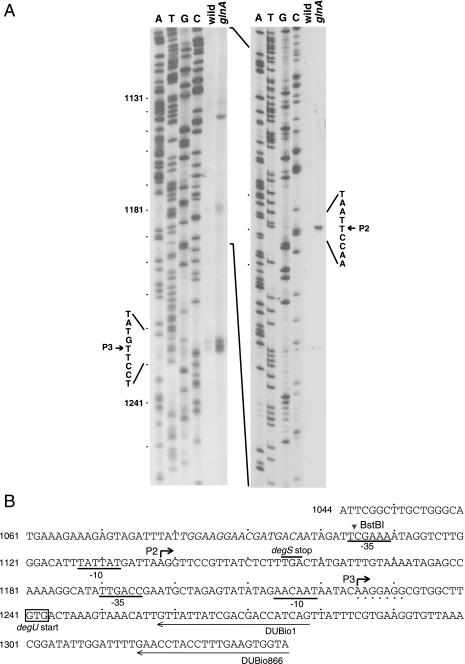
Transcriptional initiation sites of degU.
To correlate degU-lacZ expression with the DNA sequence, we searched for promoters upstream of degU. The RNA samples from T2 used for Northern analysis were subjected to primer extension analysis to determine the transcriptional initiation sites of degU. With the RNA obtained from the wild-type cells and a biotin-labeled primer, DUBio866 (Fig. 4B), we detected primer extension products that were separated by 1 nt (Fig. 4A, left panel). Since the faster-moving band was most likely produced by nibbling, we assigned the initiation site of degU in the wild-type cell to the nucleotide 15 bases upstream of the degU start codon (Fig. 4B). We designated the promoter for this transcription initiation site P3. With the RNA from the glnA cells, another discrete band was detected upstream of the P3 start site (Fig. 4A, left panel). To map this site more precisely, we used another biotin-labeled primer, DUBio1, and found that the transcriptional start site was 102 nucleotides upstream of the degU start codon (Fig. 4A, right panel, and B). We designated this promoter P2. The faint band seen between the two bands with the RNA from the glnA cells (Fig. 4A, left panel) may not represent the promoter function, since it was not detected with primer DUBio1 (Fig. 4A, right panel). The two transcriptional initiation sites are preceded by putative −10 and −35 regions recognized by σA-type RNA polymerase (Fig. 4B).
FIG. 4.
Determination of transcriptional start sites of degU. (A) Primer extension analysis of degU promoters. The numbers on the left indicate the positions of the nucleotides relative to the first nucleotide of the degS coding sequence. RNAs obtained at T2 from CU741 (wild type) and CU741G (glnA mutant) were subjected to primer extension analysis with either primer DUBio866 or primer DegUBio1 (left or right panel, respectively). The arrowheads indicate the nucleotides at which transcription initiates. The experimental conditions are described in Materials and Methods. (B) Nucleotide sequence around the degS-degU intercistronic region. The nucleotides shown in italics and bent arrows denote the TnrA box and transcriptional start sites, respectively.
The degU-lacZ fusion in strain AY9734M did not show promoter activity (Fig. 2G). Sequence analysis revealed a T-to-C mutation at nt 1,191, which is within the −35 region of the P3 promoter. In strain AY3920, the deletion had extended from the 3′ end to nt 1,221, which is adjacent to the −10 sequence of the P3 promoter, and this sequence organization may explain the loss of degU-lacZ expression shown in Fig. 2I. Expression of degU-lacZ was observed in strain AY6234 (Fig. 2K) but not in AY8134 (Fig. 2L). The 5′ end of the degU region contained in these constructs is at positions −64 and −45, respectively, with respect to the P3 promoter. It is possible that the loss of the promoter activity in AY8134 was caused by the deletion of a sequence with which some positive regulator interacts, and we indeed found that the candidate for the regulator is most likely DegU or phosphorylated DegU (see below).
It has been shown previously by degU-lacZ fusion analysis that there are two promoters for the expression of the degSU operon, i.e., the major and minor promoters, located upstream of degS and in a 3′ region of the degS coding region, respectively, and the transcriptional initiation site of the major promoter was determined by primer extension analysis (20). We term this promoter P1 in this paper.
