Abstract
Nociceptive neurons play an important role in ischemia by sensing and transmitting information to the CNS and by secreting peptides and nitric oxide, which can have local effects. While these responses are probably primarily mediated by acid sensing channels, other events occurring in ischemia may also influence neuron function. In this study, we have investigated the effects of anoxia and anoxic aglycemia on Ca2+ regulation in sensory neurons from rat dorsal root ganglia. Anoxia increased [Ca2+]i by evoking Ca2+ release from two distinct internal stores one sensitive to carbonyl cyanide p-(trifluoromethoxy) phenylhydrazone (FCCP) and one sensitive to caffeine, cyclopiazonic acid (CPA), and ryanodine [assumed to be the endoplasmic reticulum (ER)]. Anoxia also promoted progressive decline in ER Ca2+ content. Despite partially depolarizing mitochondria, anoxia had relatively little effect on mitochondrial Ca2+ uptake when neurons were depolarized but substantially delayed mitochondrial Ca2+ release and subsequent Ca2+ clearance from the cytosol on repolarization. Anoxia also reduced both sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA) activity and Ca2+ extrusion [probably via plasma membrane Ca2+-ATPase (PMCA)]. Thus anoxia has multiple effects on [Ca2+]i homeostasis in sensory neurons involving internal stores, mitochondrial buffering, and Ca2+ pumps. Under conditions of anoxic aglycemia, there was a biphasic and more profound elevation of [Ca2+]i, which was associated with complete ER Ca2+ store emptying and progressive, and eventually complete, inhibition of Ca2+ clearance by PMCA and SERCA. These data clearly show that loss of oxygen, and exhaustion of glycolytic substrates, can profoundly affect many aspects of cell Ca2+ regulation, and this may play an important role in modulating neuronal responses to ischemia.
INTRODUCTION
Nociceptive neurons play an important role in mediating responses to tissue ischemia (Armour 1999; Longhurst et al. 2001). Not only do they sense and transmit information that can elicit central responses but they also secrete substance P (SP), calcitonin gene-related peptide (CGRP), and nitric oxide, which have local effects. The importance of these responses in ischemia is clearly illustrated in the heart where sensory neurons are reported to be involved in: the transmission of myocardial pain (Armour et al. 1994; Benson et al. 1999; Brown 1967; Huang et al. 1996; White 1957), evoking cardio-cardiac and sympathetic autonomic reflexes (Longhurst et al. 2001; Malliani 1990), mediating coronary vasodilatation through the release of NO, CGRP and SP (Ledda et al. 1993; Owman 1990; Yamamoto et al. 2003), CGRP-evoked chronotropic and ionotropic responses (Ledda et al. 1993), and CGRP-mediated preconditioning (Li and Peng 2002).
Both excitatory and secretory responses to ischemia are thought to be mediated primarily by the activation of acid sensing channels in the cell membrane that causes membrane depolarization and calcium entry (Benson et al. 1999; Bevan and Geppetti 1994; Bevan and Yeats 1991; Caterina et al. 1997; Davies et al. 1988; Immke and McCleskey 2001a,b; Konnerth et al. 1987; Krishtal and Pidoplichko 1980, 1981; Longhurst et al. 2001; Liu et al. 2004; Pan et al. 1999; Waldmann and Lazdunski 1998). Acidosis, however, is not the only consequence of ischemia. In animal models of myocardial ischemia, oxygen levels at the ischemic focus fall rapidly to zero with a surrounding hypoxic border zone (Rumsey et al. 1994; Walfridsson and Lewis 1987). Indeed it is partly the lack of oxygen that causes tissue acidosis by necessitating anaerobic respiration. In addition to anoxia/hypoxia, ischemia also leads to loss of other metabolic substrates, ionic redistribution between intracellular and extracellular compartments, and the release of various substances from other cellular elements (see e.g., Opie 1991). Many of these factors are likely to influence neuronal function. In particular the consequences of anoxia and hypoxia are potentially far reaching because there are widespread reports of ion channels being sensitive to oxygen (Lopez Barneo 1994; Patel and Honoré 2001; Peers 1997; Weir et al. 2005; Yuan 2001), the mitochondrion plays a major role in cell calcium homeostasis (Nicholls 2005), and ATP depletion can influence the activity of many ion channels and transporters (Hilgemann 1997). Indeed in the CNS, ischemia has profound effects on neuronal ion homeostasis, electrical signaling, and Ca2+ signaling (Budd and Nicholls 1996a; Erecinska and Silver 1994; Hansen 1985; Sims 1995; Yao and Haddad 2004). There have been relatively few studies on the effects of hypoxia, anoxia, or aglycemia on sensory neuron function, but inhibitors of oxidative phosphorylation are reported to alter cell Ca2+ homeostasis (Duchen et al. 1990), and a few recent studies suggest that ion channels may also be regulated by hypoxia in these neurons (Gruss et al. 2006; Lukyanetz et al. 2003).
In this study, we have investigated the effects of anoxia and of anoxic aglycemia on Ca2+ regulation in small capsaicin-sensitive neurons isolated from rat cervico-thoracic dorsal root ganglia (DRG). Our results show that anoxia has multiple effects on cellular [Ca2+]i homeostasis involving store release, mitochondrial buffering, and inhibition of Ca2+ pumps but no obvious activation of Ca2+ influx pathways. Despite these events, Ca2+ signaling was not grossly abnormal in anoxia alone. In contrast, prolonged anoxic aglycemia lead to a substantive rise in [Ca2+]i and total loss of Ca2+ store and Ca2+ pump function. These data indicate that altered calcium signaling in response to anoxia may well play an important role in modulating sensory neuron responses to ischemia.
METHODS
Neuron dissociation
Adult Wistar rats of either sex aged between 6 and 8 wk (130–170 g) were killed by an overdose of halothane (4%) followed by exsanguination in accordance with schedule 1 of the United Kingdom Animals (Scientific Procedures) Act 1986. Cervico-thoracic DRGs (C4-Th6) were removed under sterile conditions and were immediately transferred into cooled Ca2+- and Mg2+-free phosphate-buffered saline (PBS), pH 7.4. After cleaning, the ganglia were incubated in a medium comprising 10 mg collagenase type I (208 U/mg, Worthington, CLS-1, MON4393), 1 mg trypsin (9.3 U/mg, Sigma, T-4665) in PBS and with 60 μM CaCl2 and 33 μM MgCl2 for 35 min at 37°C. Following enzyme treatment, ganglia were washed once in PBS (Ca2+- and Mg2+-free) and once in DMEM (containing 10% FBS, 1.2 mM l-glutamine) before mechanical trituration in 1.5 ml of DMEM. The dissociated cells were then washed twice by centrifugation (at 1,000 g for 5 min) followed by resuspension in fresh DMEM. Following the final wash, the cell pellet was resuspended in 500 μl Basal TNB-100 culture medium containing protein-lipid-complex (Biochrom, Berlin, Germany), penicillin (100 IU/ml), streptomycin (100 μg/ml), and 10 μM/ml NGF. Following a second trituration, the neurons were seeded onto poly-l-lysine and laminin-coated coverslips and incubated in sterile culture dishes in a humidified chamber at 37°C and 5% CO2-95% air for 2 h. After this incubation period, a further 3 ml TNB was added to each culture dish. Neurons were kept in the incubator for 30 min to 24 h before use.
Fluorescence measurements
Fluorescence measurements were performed using a microspectrofluorimeter based on a Nikon Diaphot 200 equipped with a monochromator (Cairn Instruments, Kent, UK) and a xenon lamp to provide an excitation light source and cooled (−20°C) photomultiplier tubes (PMT; Thorn EMI) to detect emitted fluorescence. Fura-2 was excited alternately at 340 and 380 nm (±8 nm) for 250 ms at each wavelength with the cycle repeated at 1 Hz. Fura-2 fluorescence was filtered at 510 ± 20 nm. Rhodamine 123 was excited continuously at 495 nm, and its emitted fluorescence filtered at 525 ± 10 nm. The output from the PMT's was integrated over each illumination period (Fura-2) or just averaged over 500 ms. (Rh123) and recorded on a microcomputer using a micro 1401 and Spike 4 software (Cambridge Electronic Design). For Fura-2, the fluorescence ratio (340 nm/380 nm) was also calculated and recorded using Spike 4 software.
Selection and superfusion of neurons
Neurons were placed in a recording chamber with a volume of ∼100 μl. This chamber was perfused at ∼2 ml/min. Solutions were delivered from reservoirs kept in a water bath to the recording chamber via medical grade stainless steel tubing articulated by short sections of pharmed tubing (Norton Performance Plastics). A mechanically driven two-way tap was placed within a few inches of the recording chamber. A heating coil was placed around a short section of tubing between the tap and the chamber to ensure solutions remained at 37°C. This arrangement allowed rapid solution exchange and tight control over solution gas content and temperature.
Sensory neurons were selected initially on the basis of soma size (15–30 μm), and in experiments using Fura-2, their response to capsaicin (10–100 nM for 10 s) was tested at the end of each experiment. In these studies, >80% of neurons selected on these size criteria proved to be capsaicin positive (i.e., capsaicin evoked a robust increase in [Ca2+]i). Only these neurons were included in our studies on [Ca2+]i regulation.
Fura-2 in vitro calibration
The effects of Mg2+ on Fura-2 fluorescence were investigated in vitro. Fura-2 (Molecular Probes, Leiden, The Netherlands) was dissolved at a final concentration of 5 μM (prepared from a stock solution in DMSO), in a calibration buffer containing (in mM): 150 KCl, 5 NaCl, 20 HEPES, and 1 EGTA. To this, varying amounts of CaCl2 and MgCl2 were added to achieve a final free-Ca2+ concentration of 100 nM and free-Mg2+ concentrations of (in mM) 0, 0.3, 1, 3, 10, 30 (as calculated using WINMAXC version 2.05). Calibration solution pH was 7.2 at room temperature (21–23°C). Twenty microliters of each calibration solution was placed in a small petri dish, and the Fura-2 fluorescence ratio determined using the preceding microspectrofluorimeter.
Loading and calibration of Fura-2
Neurons were loaded with Fura-2 by incubating them in either a HEPES-buffered saline (for in vivo calibrations) or a bicarbonate-buffered saline (for experiments) containing 5 μM Fura-2-AM (Molecular Probes) at room temperature for 25 min in a dark chamber. The HEPES-buffered saline used comprised (in mM): 20 HEPES, 11 glucose, 4.5 KCl, 1 MgCl2, 2.5 CaCl2, and 117 NaCl, pH 7.4 at room temperature.
In vivo calibrations were performed by incubating Fura-2-loaded neurons in a 0-Ca2+ calibration medium containing (in mM) 150 KCl, 5 NaCl, 1 EDTA, and 1 EGTA and 10 μM ionomycin (Sigma, Dorset, UK), for 10–20 min at room temperature. After this preincubation the neurons were placed in the perfusion chamber of the microspectrofluorometer and perfused with zero-Ca2+ medium (+1 μM ionomycin) at 37°C. After a 5-min perfusion, Fura-2 fluorescence was recorded in five identified sensory neurons. The ratio of fluorescence obtained under these conditions was deemed equivalent to the calibration constant Rmin (Grynkiewicz et al. 1985). The perfusate was then changed to a high-Ca2+ calibration medium containing (in mM) 150 KCl, 5 NaCl, and 10 CaCl2 and 1 μM ionomycin. The change in fluorescence ratio was followed in one of the five identified neurons until it reached a new stable value and then the fluorescence ratio in it, and in the other four identified neurons, was recorded and deemed to be equivalent to the calibration constant Rmax. The ratio of fluorescence at 380 nm in 0-Ca2+ medium divided by that obtained in high-Ca2+ medium (Sf2/Sb2) was also calculated for each neuron. The mean values obtained for Rmin, Rmax, and (Sf2/Sb2) were then used to calibrate measurements of the fluorescence ratio in subsequent experiments using the equation [Ca2+] = (R − Rmin)/(Rmax − R) × Sf2/Sb2 × Kd (Grynkiewicz et al. 1985).
Measurement of mitochondrial membrane potential with Rhodamine 123
Changes in mitochondrial membrane potential were detected using Rhodamine 123 (Rh123). This is a membrane permeant cation that is strongly sequestered in mitochondria due to their negative membrane potential. In concentrated solutions, as occur within the mitochondrion, Rh123 fluorescence is quenched. If the mitochondria become depolarized, Rh123 is redistributed from the mitochondrion to the cytosol where it becomes diluted and as a consequence fluorescence increases. Measurements of total Rh123 fluorescence from an intact cell can therefore be used to follow changes in mitochondrial membrane potential (Ψm) (Toescu and Verkhratsky 2000). Neurons were loaded with Rh123 (5 μM, Sigma, Dorset, UK) in bicarbonate-buffered medium at room temperature for 12 min. The cells were then transferred to the perfusion chamber described in the preceding text. Because Rh123 is relatively poorly retained by cells, a correction for both baseline drift and decline in maximum signal amplitude was carried out. This consisted of taking measurements of baseline fluorescence under control conditions and peak signal amplitude measured during brief application of 1 μM FCCP (an uncoupler which fully depolarizes the mitochondria) at the beginning and end of each recording. Each recording was limited to a maximum of 20-min duration. The time-dependent decline in baseline fluorescence was modeled as a linear process and was subtracted from the recording. The decline in maximum signal amplitude (measured in presence of FCCP) was also modeled as a linear process. The baseline subtracted signal was then divided by the time-dependent maximum signal and multiplied by 100 to convert the recorded signal to a percentage of the maximum signal attainable by full mitochondrial depolarization (%ΔΨm).
Solutions
Standard bicarbonate-buffered tyrode solutions contained (in mM) 117 NaCl, 4.5 KCl, 2.5 CaCl2, 1 MgCl2, 23 HCO3−, 11 glucose. Glucose-free Tyrode solution was prepared by replacing glucose with 11 mM sucrose. In Ca2+-free solutions, CaCl2 was omitted and 1 mM EGTA added. High-K+ Tyrode contained 50 mM KCl and 71.5 mM NaCl; all other constituents remained the same. Equilibration of these solutions with 5% CO2-95% air, achieved normoxic conditions with pH 7.4 at 37°C. Hypoxic solutions were generated by equilibration with 5% CO2-95% N2 (PO2 = 2 torr). Anoxic solutions were obtained by the further addition of 0.5 mM Na2S2O4 (Sato et al. 1991) following 15–30 min prior equilibration with 5% CO2-95% N2 (pH: 7.39 ± 0.2, n = 23). All solutions were equilibrated with appropriate gas mixes at 37°C in a water bath for ≥30 min before use.
Drugs
Ryanodine was from Tocris (Avonmouth, UK). All other chemicals were from either Sigma (Poole, UK) or VWR/BDH (Nottingham, UK). Cyanide containing solutions were prepared by adding solid NaCN to pre equilibrated Tyrode solution immediately before use. CN− solutions were not used for >30 min (CN− in buffered solutions is volatile and can be very rapidly lost from solution; see Wyatt and Buckler 2004). Cyclopiazonic acid (CPA) and thapsigargin containing solutions were prepared from stock solutions in DMSO. Capsaicin and FCCP were added from stock solutions in ethanol. The maximum concentration of solvent in Tyrodes were 50 μM DMSO, 10 μM ethanol.
Statistics
Values are expressed as means ± SE. Statistical significance was tested using the paired Student's t-test, or Wilcoxon ranks signed test for experiments with non-Gaussian distribution. Statistical testing of in vitro calibration data were performed using one-way ANOVA, and post hoc analyses were carried out using Bonferroni′s multiple comparison, calculated by SPSS 12.0 software for windows. Level of significance was set at P < 0.05.
RESULTS
Effects of Mg2+ on Fura-2
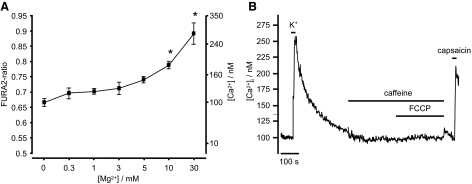
Exposure of sensory neurons to anoxia, aglycemia or metabolic poisons can increase [Mg2+]i from a resting level of 1.39 ± 0.1 mM to ≤3.48 ± 0.35 mM (due to MgATP hydrolysis) (Henrich and Buckler 2007). To confirm that changes in intracellular magnesium of this magnitude would not interfere with the measurement of [Ca2+]i using Fura-2, we conducted an analysis of the effects of [Mg2+] on Fura-2 fluorescence in vitro. In calibration solutions containing 100 nM Ca2+, Fura-2 fluorescence was not significantly enhanced by Mg2+ in the range between 0.3 and 5 mM, but above this concentration, a significant increase in fluorescence ratio was observed (1-way ANOVA, Post Hoc, Bonferroni test, see Fig. 1). Thus at intracellular calcium levels similar to those found in these cells under resting conditions changes in [Mg2+]i should not significantly interfere with the measurement of [Ca2+]i using Fura-2. This was further confirmed by testing the effects of the uncoupler FCCP (which also increases cytosolic [Mg2+]i) on Fura-2 fluorescence under conditions in which cytosolic Ca2+ was heavily buffered using bis-(o-aminophenoxy)-N,N,N',N'-tetraacetic acid (BAPTA). Under these conditions, FCCP had negligible effects on measured [Ca2+]i (Δ[Ca2+]i = 1.2 ± 0.2 nM, n = 4, Fig. 1B).
FIG. 1.
A: effects of increasing Mg2+ ion concentration on Fura-2 fluorescence ratio in vitro. Ordinate shows both fluorescence ratio and equivalent Ca2+ ion concentration (calculated using standard calibration equation see methods). Data (means ± SE, n = 3) were obtained from a calibration buffer containing 100 nM free Ca2+ and varying levels of Mg2+. Note that increasing [Mg2+] to ≥10 mM significantly increases the Fura-2 fluorescence ratio (and thus estimated [Ca2+]), but at concentrations below this, Mg2+ has no significant effect (*P < 0.05; one-way ANOVA followed by post hoc Bonferroni test). B: effects of elevation of cytosolic [Mg2+] on [Ca2+]i estimation in an isolated neuron co-loaded with Fura-2-AM and the calcium chelator bis-(o-aminophenoxy)-N,N,N',N'-tetraacetic acid-AM (BAPTA-AM). BAPTA greatly attenuated the effects of depolarization (50 mM KCl; 10 s)-induced Ca2+ influx and abolished the effects of caffeine (30 mM) on [Ca2+]i, confirming that BAPTA loading was extremely effective in buffering changes in [Ca2+]i (compare with Figs. 2, 3, and 8–11). Under these conditions, application of FCCP (1 μM) which causes an abrupt increase in cytosolic [Mg2+] of around 0.4 mM (unpublished data) caused only a tiny apparent rise in [Ca2+]i of 7 ± 2 nM (n = 4). The response to capsaicin (100 nM for 10 s.) confirms the identity of this cell as a sensory neuron.