Involvement of TnrA in P2 and P3 promoter expression.
From the results showing that degU-lacZ expression was observed in the glnA disruption mutant AY4407G but not in AY9707G (Fig. 2C and D) and that the DNA region at the amyE locus in AY4407G contains only the P2 promoter (Fig. 1), we concluded that the P2 promoter is under glnA regulation.
We next studied how the glnA mutation affects degU expression through the P3 promoter, as exemplified in AY5134 and AY5134G. It is well documented that the glnA gene product regulates the activities of GlnR and TnrA, through which many genes are regulated (8, 42). To investigate whether the glnA effect on degU expression from the P3 promoter occurs through any of these regulatory proteins, we first examined the effect of the glnR57 mutant, which carries an in-phase deletion in glnR (32), and found that it had no effect on degU-lacZ expression in strain AY5134 (data not shown). On the other hand, the elevated expression of degU-lacZ in the glnA-deleted AY5134G strain was diminished to the level seen in the wild-type strain, AY5134, by additional disruption of tnrA (AY5134GT) (Table 3). The tnrA deletion alone in strain AY5134T did not affect degU-lacZ expression. We concluded from these results that the degU-lacZ fusion in AY5134G is expressed via two pathways, with one being the P3 promoter and the other a direct or indirect stimulatory effect on P3 through the GlnA-TnrA route.
TABLE 3.
Effects of glnA and tnrA deletion on degU-lacZ expressiona
| Strain | Relevant genotype | β-Galactosidase activity |
|---|---|---|
| AY5134 | Wild type | 35 |
| AY5134G | glnA::Bsr | 81 |
| AY5134T | tnrA::Cmr | 40 |
| AY5134GT | glnA::BsrtnrA::Cmr | 39 |
| AY4407 | Wild type | 1.1 |
| AY4407G | glnA::Bsr | 33 |
| AY4407T | tnrA::Cmr | 1.0 |
| AY4407GT | glnA::BsrtnrA::Cmr | 1.0 |
Cells were grown in glutamine-containing Schaeffer's medium as described in Materials and Methods. β-Galactosidase activities from T−1 to T5 were determined (Miller units), and the highest values attained are shown.
To examine whether TnrA is also involved in P2 promoter expression, strain AY4407 and its derivatives carrying a glnA and/or tnrA deletion were examined. It was shown that P2 promoter expression was dramatically increased in the glnA strain AY4407G, in contrast to that in the wild-type strain, where no enzymatic activity was observed (Table 3). The enhanced expression in AY4407G was abolished by additional disruption of tnrA, as shown in strain 4407TG (Table 3), indicating that P2 is regulated by the GlnA-TnrA route.
Mutational analysis of the possible TnrA recognition sequence upstream of the P2 promoter.
The involvement of TnrA in P2 promoter expression and the loss of its activity by deletion of the sequence between nt 1,044 and nt 1,097 (strains AY4407 and AY9707 in Fig. 1 and 2C and D) suggested a TnrA target in this region. A computer search for the recognition sequence of TnrA, TGTNAN7TNACA (23, 42), or its updated version, TGTNANAWWWTMTNACA (47), revealed a sequence with partial homology (TGGAAGGAACGATGACA [underlined nucleotides match the requirement]) spanning nt 1,081 to 1,097 (positions −57 to −41 with respect to the transcriptional start site of P2) (Fig. 4B). When the nucleotide sequence was changed to TGGACGGAACGACGGTT(underlined nucleotides were changed), the promoter activity was lost in the mutant strain AY4407M and its glnA derivative (Fig. 1 and 2E), suggesting that this nucleotide sequence is the target of TnrA. It has been shown that transcriptional activation by TnrA depends on the target DNA sequence located closely to and upstream of the −35 sequence (43). The above result is in accordance with this precedent, although the nucleotide sequence in this case somewhat deviates from the updated version of the consensus sequence.
Expression of P2 promoter under nitrogen-limited conditions.
Since the P2 promoter is activated by TnrA, it may also be stimulated in nitrogen-limiting medium. To test this notion, we grew AY4407 carrying the P2-lacZ fusion in glutamate-containing BSS medium, with or without the addition of NH4Cl, and estimated the β-galactosidase activity. As shown in Fig. 5, the expression of the P2-lacZ fusion was elevated only under the nitrogen-limiting conditions, and this enhanced expression was abolished by tnrA deletion. These results show that the P2 promoter is nitrogen regulated.
FIG. 5.
Expression of P2 promoter during nitrogen-limited growth. Cells were grown overnight in BSS medium containing 0.2% glutamate and Nm at 10 μg/ml and transferred as a 1% inoculum to the same medium, with (solid symbols) and without (open symbols) the addition of NH4Cl. Experimental procedures are described in Materials and Methods. Open circles, AY5134; solid circles, AY5134; open squares, AY5134T (tnrA mutant); solid squares, AY5134T (tnrA mutant).
Involvement of DegU in P3 but not in P2 promoter expression.
It was proposed that degU expression is under positive autoregulation by its own gene product (16). We thus examined the effect of degU deficiency in strains AY4407 and AY5134, carrying the P2 and P3 promoters, respectively. The results showed that the elevated expression of the P2-lacZ fusion by glnA disruption (AY4407G) was not affected by degU disruption (AY4407GU), indicating that P2 promoter expression is not regulated by DegU. In contrast, P3-lacZ expression in both the wild-type and glnA strains (AY5134 and AY5134G, respectively) was reduced to the background level by degU disruption (AY5134U and AY5134GU) (Table 4). These results show that the expression of the P3 promoter is dependent on DegU and rule out the possibility that the stimulation of the P3 promoter by the glnA mutation in strain AY5134 (Table 3) is due to the effect of TnrA. Thus, it can be concluded that the expression of P3-lacZ at the amyE locus is under positive regulation of DegU irrespective of whether the transcription is stimulated or not by glnA deletion, whereas the P2-lacZ construct placed at the amyE locus is expressed from its own promoter, independent of DegU.
TABLE 4.
Effects of degU deletion on P2 and P3 promoter expressiona
| Strain | Relevant genotype | β-Galactosidase activity |
|---|---|---|
| P2-lacZ strains | ||
| AY4407 | Wild type | 1.1 |
| AY4407G | glnA::Bsr | 36 |
| AY4407U | degU::Cmr | 1.3 |
| AY4407GU | glnA::BsrdegU::Cmr | 45 |
| P3-lacZ strains | ||
| AY5134 | Wild type | 36 |
| AY5134G | glnA::Bsr | 83 |
| AY5134U | degU::Cmr | 2.1 |
| AY5134GU | glnA::BsrdegU::Cmr | 2.3 |
| AY6234 | Wild type | 30 |
| AY6234U | degU::Cmr | 2.2 |
Cells were grown in glutamine-containing Schaeffer's medium as described in Materials and Methods. The table contains two sets of data obtained from two separate experiments, one for strains carrying the P2-lacZ fusion and the other for those containing the P3-lacZ fusion. β-Galactosidase activities from T−1 to T5 were determined (Miller units), and the highest values attained are shown.
It should be noted that a deletion up to nt 1,161 carried in strain AY6234 is also subject to DegU regulation (Table 4), indicating that the DegU target is located downstream of this nucleotide.
Involvement of DegS in degU expression.
We next investigated whether DegS is involved in degU expression. Kobayashi reported that phosphorylated DegS regulates degU expression, using a nonsense degS mutant and a degU mutant carrying a D-to-A mutation at codon 56, the site of phosphorylation (16). The degS mutant we used carried an in-phase degS deletion, degS619, in which codons 11 through 170 are deleted, eliminating a possible polar effect on the expression of the downstream degU gene. As shown in Table 5, there was almost no degU-lacZ expression in the degS619 mutant (AY5134S) compared with the expression seen in the wild-type strain (AY5134), confirming the previous results of Kobayashi.
TABLE 5.