Effects of anoxia on resting [Ca2+]i in DRG neurons
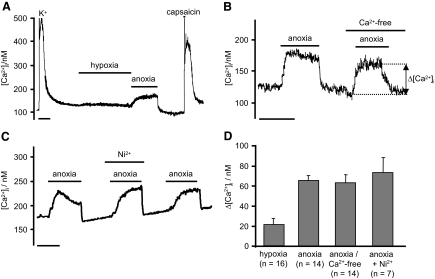
Capsaicin-sensitive neurons had a resting [Ca2+]i of 163 ± 5 nM (n = 79). Hypoxia (pO2: 2 Torr) had no significant effect on [Ca2+]i (n = 16), but anoxia caused an abrupt rise in [Ca2+]i of 66 ± 5 nM to a stable level of 239 ± 9 nM, P < 0.001, n = 49 (see e.g., Fig. 2). This rise in [Ca2+]i was usually complete within 1 min. When anoxia was applied for prolonged periods, the rise in [Ca2+]i measured after 18 min (56 ± 5 nM) was not different to that observed after the first 3 min (60 ± 6 nM, n = 6, P = 0.595), indicating that following an initial rapid rise [Ca2+]i remains relatively stable under anoxic conditions. [Ca2+]i recovered immediately back to baseline on reoxygenation (see Fig. 2). This [Ca2+]i response to anoxia was repeatable (Fig. 2).
FIG. 2.
A: effects of hypoxia and anoxia on [Ca2+]i in a sensory neurons. B: effects of Ca2+-free medium on the [Ca2+]i response to anoxia. The anoxia induced rise in [Ca2+]i was measured as the difference between baseline [Ca2+]i measured before exposure to anoxia and the [Ca2+]i attained during exposure to anoxia. C: effects of Ni2+ (2.5 mM) on the anoxic evoked rise in [Ca2+]i. D: summary of effects of anoxia on Δ[Ca2+]i, defined as the difference between baseline [Ca2+]i and that attained at the end of the anoxic exposure (e.g., as in B) under control conditions, in Ca2+-free medium, and in the presence of 1 mM Ni2+. Time scale bars are 200 s.
The anoxia-induced rise in [Ca2+]i was not reduced in Ca2+-free solution (Δ[Ca2+]i = 64 ± 4 nM, n = 28, Fig. 2C) or in the presence of 1 mM Ni2+ (Δ[Ca2+]i = 90 ± 23 nM +1 mM Ni2+ vs. 103 ± 21 nM, control, n = 6, P = 0.463), indicating that this rise in [Ca2+]i was not mediated by enhanced Ca2+ influx.
Intracellular Calcium stores contribute to the anoxic-evoked rise in [Ca2+]i
Having excluded Ca2+ influx as a potential cause of the anoxia induced rise in [Ca2+]i, we sought to determine the role of intracellular Ca2+ stores. The [Ca2+]i response to anoxia was therefore measured under control conditions and following depletion of specific Ca2+ stores. All of these following experiments were conducted in a Ca2+-free tyrode solution.
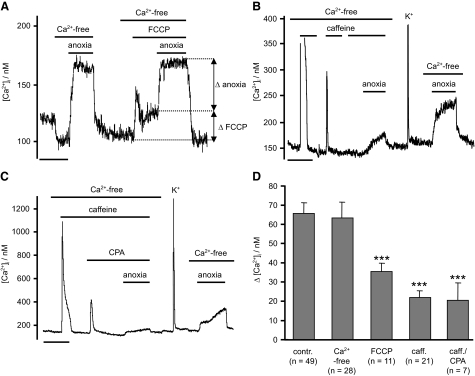
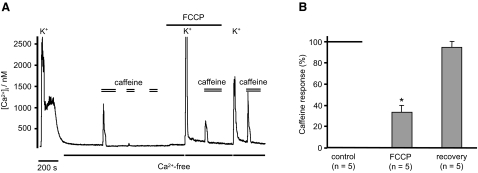
We investigated a role for mitochondria by using the uncoupler FCCP to depolarize the mitochondria and thus release any Ca2+ stored within (Shishkin et al. 2002; Thayer and Miller 1990). In experiments using Rh123, 1 μM FCCP appeared to produce a maximal mitochondrial depolarization (n = 3 data not shown). Application of 1 μM FCCP alone caused a significant rise in [Ca2+]i from 174 ± 13 to 214 ± 18 nM (n = 11, P < 0.001). Subsequent exposure to anoxia, in the continued presence of FCCP, caused a further rise in [Ca2+]i of 36 ± 4.5 nM (n = 11, P < 0.01, Fig. 3, A and D). This anoxia-induced increase in [Ca2+]i was significantly less than that seen in the absence of FCCP (P < 0.001, Fig. 3). The combination of FCCP and anoxia, however, increased [Ca2+]i to a final level similar to that observed in anoxia alone ([Ca2+]i = 243 ± 19 nM, anoxia + FCCP vs. 238 ± 13 nM, anoxia alone, n = 11, P = 0.949). Therefore prior depletion of mitochondrial stores with FCCP diminishes the response to anoxia by an amount equivalent to the rise in [Ca2+]i caused by FCCP alone. Consequently part of the Ca2+ response to anoxia seems to be dependent on some aspect of mitochondrial function, but there is also another component.
FIG. 3.
Intracellular Ca2+ stores contribute to anoxic rise in [Ca2+]i. A: effects of mitochondrial calcium depletion using FCCP (1 μM), on anoxia-induced rise in [Ca2+]i. FCCP alone induces first a transitory and then a sustained rise in [Ca2+]i (ΔFCCP). Following FCCP treatment, anoxia induced a further rise in [Ca2+]i to approximately the same level as under control conditions. Note, however, that the incremental rise in [Ca2+]i in response to anoxia (Δ anoxia) is reduced in the presence of FCCP (n = 11, ***P < 0.001). B and C: effects of ER calcium store depletion and reloading on the [Ca2+]i response to anoxia. ER Ca2+ stores were depleted either by repeated exposure to caffeine (30 mM), or caffeine and CPA (10 μM), in a Ca2+-free medium. Following removal of caffeine and CPA, stores were refilled by brief (∼5 s) exposure to 50 mM extracellular K+ in a normal Ca2+-containing medium before obtaining a control response to anoxia. Note that the [Ca2+]i response to anoxia is diminished following store depletion compared with that following store reloading. D: summary of anoxia-induced rise in [Ca2+]i measured in normal Ca2+-containing medium, in Ca2+-free medium, following mitochondrial Ca2+ store depletion (FCCP), and following ER Ca2+ store depletion (caffeine or caffeine and CPA). Note that both ER and mitochondrial store depletion significantly (***P < 0.001, Mann-Whitney U test) diminish the anoxia-induced rise in [Ca2+]i but neither fully inhibit it. Time scale bars in A–C are 200 s.
DRG sensory neurons contain caffeine-sensitive stores (Usachev et al. 1993). Depletion of these stores with 30 mM caffeine did not abolish the [Ca2+]i response to anoxia but did significantly reduce it compared with that seen when stores were not depleted (Δ[Ca2+]i = 22 ± 3.5 nM, n = 21 post caffeine, P < 0.001, see Fig. 3D). Similar results were also obtained when both CPA, an inhibitor of the endoplasmic reticulum Ca2+ pump (SERCA), and caffeine were used together to deplete Ca2+ stores; i.e., the rise in [Ca2+]i in response to anoxia was reduced (to 21 ± 3.4 nM, n = 7, P < 0.001), but was not abolished (Fig. 3).
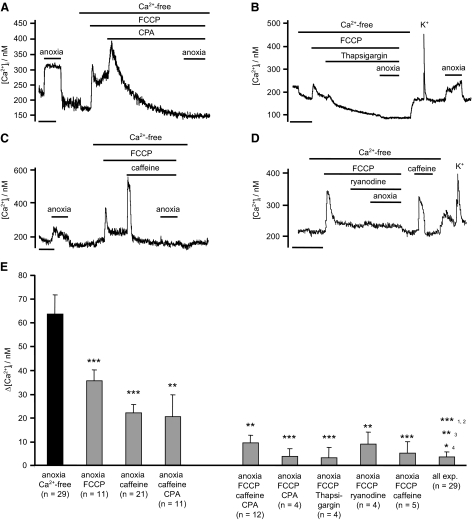
The preceding data suggested that part of the response to anoxia could be mediated by Ca2+ release from caffeine-sensitive internal stores. To determine whether any other internal stores could be involved, we depleted both caffeine/CPA-sensitive stores and mitochondrial stores. In this series of experiments, FCCP and caffeine (and/or CPA) were applied sequentially but in a random order. Irrespective of the order of addition the combination of FCCP with caffeine and or CPA completely prevented any anoxia induced rise in [Ca2+]i, (Δ[Ca2+]i = 9 ± 3.4 nM, n = 12, NS; see Fig. 4). The anoxia-induced rise in calcium could also be inhibited by a combination of FCCP plus thapsigargin (100 nM), an irreversible inhibitor of SERCA (n = 4, Fig. 4B), and by FCCP in combination with 25 μM ryanodine (n = 4, Fig. 4D). Note that ryanodine did not deplete ER stores of Ca2+ (as evidenced by the observation that after removal of ryanodine, caffeine evoked a large rise in [Ca2+]i, Fig. 4D) but none the less reduced Ca2+ release in response to anoxia.