Effects of degS deletion and overexpression of degU on degU-lacZ expressiona
| Strain | Relevant genotype | IPTG induction | β-Galactosidase activity |
|---|---|---|---|
| AY5134 | Wild type | 35 | |
| AY5134S | degS619 | 2.2 | |
| AY5134GS | degS619 glnA::Bsr | 7.7 | |
| AY5134S(pDG148-degU) | degS619 | − | 2.8 |
| AY5134S(pDG148-degU) | degS619 | + | 354 |
| AY5134HY | degS200(Hy) | 253 | |
| AY101 | amyE::lacZ | 1.2 |
Cells were grown in glutamine-containing Schaeffer's medium as described in Materials and Methods. β-Galactosidase activities from T−1 to T5 were determined (Miller units), and the highest values attained are shown. IPTG (0.2 mM), when used, was added at the beginning of culture.
To investigate whether the increase in degU expression by glnA deletion has any effect on degU-lacZ expression in the degS619 background, we introduced the glnA::Bsr mutation into the strain carrying degS619 to construct AY5134GS, and it was shown that there was a low but significant increase in degU-lacZ expression (Table 5). These results suggested that DegU might stimulate its own expression without phosphorylation by DegS. In order to examine this possibility further, we amplified DegU in the degS619 strain AY5134S carrying pDG148-degU, in which degU expression is under the control of the IPTG (isopropyl-β-d-thiogalactopyranoside)-inducible Pspac promoter. As shown in Table 5, the addition of IPTG resulted in >100-fold increased expression of degU.
DegS has both phosphorylation and dephosphorylation activities toward DegU and DegU-phosphate, respectively, and the latter activity in the degS200(Hy) mutant is greatly reduced, leading to stabilization of DegU-phosphate in the cell (6, 39). We therefore expected that the mutation might cause an increase in the degU expression level, and the result shown in Table 5 indicates that this was indeed the case.
It can be concluded from these results that although phosphorylation of DegS causes positive autoregulation of degU, unphosphorylated DegU has the same capacity, although it is less efficient at low concentrations.
In strain AY6234 carrying pDG148-degU, the addition of IPTG also stimulated degU-lacZ expression to the same extent as that exhibited by AY5134 carrying the same plasmid, whereas no stimulation was observed in strain AY8134 (data not shown). These results and the data in Table 4 indicate that the target of DegU is present in a DNA region downstream of nt 1,162 but upstream of or including nt 1,181.
P1 promoter without glnA or degU regulation.
To investigate whether the P1 promoter before the degS gene is subject to regulation by the glnA or degU gene product, we created a degS-lacZ fusion at the amyE locus and introduced this mutation into the constructed strain (see Materials and Methods). It was shown that disruption of neither glnA nor degU affected degS-lacZ expression (Table 6), indicating that unlike the P2 and P3 promoters, the P1 promoter is not subject to regulation by these gene products.
TABLE 6.
Effects of glnA and degU mutations on expression of degS-lacZ placed at the amyE locusa
| Strain | Relevant genotype | β-Galactosidase activity |
|---|---|---|
| AYDS11 | Wild type | 8.6 |
| AYDS11G | glnA::Bsr | 8.0 |
| AYDS11U | degU::Cmr | 8.6 |
Experimental procedures are the same as those described in the footnote to Table 3.
DISCUSSION
We have shown in this study that degU expression is driven by two promoters, P2 and P3, located within the C-terminal region of the degS coding sequence and upstream of degU, respectively, and that the expression of P2- and P3-lacZ fusions placed at the amyE locus is stimulated by glnA deletion. It was also shown that degU expression was abolished by deletion of degS or degU and stimulated by an increased level of DegU or phosphorylated DegU.
Msadek et al. reported previously that there are two promoters for the expression of the degSU operon, with the major promoter located upstream of degS (here we call it P1) and the minor one located upstream of the BstBI site within the degS sequence (20) (Fig. 4B). The minor promoter activity is lost when the DNA region upstream of the BstBI site is removed (20). This upstream region is necessary for the P2 promoter in our study, suggesting that their minor promoter corresponds to the P2 promoter. On this basis, the P3 promoter found in this study is one that has not been detected previously. However, since the discrepancy could have arisen by a strain difference and/or the medium used, the relationship between the minor promoter and those found here, the P2 and P3 promoters, is not clear at present.