FIG. 4.
Effects of Ca2+ store depletion on anoxia induced [Ca2+]i rise. A–C: neurons were superfused with a Ca2+-free buffer and then mitochondrial Ca2+ stores were depleted by application of FCCP (1 μM), followed by the depletion of ER Ca2+ stores using; CPA (10 μM, A); thapsigargin (100 nM, B); or caffeine (30 mM, C). D: effects of FCCP and ryanodine (25 μM) on [Ca2+]i response to anoxia. E: summary of effects of combining mitochondrial store depletion with either ER store depletion or inhibition of ryanodine receptors on the [Ca2+]i response to anoxia. Note that while depletion of mitochondrial stores or ER stores alone inhibits the Ca2+ response to anoxia by 40–60%, the combination of mitochondrial store depletion and ER store depletion (or RyR inhibition) blocks >80% of the response to anoxia. Data are mean + SE with number of experiments in parenthesis. Significance was assessed by reference to control data in the same neuron for each individual experiment using Student's paired t-test (*P < 0.05, **P < 0.01, ***P < 0.001). Pooled data (all experiments) for FCCP + caffeine, FCCP + CPA, FCCP + caffeine + CPA, FCCP + thapsigargin and FCCP + ryanodine, was compared against control (1), FCCP alone (2), caffeine alone (3), and caffeine + CPA (4), using Student's unpaired t-test. Time scale bars in A–D: 200 s.
Mitochondrial function and Ca release
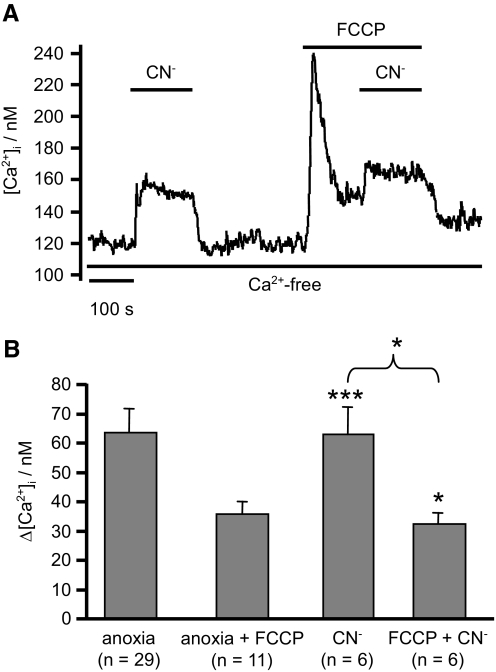
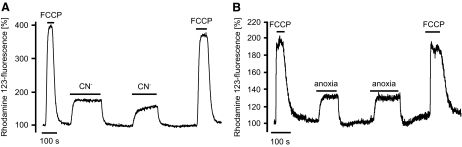
The observation that part of the [Ca2+]i response to anoxia was abolished by application of FCCP led us to further investigate the role of mitochondria in mediating Ca2+ release. Application of the electron transport inhibitor cyanide (2 mM) in a Cao2+-free medium also led to an abrupt increase in [Ca2+]i (63 ± 9 nM, n = 6, P < 0.001) similar to that observed for anoxia (Fig. 5A). The effect of cyanide was partially inhibited (reduced to 32 ± 4 nM, n = 6, P < 0.05) by prior mitochondrial depolarization with FCCP (Fig. 5B). These data suggest that both anoxia and cyanide might induce some Ca2+ release through mitochondrial depolarization. The effects of anoxia and cyanide on mitochondrial membrane potential (ψm) were therefore assessed using Rh123 (see methods). FCCP (1 μM) was used as a reference to evoke full mitochondrial depolarization and thus a maximal increase in Rh123 fluorescence (see methods). Anoxia evoked a rapid increase in Rh123 fluorescence but only to 25 ± 7% of the maximum seen with FCCP (Fig. 6 ; n = 6). CN− (2.5 mM) similarly increased Rh123 fluorescence by 24 ± 5% (n = 8) of maximum. These effects were rapidly reversible on reoxygenation or removal of CN−. Thus both anoxia and CN− induce rapid but only partial depolarization of ψm, which may be sufficient to evoke some mitochondrial Ca2+ release (but see discussion).
FIG. 5.
Effects of cyanide on [Ca2+]i. A and B: effects of 2 mM CN− on [Ca2+]i in a Ca2+-free medium both in the absence and presence of FCCP (1 μM). CN− produces an abrupt increase in [Ca2+]i similar to that seen with anoxia, indicating Ca2+ release from internal stores. Note that this response is only partially occluded by prior treatment with FCCP. Data on the effects of anoxia alone and anoxia in the presence of FCCP (taken from Fig. 3) are included for comparison. Values are means + SE with numbers of observations in parenthesis. Statistical significance was assessed using Student's t-test (*P < 0.05, **P < 0.01, ***P < 0.001).
FIG. 6.
The effects of anoxia and cyanide on mitochondrial membrane potential were assessed using the fluorescent compound rhodamine 123 (Rh123, see methods). Rh123 fluorescence intensity in A and B is expressed simply as a percentage of the initial baseline value. To quantify the effects of anoxia and cyanide on mitochondrial membrane potential, FCCP (1 μM) was applied to obtain reference values for Rh123 fluorescence corresponding to full mitochondrial depolarization (see methods). The application of either CN− (2.5 mM), A, or anoxia, B, evoked an increase in Rh123 fluorescence (mitochondrial depolarization) equivalent to ∼25% of the maximal FCCP-evoked response. Depolarization in response to both cyanide and anoxia was rapid in onset but reached a stable level within a minute that was sustained throughout the exposure period (∼200 s).
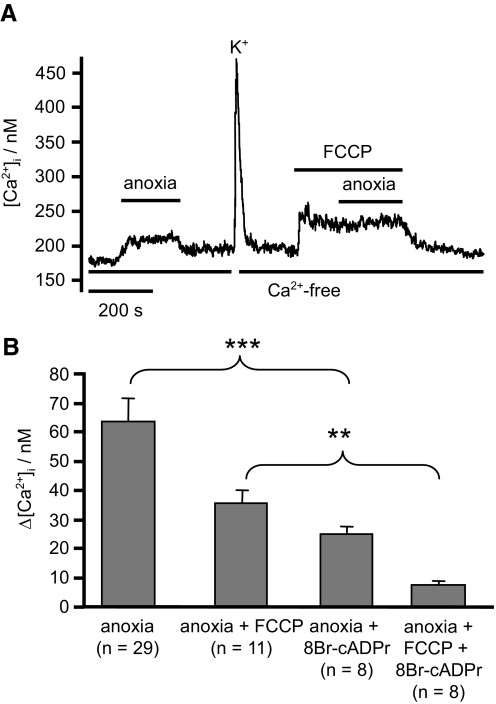
The preceding studies with cyanide also indicate that inhibition of electron transport can promote Ca2+ release via another pathway (i.e., independent of mitochondrial depolarization). In pulmonary vascular smooth muscle, it has been suggested that hypoxia induced increase in NADH might stimulate cyclic-ADP-ribose (cADPR) production (Dipp and Evans 2001; Evans and Dipp 2002) and thus evoke Ca2+ release from the sarcoplasmic/endoplasmic reticulum. Because a significant fraction of the [Ca2+]i response to anoxia in DRG neurons appears to be dependent on ER function (see preceding text and discussion) and because the effects of anoxia are mimicked by electron transport inhibition using cyanide, we sought to test for the possible involvement of cADPR in mediating the Ca2+ response to anoxia. Pretreatment of sensory neurons with the cADPR antagonist 8-bromo-cyclic-ADP-ribose (8-Br-cADPR, 100 μM) for 10 min significantly reduced [Ca2+]i responses to anoxia both in the absence and presence of FCCP (Fig. 7) when compared with [Ca2+]i responses in untreated neurons.
FIG. 7.
Effects of anoxia and 8-bromo-cyclic-ADP-ribose (8-BrcADPr) on [Ca2+]i. A and B: effects of pretreatment with 100 μM 8-Br-cADPr (for 10 min prior to experimentation) on [Ca2+]i response to anoxia and anoxia in the presence of FCCP (experiments conducted in Ca2+-free tyrode). Data on the effects of anoxia alone and anoxia in the presence of FCCP (taken from Fig. 3) are included for comparison. Values are means + SE with number of observations in parenthesis. Statistical significance was assessed using Student's t-test (*P < 0.05, **P < 0.01, ***P < 0.001).
Effects of anoxia on mitochondrial Ca2+ buffering
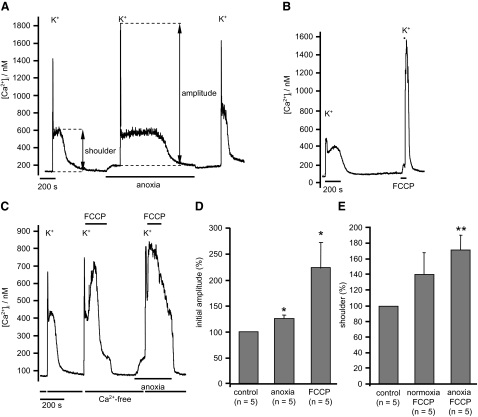
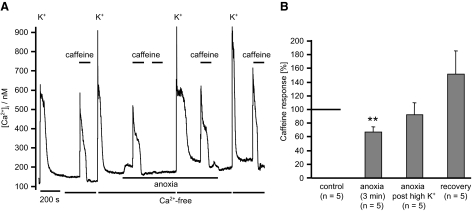
Given that anoxia depolarizes mitochondria, albeit only partially, we also investigated the effects of anoxia on mitochondrial Ca2+ buffering. Voltage-gated Ca2+ entry was triggered by a brief (5 s) application of a high-K+ tyrode (50 mM), containing normal levels of extracellular Ca2+ (2.5 mM). The peak [Ca2+]i attained under these conditions is determined by the rate of voltage-gated Ca2+ influx, Ca2+ efflux, Ca2+ buffering, and Ca2+ uptake by mitochondria. On subsequent removal of the high-K+ medium, there is initially a very rapid decline in [Ca2+]i. This is followed a few seconds later, and after [Ca2+]i has fallen to 357 ± 128 nM, by a secondary slow rise in [Ca2+]i to a plateau and then a much slower decline in [Ca2+]i (see e.g., Fig. 8, A and B). This secondary phase, or shoulder, in [Ca2+]i has been attributed to the release of Ca2+ from mitochondria that had previously been accumulated during exposure to high K+ when [Ca2+]i is very high (Werth and Thayer 1994). The role of mitochondrial Ca2+ buffering in these events is most readily demonstrated by depolarizing the mitochondria using an uncoupler and thus preventing Ca2+ uptake (Thayer and Miller 1990). Figure 8B confirms that brief application of FCCP prior to and during application of high-K+ tyrode caused a substantial increase in the [Ca2+]i response to depolarization (by 224 ± 50%, n = 5, P < 0.05) and the abolition of the mitochondrial Ca2+ release phase during recovery of [Ca2+]i. These observations are consistent with the mitochondrion normally buffering large amounts of Ca2+ during voltage-gated Ca2+ influx in these neurons.
FIG. 8.
Effects of anoxia on mitochondrial Ca2+ buffering. A: mitochondrial Ca2+ buffering was assessed by evoking voltage-gated Ca2+ entry with a 5-s pulse of a 50 mM potassium tyrode containing 2.5 mM [Ca2+]o. The initial Ca2+ response was quantified as the difference between resting [Ca2+]i immediately before, and the maximum [Ca2+]i attained during, high-K+ exposure. On high-K+ removal, there is an initial rapid decline in [Ca2+]i followed by a secondary slow rise and/or plateau in [Ca2+]i that was sustained for several minutes. The amplitude of this “shoulder” was also quantified relative to resting [Ca2+]i immediately before high-K+ exposure. B: effects of abolishing mitochondrial Ca2+ buffering with FCCP (1 μM). C: estimation of mitochondrial Ca2+ uptake during high-K+ exposure. Cells were first exposed to a high-K+ solution containing normal Ca2+ for 5 s and then returned to Ca2+-free medium, immediately following the initial decline in [Ca2+]i, i.e., at the onset of the shoulder, FCCP was rapidly applied. Mitochondrial Ca2+ uptake/FCCP-evoked Ca2+ release was then quantified as the difference between the maximal value of [Ca2+]i attained during FCCP exposure minus baseline [Ca2+]i immediately before application of the high-K+ solution. D: summary of effects of both anoxia and FCCP on initial [Ca2+]i transient amplitude in high-K+ medium. Data are normalized to the control transient amplitude. E: summary of the effects of anoxia on mitochondrial Ca2+ uptake during high-K+ exposure (experimental protocol as in C). All statistical tests were conducted using paired Student's t-test, *P < 0.05.
When we depolarized neurons under anoxic conditions, the initial Ca2+ transient was slightly enhanced compared with that obtained under normoxic conditions to 117 ± 4% (n = 10, P < 0.05, Fig. 8). The level of [Ca2+]i attained during the second slow phase (shoulder) when calculated as a rise in [Ca2+]i relative to baseline was reduced to 83 ± 5.5% of control (n = 10, P < 0.01), but when expressed as an absolute level was not significantly altered (89 ± 4% of control, P = 0.07). These observations were consistent with our expectations that anoxia might impair mitochondrial Ca2+ buffering but were notably rather minor. The most obvious effect of anoxia however was a near doubling of the duration of the mitochondrial Ca2+ release phase from 119 ± 22 s under control conditions to 235 ± 64 s under anoxic conditions (n = 8, P < 0.05, measured at half height, see Fig. 8). These data could indicate either a paradoxical increase in the Ca2+ load buffered by the mitochondria during anoxia or slowed Ca2+ clearance.
To semi-quantify the amount of Ca2+ taken up by mitochondria, we used uncouplers to induce rapid mitochondrial Ca2+ release. Cells were briefly exposed to a high-K+ solution (containing 2.5 mM Ca2+) and then returned to a normal Ca2+-free tyrode and [Ca2+]i allowed to recover until the onset of the second phase (shoulder) at which point 1 μM FCCP was applied to rapidly dump any Ca2+ stored in the mitochondrion back into the cytosol (see Fig. 8C). This protocol results in a substantial rise in [Ca2+]i on FCCP application that was quantified, relative to baseline [Ca2+]i, as an index of the amount of Ca2+ stored in the mitochondrion. Anoxia had no significant effect on this measure of mitochondrial Ca2+ load (n = 5, P = 0.293, see Fig. 8E). We could not therefore find any corroborating evidence that anoxia increased the amount of Ca2+ stored within the mitochondria.
Effects of anoxia on ER Ca2+ stores
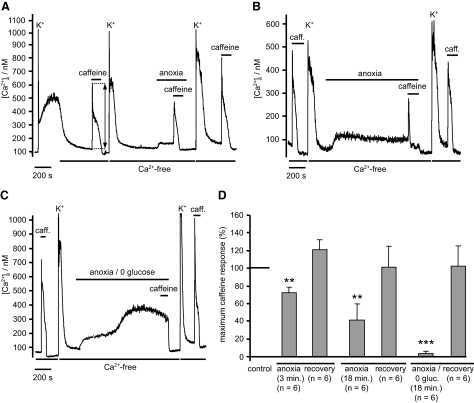
In view of the fact that anoxia appears to induce Ca2+ release from the ER, we sought to determine the extent to which anoxia might deplete those stores of Ca2+. To assess store content, we applied 30 mM caffeine and measured the resultant rise in [Ca2+]i, which is caused by caffeine-induced Ca2+ release. These experiments were carried out in Ca2+-free tyrode to exclude any contribution from capacitive Ca2+ entry. Store filling before, and between, caffeine exposures was facilitated by brief (5 s) application of a high-K+ tyrode containing normal levels of [Ca2+]o. Following 3 min of anoxia, the maximum amplitude of the caffeine response was significantly reduced to 78 ± 5% (n = 6, P < 0.01) of control. Following re-oxygenation the response to caffeine recovered to 120 ± 11% (n = 6, P = 0.128) compared with control (Fig. 9, A and D) . Protracted exposure to anoxia (18 min) resulted in a further reduction in the Ca2+ response to caffeine to 41 ± 11% (n = 6, P < 0.01) of control (exposure of neurons to Ca2+-free medium under normoxic conditions for 20 min only reduced the response to caffeine to 82 ± 6%, n = 6). These data indicate that anoxia causes a slow depletion of ER Ca2+ store content. This effect of anoxia was reversible, i.e., even after extended exposure to anoxia on re-oxygenation and re-loading in high-K+ tyrode, the caffeine releasable Ca2+ store recovered fully (to 93 ± 17% of control, n = 6, P = 0.693, Fig. 9, B and D). Indeed even during prolonged anoxia, caffeine-sensitive stores could be almost completely refilled (to 92 ± 18% of control, n = 5, NS) by high-K+-induced voltage-gated Ca2+ entry (Fig. 10). Thus although anoxia evokes Ca2+ release or leak from ER stores, it does not cause major disruption of store function.
FIG. 9.