We have observed that the P2 and P3 promoters are activated by glnA mutation (Fig. 2; Tables 3 and 4). We interpret these results as follows. The deletion of glnA resulted in activation of TnrA, which then induced the P2 promoter activity, probably by binding to the target sequence upstream of the P2 promoter (Fig. 2E and 4B). An increase in the degU transcript from the P2 promoter contributed to amplification of degU expression, resulting in increased levels of DegU. In strains carrying the P3-lacZ fusion at the amyE locus, these events occurring at the native, chromosomal degU locus supplied increased amounts of DegU to the P3 promoter, resulting in enhanced expression of the P3 promoter, since it is positively regulated by DegU (Table 4). When the P2 promoter is also present before the degU-lacZ construct (strains AY4450 and AY4434), the transcription from the P2 promoter supplies additional degU transcripts, which results in a further increase in degU expression. Since the expression of the P2 promoter was higher at the end of the exponential growth phase (Fig. 2C), it seems reasonable that the degU-lacZ expression levels in strains AY4450 and AY4434 were higher during and at the end of exponential growth phase than those in the cells carrying only the P3-lacZ fusion at the amyE locus (compare Fig. 2A and B with Fig. 2F, H, J, and K).
It is tempting to speculate that the P2 promoter works in nitrogen-limited environments, since the limited ammonium supply in the medium caused an increase in expression of this promoter (Fig. 5). However, the low level of increase in degU expression may not be sufficient to increase the expression of aprE, since even the high-level expression of degU driven by IPTG induction of pDG148-degU used in this study failed to enhance aprE expression (27). It would rather suggest that degU expression through the P2 promoter might play some role in competence development, as shown by an increase in comK expression (data not shown) and the transformation efficiency during nitrogen-limited growth (14). Since the P3 promoter activity is increased by the presence of the intact degS and, even more dramatically, degS200(Hy) genes (Table 5) and since high levels of phosphorylated DegU are necessary for aprE expression (40), the role of the P3 promoter may be to supply phosphorylated DegU to the target genes through the DegS-DegU phosphorylation system. However, quantitative studies are required to know the extents to which the three promoters contribute to aprE expression and competence development, which require phosphorylated and unphosphorylated DegU, respectively.
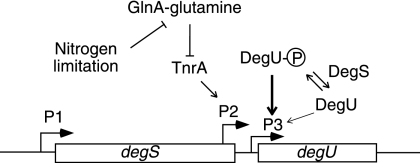
A current model for degU expression is presented in Fig. 6, which is based on the present findings and the model presented by Kobayashi (16). Transcription from the P1 promoter without regulation by the GlnA-TnrA and DegS-DegU systems covers the entire degSU operon, probably without attenuation at the intercistronic region. When the cell encounters nitrogen-limiting conditions, the P2 promoter is stimulated, resulting in enhanced expression of degU. The P3 promoter is under positive autoregulation of DegU. Efficient autoregulation requires phosphorylated DegU, which binds to the promoter region of degU (16), although its unphosphorylated form also has some enhancing activity, as shown by an increase in expression of degU-lacZ in glnA- and degS-deficient cells and in degS cells containing high levels of unphosphorylated DegU (Table 4). In the degS200(Hy) mutant, in which the dephosphorylation activity of DegU-phosphate is low, the phosphorylated DegU molecules activate the P3 promoter to increase the degU mRNA level and enhance aprE expression by binding to the upstream region of the aprE gene (12, 34).
FIG. 6.
Schematic representation of degSU operon regulation. For details, see Discussion. The bent arrows show the three promoters. The arrows and T bars indicate positive and negative regulation of the targets, respectively. The dark and light arrows pointing to P3 depict positive regulation with high and low efficiencies, respectively. The arrows are also used to show phosphorylation and dephosphorylation of DegU by DegS. The map is not drawn to scale.
Acknowledgments
We thank A. L. Sonenshein and K. Kobayashi for a bacterial strain and a plasmid, respectively. We also thank anonymous referees for comments and suggestions to improve the manuscript and S. Yasumoto for technical assistance.
This work was supported by a grant-in-aid for scientific research (C) from the Ministry of Education, Science, Sports and Culture of Japan.
Footnotes
Published ahead of print on 23 May 2008.
REFERENCES
- 1.Amati, G. A., P. Bisicchia, and A. Galizzi. 2004. DegU-P represses expression of the motility fla-che operon in Bacillus subtilis. J. Bacteriol. 1866003-6014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belitsky, B. R., and A. L. Sonenshein. 1998. Role and regulation of Bacillus subtilis glutamate dehydrogenase genes. J. Bacteriol. 1806298-6305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belitsky, B. R., L. V. Wray, Jr., S. H. Fisher, D. E. Bohannon, and A. L. Sonenshein. 2000. Role of TnrA in nitrogen source-dependent repression of Bacillus subtilis glutamate synthase gene expression. J. Bacteriol. 1825939-5947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bron, S., L. Janniere, and S. D. Ehrlich. 1988. Restriction and modification in Bacillus subtilis Marburg 168: target sites and effects on plasmid transformation. Mol. Gen. Genet. 211186-189. [DOI] [PubMed] [Google Scholar]
- 5.Chasin, L. A., and B. Magasanik. 1968. Induction and repression of the histidine-degrading enzymes of Bacillus subtilis. J. Biol. Chem. 2435165-5178. [PubMed] [Google Scholar]
- 6.Dahl, M. K., T. Msadek, F. Kunst, and G. Rapoport. 1992. The phosphorylation state of the DegU response regulator acts as a molecular switch allowing either degradative enzyme synthesis or expression of genetic competence in Bacillus subtilis. J. Biol. Chem. 26714509-14514. [PubMed] [Google Scholar]
- 7.Ferson, A. E., L. V. Wray, Jr., and S. H. Fisher. 1996. Expression of the Bacillus subtilis gabP gene is regulated independently in response to nitrogen and amino acid availability. Mol. Microbiol. 22693-701. [DOI] [PubMed] [Google Scholar]
- 8.Fisher, S. H. 1999. Regulation of nitrogen metabolism in Bacillus subtilis: vive la différence. Mol. Microbiol. 32223-232. [DOI] [PubMed] [Google Scholar]
- 9.Fukuchi, K., Y. Kasahara, K. Asai, K. Kobayashi, S. Moriya, and N. Ogasawara. 2000. The essential two-component regulatory system encoded by yycF and yycG modulates expression of the ftsAZ operon in Bacillus subtilis. Microbiology 1461573-1583. [DOI] [PubMed] [Google Scholar]
- 10.Hahn, J., A. Luttinger, and D. Dubnau. 1996. Regulatory inputs for the synthesis of ComK, the competence transcription factor of Bacillus subtilis. Mol. Microbiol. 21763-775. [DOI] [PubMed] [Google Scholar]
- 11.Hamoen, L. W., A. F. Van Werkhoven, G. Venema, and D. Dubnau. 2000. The pleiotropic response regulator DegU functions as a priming protein in competence development in Bacillus subtilis. Proc. Natl. Acad. Sci. USA 979246-9251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Henner, D. J., E. Ferrari, M. Perego, and J. Hoch. 1988. Localization of the targets of the hpr-97, sacU32(Hy), and sacQ36(Hy) mutations in upstream regions of the subtilisin promoter. J. Bacteriol. 170296-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Itaya, M., I. Yamaguchi, K. Kobayashi, T. Endo, and T. Tanaka. 1990. The blasticidin S resistance gene (bsr) selectable in a single copy state in the Bacillus subtilis chromosome. J. Biochem. 107799-801. [DOI] [PubMed] [Google Scholar]
- 14.Jarmer, H., R. Berka, S. Knudsen, and H. H. Saxild. 2002. Transcriptome analysis documents induced competence of Bacillus subtilis during nitrogen limiting conditions. FEMS Microbiol. Lett. 206197-200. [DOI] [PubMed] [Google Scholar]
- 15.Kobayashi, K. 2007. Bacillus subtilis pellicle formation proceeds through genetically defined morphological changes. J. Bacteriol. 1894920-4931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kobayashi, K. 2007. Gradual activation of the response regulator DegU controls serial expression of genes for flagellum formation and biofilm formation in Bacillus subtilis. Mol. Microbiol. 66395-409. [DOI] [PubMed] [Google Scholar]
- 17.Koumoutsi, A., X. H. Chen, J. Vater, and R. Borriss. 2007. DegU and YczE positively regulate the synthesis of bacillomycin D by Bacillus amyloliquefaciens strain FZB42. Appl. Environ. Microbiol. 736953-6964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kunst, F., T. Msadek, and G. Rapoport. 1994. Signal transduction network controlling degradative enzyme synthesis and competence in Bacillus subtilis, p. 1-20. In P. Piggot, C. P. Moran, Jr., and P. Youngman (ed.), Regulation of bacterial differentiation. American Society for Microbiology, Washington, DC.