Anoxia and anoxic aglycemia deplete ER Ca2+ stores. ER Ca2+ store content was estimated by measuring the amplitude of the [Ca2+]i response to caffeine application (30 mM for 2 min) in a Ca2+-free medium. When required, ER Ca2+ store loading was facilitated by brief exposure to a high-K+ (50 mM) solution containing normal extracellular [Ca2+]. A: effects of brief (3 min) anoxia on ER Ca2+ content. B: effects of prolonged anoxia (18 min) on ER Ca2+ content. Note peak response to caffeine was significantly reduced under anoxic conditions compared with that observed before or after anoxia. C: effects of prolonged (18 min) anoxia and aglycemia on [Ca2+]i and ER Ca2+ content. Note that anoxic aglycemia causes a biphasic rise in [Ca2+]i; following the second phase rise in [Ca2+]i application of caffeine failed to elicit any further rise in [Ca2+]i. The response to caffeine recovered on reintroduction of both oxygen and glucose. D: summary of effects of anoxia and anoxic aglycemia on the response to caffeine expressed as a percentage of the preintervention control. Data are means + SE with number of observations in parenthesis. Statistical significance was assessed using Student's paired t-test (**P < 0.01, ***P < 0.0001). caff, caffeine.
FIG. 10.
Refilling of the ER Ca2+ store during anoxia. A: the amplitude of the [Ca2+]i response to caffeine application (30 mM for 2 min) in a Ca2+-free medium was used as an assay of the extent of ER Ca2+ store filling. ER store loading was facilitated by brief exposure to a high-K+ solution containing normal extracellular [Ca2+]. A control caffeine response was first determined under normoxic conditions. Stores were then refilled in high K+, and the neuron transferred to an anoxic solution. Ca2+ release was then evoked a 2nd time by application of caffeine in a Ca2+-free medium. A 3rd pulse of caffeine confirmed that the stores were effectively empty. The neuron was then exposed to high-K+ solution (containing Ca2+) while still in anoxia followed by a fourth exposure to caffeine. Note that this 4th exposure to caffeine evoked a robust [Ca2+]i response, similar to 1st control response demonstrating that the ER Ca2+ store can be effectively refilled under anoxic conditions. Finally the cell was returned to normoxia and stores reloaded and then discharged again by sequential application of high K+ followed by caffeine. B: summary of effects of anoxia on caffeine response after 3 min of anoxia, following ER store refilling under anoxic conditions and following store refilling post anoxia. Histograms depict the normalized mean values as [%] + SE (**P < 0.01, Student's paired t-test). Numbers of experiments are given in parenthesis.
Mitochondrial buffering assists the refilling of caffeine-sensitive Ca2+ stores
We briefly investigated whether mitochondrial Ca2+ buffering could influence ER store loading by looking at the effects of FCCP on store loading and caffeine-evoked Ca2+ release. ER stores were depleted by repetitive caffeine applications (Fig. 11) and then FCCP (1 μM) was applied and voltage-gated Ca2+ entry triggered by a high-K+ pulse (50 mM, 5 s). The following response to caffeine (30 mM, still in the presence of FCCP), was substantively reduced to 33 ± 6% (n = 5, P < 0.05) of control. Following subsequent removal of FCCP, a rest period of 3 min, a further high-K+ pulse (50 mM, 5 s), the caffeine response recovered to 95 ± 6% (n = 5, Fig. 11) of control. Because store refilling was near normal under anoxic conditions (see preceding text), these data suggest a specific role for mitochondrial Ca2+ buffering in facilitating store loading.
FIG. 11.
Refilling of caffeine-sensitive Ca2+ stores depends partly on mitochondrial function. A: an initial high-K+ pulse (50 mM for 2 s) was applied to facilitate ER Ca2+ store filling. The extent of store filling was then assessed by application of caffeine (30 mM) in Ca2+-free medium. A 2nd and 3rd application of caffeine confirmed that the ER stores were now empty. FCCP (1 μM) was then applied to inhibit mitochondrial Ca2+ buffering and another a high K+ pulse (50 mM in normal Ca2+ medium) followed by caffeine was applied to refill and then empty ER Ca2+ stores. Finally exposure to FCCP was terminated and ER Ca2+ stores again refilled and then discharged by sequential exposure to high K+ followed by caffeine. Note reduction in the caffeine response during exposure to FCCP. B: summary of the effects of FCCP on ER store filling/caffeine response obtained using the protocol in A. Responses to caffeine are expressed as a percentage of the initial control response to caffeine. Values are means ± SE with number of observations in parenthesis. Statistical significance was assessed using Student's paired t-test (*P < 0.05).
Effects of anoxia on cytosolic Ca2+ clearance
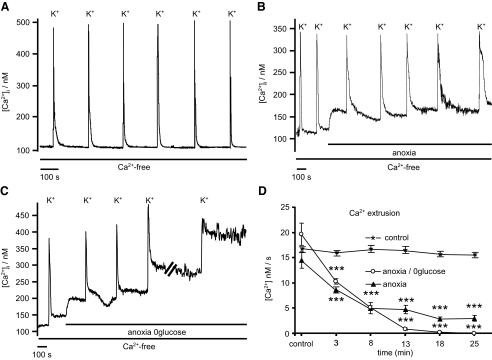
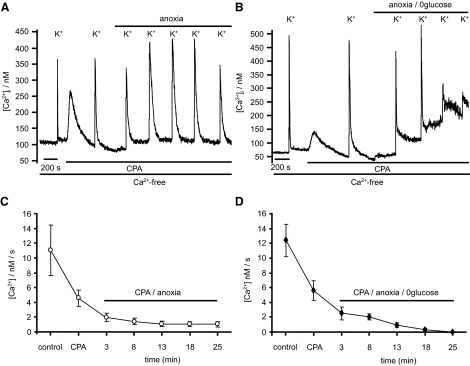
During the preceding studies, it was noted that [Ca2+]i recovery following high-K+ depolarization (i.e., during the mitochondrial Ca2+ release phase) was slower under anoxic conditions (Fig. 8A). Because this could not be attributed to an increased mitochondrial Ca2+ load, it must reflect some effect of anoxia on mitochondrial Ca2+ release or cytosolic Ca2+ clearance/extrusion mechanisms. We therefore sought to determine the effects of anoxia on cytosolic Ca2+ clearance under conditions in which mitochondrial Ca2+ buffering is thought to be insignificant. To achieve this, cells were depolarized in a high-K+ (50 mM) tyrode with extracellular calcium concentration reduced to 250 μM (high-K+-low-Ca2+). Brief (5 s) application of this solution induced a rapid rise in [Ca2+]i that was limited to a peak of <500 nM (mean = 274 ± 8 nM, n = 9). [Ca2+]i recovery following removal of high-K+-low-Ca2+ tyrode was monophasic (Fig. 12A), confirming that the threshold for significant mitochondrial Ca2+ buffering was not breached (Nicholls and Budd 2000). Cytosolic Ca2+ clearance was quantified during this recovery phase as the rate of change in [Ca2+]i measured over a small range of [Ca2+]i between 150 and 200 or 200 and 250 nM (see legend to Fig. 12). Repeated exposures to this solution under control conditions evoked similar [Ca2+]i responses with reproducible recovery/Ca2+ clearance rates (Fig. 12D). Anoxia reduced the amplitude of these high-K+-low-Ca2+ induced [Ca2+]i transients to 78 ± 5% (n = 9, P < 0.01) of control and significantly slowed Ca2+ clearance from 14.8 ± 1.34 nM/s (n = 23) under control conditions, to 8.38 ± 1.1 nM/s (n = 12, P < 0.001) after 3 min of anoxia and 2.98 ± 0.6 nM/s after 25 min of anoxia (see Fig. 12, B and D).
FIG. 12.
Ca2+ extrusion during anoxia and anoxic aglycemia. To measure Ca2+ extrusion/clearance from the cytosol, cells were subject to a moderate [Ca2+]i increase by brief exposure to a depolarizing solution containing 50 mM K+ and low extracellular [Ca2+] (250 μM). [Ca2+]i recovery was then observed in a Ca2+-free solution as an index of Ca2+ extrusion. A: control response to repeated application of high-K+, low Ca2+ medium. B: effects of anoxia on [Ca2+]i recovery. C: effects of anoxic aglycemia on [Ca2+]i recovery. D: time dependence of the effects of; *, control conditions; ▴, anoxia; and ○, anoxic aglycemia on Ca2+ extrusion. Data points are means ± SE of [Ca2+]i recovery rates calculated between 150 and 200 nM [Ca2+]i (*, control conditions; ▴, anoxia) or between 200 and 250 nM [Ca2+]i (○, anoxic aglycemia) measured under control conditions and at various time points following introduction of anoxia or anoxic aglycemia. Statistical significance, relative to control (initial) recovery rates, were determined by 1-way ANOVA (***P < 0.001, **P < 0.01, *P < 0.05).
Because Ca2+ clearance can occur via the plasma membrane and/or uptake into the ER, we sought to determine which of these pathways was being inhibited by anoxia. Inhibition of SERCA under normoxic conditions with either thapsigargin (100 nM) or CPA (10 μM) reduced Ca2+ clearance rates from 14.8 ± 1.3 to 4.62 ± 0.85 nM/s (n = 11, P < 0.01, Fig. 13), confirming that ER Ca2+ uptake contributes significantly to Ca2+ clearance under these experimental conditions (see Fig. 13 and discussion). The remaining Ca2+ clearance presumably represents Ca2+ efflux across the plasma membrane. In the presence of thapsigargin or CPA, anoxia (3 min) further reduced Ca2+ clearance to 2.2 ± 0.48 nM/s (n = 11 P < 0.001, Figs. 13 and 14). Thus anoxia inhibits plasma membrane Ca2+ clearance by ∼53%.
FIG. 13.
Ca2+ extrusion during CPA application. Neurons were depolarized with a brief (5 s) application of 50 mM K+ low Ca2+ (250 μM) solution, and cytosolic Ca2+ clearance was observed in a Ca2+-free medium. Ca2+ uptake by SERCA was then inhibited with CPA (10 μM). The remaining Ca2+ clearance was assumed to represent efflux across the plasma membrane. A: effects of anoxia on CPA resistant Ca2+ clearance. B: effects of anoxic aglycemia on CPA-resistant Ca2+ clearance. C: effects of anoxia on Ca2+ extrusion. Data are means ± SE of [Ca2+]i recovery rates (calculated between 150 and 200 nM [Ca2+]i) measured under control conditions in the presence of CPA and at various time points following introduction of anoxia. D: effects of anoxic aglycemia on Ca2+ extrusion. Data are means ± SE of [Ca2+]i recovery rates (calculated between 200 and 250 nM [Ca2+]i) under control conditions in the presence of CPA and at various time points following the introduction of anoxic aglycemia. Each point depicts the means ± SE of 6 (C) or 5 (D) individual experiments.
FIG. 14.
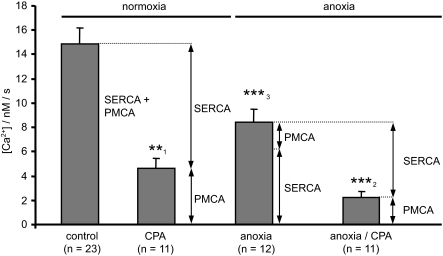
Effects of anoxia on PMCA and SERCA mediated Ca2+ clearance. Summary of data from Figs. 12 and 13. Left: total Ca2+ clearance ([Ca2+]i recovery rate) in Ca2+-free medium under normoxic conditions (control; 14.8 ± 1.34 nM/s; n = 23). Ca2+ clearance was reduced to 4.62 nM/s by the SERCA inhibitors CPA and thapsigargin (**P < 0.01). Exposure of neurons to anoxia (3 min) without CPA or thapsigargin treatment reduced the total neuronal Ca2+ clearance to 8.38 ± 1.1 nM/s (n = 12, ***P < 0.001). CPA-resistant Ca2+ clearance was also reduced after 3-min exposure to anoxia to 2.2 ± 0.48 nM/s (n = 11, ***P < 0.001). Ca2+ clearance in the presence of CPA (i.e., CPA resistant) was assumed to correspond to that mediated by the PMCA, whereas the CPA sensitive component (control –CPA) was assumed to be mediated by SERCA. ↔, the contribution of each individual Ca2+ clearance pathway to the total Ca2+ clearance under both normoxic and anoxic conditions. Numbers of experiments are given in parenthesis. Statistical significance was assessed using Student's paired t-test, [***P < 0.001, **P < 0.01, *P < 0.05) compared with control (normoxia; 1); compared with normoxia / CPA (2); compared with control (normoxia; 3)].
To estimate Ca2+ clearance by SERCA, we subtracted Ca2+ clearance rates measured in the presence of CPA or thapsigargin from total cytosolic Ca2+ clearance rates measured in the absence of CPA or thapsigargin (see Fig. 14). Ca2+ clearance via SERCA under normoxic conditions was thus estimated to be 10.2 nM/s. Anoxia (3 min) reduced this SERCA-mediated Ca2+ clearance to 6.2 nM/s or ∼60% of control. Thus anoxia appears to inhibit both SERCA and PMCA to roughly the same extent (Fig. 14).
We also attempted to assay SERCA-mediated Ca2+ clearance by inhibiting PMCA activity. It has been reported that as PMCA mediates exchange of [Ca2+]i for H+o, it can be inhibited by extracellular alkalosis (Benham et al. 1992). We found that while raising external pH to 8.8 caused a 90% inhibition of Ca2+ clearance in Ca2+-containing medium, the effects of alkalinization in Ca2+-free medium were much less pronounced and significant Ca2+ clearance remained even when the SERCA was also inhibited (unpublished results). One possible explanation for these observations is that the inhibitory effect of external alkalosis derive not simply from kinetic restrictions imposed by reduced H+o availability but also from thermodynamic constraints, i.e., at very low [H+]o Ca2+ extrusion may become energetically unfavorable in a normal Ca2+ medium (but not in a Ca2+-free medium). We could not therefore block PMCA to directly assess the effects of anoxia on SERCA-mediated cytosolic Ca2+ clearance.
Effects of anoxic aglycemia on Ca2+ regulation
In the preceding studies, it is clear that although anoxia induces significant changes, Ca2+ regulation does not become totally dysfunctional. This probably reflects the fact that while cellular ATP levels are reduced by anoxia they are not exhausted (see discussion). To determine how more complete ATP depletion affects [Ca2+]i regulation, we exposed some neurons to a combination of both anoxia and aglycemia. Under these conditions, the rise in [Ca2+]i was biphasic (see Fig. 9C). During the first phase, [Ca2+]i rose rapidly (within 1 min) to 80 ± 12 nM (n = 9) and then remained stable for 8–12 min. This initial rise in [Ca2+]i was not significantly different than that observed in response to anoxia alone (P = 0.35). This was then followed by a second more profound increase in [Ca2+]i by ≤187 ± 32 nM (n = 9). During this second phase, responses to caffeine (30 mM for 2 min) were effectively abolished (2 ± 2% of control, n = 6, P < 0.0001, Fig. 9, C and D), indicating complete depletion of ER Ca2+ stores. Ca2+ clearance rates (measured using the protocol described in the preceding text) were also greatly reduced after ∼18 min of continued anoxic aglycemia (both in the presence and absence of CPA, see Figs. 12 and 13), indicating an almost complete inhibition of both SERCA and PMCA. Ca2+ influx in response to high-K+-low-Ca2+ medium was also much reduced after prolonged anoxic aglycemia, suggesting that voltage-gated Ca2+ channels may also become inhibited under these more extreme conditions.
DISCUSSION
In this study, we have primarily investigated the effects of anoxia on sensory neuron Ca2+ regulation. In animal models of cardiac ischemia (blood vessel occlusion), oxygen levels at the ischemic focus fall rapidly to zero (Rumsey et al. 1994; Walfridsson and Lewis 1987). Lack of oxygen delivery results in inhibition of mitochondrial respiration in the surrounding tissue as assessed by NADH accumulation (Barlow and Chance 1976) and depletion of phosphocreatine and ATP (Elliott et al. 1992; Zhang et al. 2001). Surrounding this core zone there may also be a hypoxic boarder zone. Our anoxia data, however, are most relevant to events in the “core region” of ischemic tissue where oxygen delivery is so low as to be unable to support oxidative phosphorylation.
In our isolated sensory neuron model, anoxia alone results in a rapid but only partial depletion of MgATP as assessed by increase in [Mg2+]i. In contrast anoxic aglycemia causes a biphasic fall in MgATP; the first phase, which lasts ∼10 min, is similar to that observed in anoxia alone. The second phase of the MgATP response appears to culminate in near complete exhaustion of MgATP (see Henrich and Buckler 2007). The anoxia model may therefore only simulate the early stages of no-flow ischemia where there is still glycolytic substrate available. In later stages of ischemia, with exhaustion of glycolytic substrates, prolonged anoxic aglycemia would probably be a better model.
Exposure of neurons to acute anoxia lead to a rapid rise in [Ca2+]i that appeared to result from Ca2+ release from internal stores. This observation is similar to a previous study in sensory neurons in which inhibitors of oxidative metabolism (cyanide) caused Ca2+ release from internal stores (Duchen et al. 1990). They are also consistent with reports of hypoxia-evoked Ca2+ release from internal stores in hippocampal neurons (Dubinsky and Rothman 1991; Grondahl et al. 1998), astrocytes (Aley et al. 2006), and pulmonary vascular smooth muscle (Jabr et al. 1997; Kang et al. 2002). The effects of anoxia on DRG neurons were not limited to Ca2+ store release; however, it also had multiple other effects on [Ca2+]i regulation (see following text).
We were unable to corroborate a recent study claiming that hypoxia (10–40 Torr) evokes voltage-gated Ca2+ influx in DRG neurons (Lukyanetz et al. 2003). In our study, hypoxia had little effect on [Ca2+]i, and the Ca2+ responses to anoxia were not diminished by either Ca2+ channel blocker (Ni2+) or removal of extracellular Ca2+. The difference between our results and those of Lukyanetz et al. cannot be accounted for by our selective use of capsaicin sensitive neurons because we found that capsaicin-negative neurons also failed to respond to hypoxia (data not shown). We note, however, that the study of Lukyanetz at al. was conducted in medium apparently lacking any metabolic substrate, and it is unclear how long neurons were maintained in this medium.
Does anoxia promote mitochondrial Ca2+ release?