- 19.Kunst, K., and G. Rapoport. 1995. Salt stress is an environmental signal affecting degradative enzyme synthesis in Bacillus subtilis. J. Bacteriol. 1772403-2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Msadek, T., K. Kunst, D. Henner, A. Klier, and G. Rapoport. 1990. Transduction pathway controlling synthesis of a class of degradative enzymes in Bacillus subtilis: expression of the regulatory genes and analysis of mutations in degS and degU. J. Bacteriol. 172824-834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Msadek, T., F. Kunst, and G. Rapoport. 1995. A signal transduction network in Bacillus subtilis includes the DegS/DegU and ComP/ComA two-component system, p. 447-471. In J. A. Hock and T. J. Silhavy (ed.), Two-component signal transduction. ASM Press, Washington, DC.
- 22.Mukai, K., M. Kawata, and T. Tanaka. 1990. Isolation and phosphorylation of the Bacillus subtilis degS and degU gene products. J. Biol. Chem. 26520000-20006. [PubMed] [Google Scholar]
- 23.Nakano, M. M., F. Yang, P. Hardin, and P. Zuber. 1995. Nitrogen regulation of nasA and the nasB operon, which encode genes required for nitrate assimilation in Bacillus subtilis. J. Bacteriol. 177573-579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ogura, M., A. Matsuzawa, H. Yoshikawa, and T. Tanaka. 2004. Bacillus subtilis SalA (YbaL) negatively regulates expression of scoC, encoding the repressor for the alkaline exoprotease gene, aprE. J. Bacteriol. 1863056-3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ogura, M., and T. Tanaka. 1996. Bacillus subtilis DegU acts as a positive regulator for comK expression. FEBS Lett. 397173-176. [DOI] [PubMed] [Google Scholar]
- 26.Ogura, M., and T. Tanaka. 1997. Bacillus subtilis ComK negatively regulates degR expression. Mol. Gen. Genet. 254157-165. [DOI] [PubMed] [Google Scholar]
- 27.Ogura, M., H. Yamaguchi, K.-I. Yoshida, Y. Fujita, and T. Tanaka. 2001. DNA microarray analysis of Bacillus subtilis DegU, ComA and PhoP regulons: an approach to comprehensive analysis of B. subtilis two-component regulatory systems. Nucleic Acids Res. 293804-3813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reitzer, L. 2003. Nitrogen assimilation and global regulation in Escherichia coli. Annu. Rev. Microbiol. 57155-176. [DOI] [PubMed] [Google Scholar]
- 29.Ruzal, S. M., and C. Sanchez-Rivas. 1998. In Bacillus subtilis DegU-P is a positive regulator of the osmotic response. Curr. Microbiol. 37368-372. [DOI] [PubMed] [Google Scholar]
- 30.Schaeffer, P. J., J. Millet, and J. Aubert. 1965. Catabolite repression of bacterial sporulation. Proc. Natl. Acad. Sci. USA 54704-711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schreier, H. J. 1993. Biosynthesis of glutamine and glutamate and the assimilation of ammonia, p. 281-298. In A. L. Sonenshein, J. A. Hoch, and R. Losick (ed.), Bacillus subtilis and other gram-positive bacteria: biochemistry, physiology, and molecular genetics. ASM Press, Washington, DC.
- 32.Schreier, H. J., S. W. Brown, K. D. Hirschi, J. F. Nomellini, and A. L. Sonenshein. 1985. Regulation of Bacillus subtilis glutamine synthetase gene expression by the product of the glnR gene. J. Mol. Biol. 21051-63. [DOI] [PubMed] [Google Scholar]
- 33.Schreier, H. J., S. H. Fisher, and A. L. Sonenshein. 1985. Regulation of expression from the glnA promoter of Bacillus subtilis requires the glnA gene product. Proc. Natl. Acad. Sci. USA 823375-3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shimane, K., and M. Ogura. 2004. Mutational analysis of the helix-turn-helix region of Bacillus subtilis response regulator DegU, and identification of cis-acting sequences for DegU in the aprE and comK promoters. J. Biochem. 136387-397. [DOI] [PubMed] [Google Scholar]
- 35.Stanley, N. R., and B. A. Lazazzera. 2005. Defining the genetic differences between wild and domestic strains of Bacillus subtilis that affect poly-gamma-dl-glutamic acid production and biofilm formation. Mol. Microbiol. 571143-1158. [DOI] [PubMed] [Google Scholar]
- 36.Tanaka, T. 1982. Transfer of a Bacillus subtilis chromosomal mutation to a homologous DNA region on a plasmid which is carried in the mutant. Agric. Biol. Chem. 461101-1102. [Google Scholar]
- 37.Tanaka, T., H. Ishida, and T. Maehara. 2005. Characterization of the replication region of plasmid pLS32 from the Natto strain of Bacillus subtilis. J. Bacteriol. 1874315-4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tanaka, T., and M. Kawata. 1988. Cloning and characterization of Bacillus subtilis iep, which has positive and negative effects on production of extracellular proteases. J. Bacteriol. 1703593-3600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tanaka, T., M. Kawata, and K. Mukai. 1991. Altered phosphorylation of Bacillus subtilis DegU caused by single amino acid changes in DegS. J. Bacteriol. 1735507-5515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Verhamme, D. T., T. B. Kiley, and N. R. Stanley-Wall. 2007. DegU co-ordinates multicellular behaviour exhibited by Bacillus subtilis. Mol. Microbiol. 65554-568. [DOI] [PubMed] [Google Scholar]
- 41.Ward, J. B., Jr., and S. A. Zahler. 1973. Genetic studies of leucine biosynthesis in Bacillus subtilis. J. Bacteriol. 116719-726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wray, L. V., Jr., A. E. Ferson, K. Rohrer, and S. H. Fisher. 1996. TnrA, a transcription factor required for global nitrogen regulation in Bacillus subtilis. Proc. Natl. Acad. Sci. USA 938841-8845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wray, L. V., Jr., J. M. Zalieckas, A. E. Ferson, and S. H. Fisher. 1998. Mutational analysis of the TnrA-binding site in the Bacillus subtilis nrgAB and gabP promoter regions. J. Bacteriol. 1802943-2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wray, L. V., Jr., J. M. Zalieckas, and S. H. Fisher. 2001. Bacillus subtilis glutamine synthetase controls gene expression through a protein-protein interaction with transcription factor TnrA. Cell 107427-435. [DOI] [PubMed] [Google Scholar]
- 45.Yanisch-Perron, C., J. Vieira, and J. Messing. 1985. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 33103-109. [DOI] [PubMed] [Google Scholar]
- 46.Yoshida, K.-I., K. Kobayashi, Y. Miwa, C.-M. Kang, M. Matsunaga, H. Yamaguchi, S. Tojo, M. Yamamoto, R. Nishi, N. Ogasawara, T. Nakayama, and Y. Fujita. 2001. Combined transcriptome and proteome analysis as a powerful approach to study genes under glucose repression in Bacillus subtilis. Nucleic Acids Res. 29683-692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yoshida, K.-I., H. Yamaguchi, M. Kinehara, Y. H. Ohki, Y. Nakaura, and Y. Fujita. 2003. Identification of additional TnrA-regulated genes of Bacillus subtilis associated with a TnrA box. Mol. Microbiol. 49157-165. [DOI] [PubMed] [Google Scholar]