Mitochondria play an important role in Ca2+ homeostasis in neurons by acting as a dynamic Ca2+ buffering system. It is, however, thought that under resting conditions, [Ca2+]i is below the set point for mitochondrial Ca2+ uptake and consequently the mitochondrion contains relatively little Ca2+ (Budd and Nicholls 1996b; Chalmers and Nicholls 2003; Nicholls 1978; Nicholls and Budd 2000; Nicholls and Scott 1980; Somlyo et al. 1985; Thayer and Miller 1990; Thayer and Wang 1995; Zoccarato and Nicholls 1982). To determine whether mitochondria contain enough Ca2+ under resting conditions to enhance [Ca2+]i, we investigated the effects of releasing mitochondrial Ca2+ with FCCP (Babcock et al. 1997; Duchen 1999; Friel and Tsien 1994). The application of FCCP both enhanced [Ca2+]i and diminished any subsequent [Ca2+]i response to anoxia or cyanide. These data therefore suggest that part of the [Ca2+]i response to anoxia is dependent on ψm.
In analyzing these data, our first concern was whether the ψm-dependent Ca2+ response could be an artifact caused by MgATP hydrolysis and elevation of cytosolic Mg2+ interfering with [Ca2+]i measurement. This seems unlikely for the following reasons, anoxia and FCCP cause only a small increase in [Mg2+]i of ∼0.5 mM from a basal level of 1.5 mM (Henrich and Buckler 2007); the selectivity ratio for Fura-2 (KdMg/KdCa) is around 53,000 (Lattanzio and Bartschat 1991), which suggests that an elevation of [Mg2+]i of 0.5 mM would be equivalent to a rise in [Ca2+]i of only 9 nM; in vitro calibration of Fura-2 shows that there is no major effect of Mg2+ between 0.3 and 3 mM (Fig. 1); and loading neurons with BAPTA almost completely abolishes the [Ca2+]i response to FCCP (Fig. 1B).
Having excluded significant interference from [Mg2+]i, the cause of the sustained elevation of [Ca2+]i in response to FCCP (and the FCCP inhibitable component of responses to anoxia and cyanide) is not immediately obvious. If it was due simply to Ca2+ release from mitochondria, that release would either have to be sustained for several minutes without decrement or other Ca2+ clearance pathways would have to be inhibited (otherwise any rise in [Ca2+]i would be transient because of the ongoing activity of Ca2+ pumps). Another possible source of Ca2+ could be displacement from internal buffers due to competition from H+ and Mg2+ both of which increase in response to anoxia and FCCP (Henrich and Buckler 2007). The fall in pHi and the rise in [Mg2+]i however both reach stable levels within a few tens of seconds of exposure to anoxia. Thus for a rise in [Ca2+]i to be sustained there would again have to be a co-incident impairment of Ca2+ clearance from the cytosol. Thus while Ca2+ release from mitochondria and/or displacement from internal buffers are attractive sources for the FCCP inhibitable part of the anoxia induced rise in [Ca2+]i, it is unlikely that either could fully account for a sustained rise in [Ca2+]i.
Effects of anoxia on mitochondrial Ca2+ buffering
The current concept of the role of mitochondria in cellular Ca2+ homeostasis is that under conditions in which cytosolic [Ca2+]i is substantially elevated, the mitochondrion avidly takes up Ca2+ via the uniporter driven by both the calcium gradient and the mitochondrial transmembrane potential (ψm). This Ca2+ load is then buffered within the inner matrix by complexing with phosphate (Chalmers and Nicholls 2003; Nicholls and Akerman 1982). If [Ca2+]i subsequently declines, stored Ca2+ is released back into the cytosol via a Na+:Ca2+ exchange. This process is clearly demonstrated by evoking voltage-gated Ca2+ influx into the cytosol (see e.g., Fig. 8). Under these conditions, mitochondria take up Ca2+ thus constraining any rise in cytosolic [Ca2+]. On repolarization, and cessation of Ca2+ influx, [Ca2+]i rapidly falls as Ca2+ continues to be taken up into the mitochondrion until it reaches a “set point” (typically ∼0.5 μM) (Nicholls 1978, 2005; Nicholls and Scott 1980) whereupon the mitochondrion ceases to take up Ca2+ and instead begins to release it. From this point onward, the decline in [Ca2+]i is halted while Ca2+ is released back into the cytosol and then pumped out of the cell or into other internal stores. This process gives rise to a characteristic “plateau,” or “shoulder” during [Ca2+]i recovery.
Given our observation that anoxia caused partial depolarization of mitochondria and appeared to induce Ca2+ release under resting conditions, we anticipated that it would have a major impact on mitochondrial Ca2+ buffering. Surprisingly, however, anoxia had very little effect on mitochondrial Ca2+ buffering during voltage-gated Ca2+ entry as assessed either by the resultant rise in [Ca2+]i or by the amount of Ca2+ stored in the mitochondria (see Fig. 8 and results). We are therefore forced to conclude that mitochondrial Ca2+ buffering capacity is relatively insensitive to moderate changes in ψm. The dynamics of the mitochondrial Ca2+ buffering process, specifically the Ca2+ release phase, were, however, substantially slowed by anoxia. While this might indicate that anoxia inhibits mitochondrial Ca2+ release, there are other possible explanations (see following text).
Effects of anoxia on ER Ca2+ release
Sensory neurons possess significant intracellular Ca2+ stores (Verkhratsky 2005). Following store depletion, the [Ca2+]i response to anoxia was reduced by ∼60% (Fig. 3). We also noted that during exposure to anoxia emptying of ER Ca2+ stores lowered basal [Ca2+]i and refilling the stores raised it again (see Fig. 10A). These data strongly suggest that ER Ca2+ stores play a major role in mediating the [Ca2+]i response to anoxia. These data alone, however, do not enable us to determine how, or indeed if, anoxia alters ER function. Resting [Ca2+]i represents a balance of Ca2+ fluxes into and out of the cytosol. Under control conditions, CPA also caused a small rise in [Ca2+]i. This observation indicates that under resting conditions, there is significant cycling of Ca2+ into and out of the ER such that on SERCA inhibition there is a net Ca2+ efflux. Studies looking at the effects of inhibition of SERCA on [Ca2+] levels in the ER itself also reveal a significant background Ca2+ leak from those stores (Solovyova and Verkhratsky 2003). Consequently the anoxia-induced elevation of [Ca2+]i that appears to be dependent on ER Ca2+ stores could result from increased store release, decreased store uptake, or simply decreased Ca2+ efflux across the plasma membrane (or indeed any combination of these events). Evidence that anoxia enhances net Ca2+ release (or leak) from the ER comes primarily from our observation that the caffeine-releasable pool of Ca2+ progressively declines during anoxia (Fig. 9, A and B). Moreover, the observation that ryanodine prevented anoxia-induced release of Ca2+ from the ER suggests that Ca2+ efflux via ryanodine receptors plays an important role in mediating this slow ER Ca2+ release. Decreased Ca2+ uptake by SERCA must, however, also be an important factor (see following text).
Although we have not investigated possible links between anoxia and ER function in any detail, it is notable that the anoxia-induced ER Ca2+ release persists in the presence of FCCP. Because the level of FCCP (1 μM) used in this experiment is sufficient to cause near maximal mitochondrial depolarization (as assessed using Rh123 fluorescence) (unpublished data), it is reasonable to assume that FCCP also fully inhibits mitochondrial ATP synthesis. The ER Ca2+ release response to anoxia cannot therefore be due to changes in energy metabolism. We have, however, noted a very similar response to the electron transport inhibitor cyanide i.e., an abrupt increase in intracellular [Ca2+]i that is only partially occluded by FCCP (see Fig. 5B). These observations suggest that inhibition of electron transport might be the key factor in promoting ER Ca2+ store release in response to anoxia and cyanide.
A model that has been advanced to explain hypoxic pulmonary vasoconstriction suggests that hypoxia-induced increase NADH could stimulate cyclic-ADP-ribose (cADPR) production (Dipp and Evans 2001; Evans and Dipp 2002). cADPR promotes Ca2+ release from the endoplasmic reticulum via ryanodine receptors (Galione 1993; Tanaka and Tashjian 1995; Thorn et al. 1994). Hypoxia-evoked ER Ca2+ release in astrocytes has also recently been attributed to cADPR-mediated activation of ryanodine receptors (Aley et al. 2006). In our studies, pretreatment of sensory neurons with 8-Br-cADPR for 10 min significantly reduced [Ca2+]i responses to anoxia both in the absence and presence of FCCP (Fig. 7) but did not abolish them. These data suggest that at least part of the FCCP resistant [Ca2+]i response to anoxia may be due to cADPR-mediated Ca2+ release via ryanodine receptors. We cannot, however, exclude the possibility that other mechanisms also contribute to ER Ca2+ release. For example it has been suggested that ryanodine receptors can act as an O2 sensors to evoke Ca2+ efflux from the SR in skeletal muscle during severe hypoxia (Eu 2000), although this has been disputed (Cheong et al. 2005).
Effects of anoxia on Ca2+ clearance
Ca2+ extrusion from the cytosol following a modest increase in [Ca2+]i was significantly and progressively inhibited in the presence of anoxia. Slowing of Ca2+ recovery during metabolic inhibition has also been observed in mouse sensory neurons (Duchen et al. 1990).
The PMCA has been reported to be the principal (nonmitochondrial) Ca2+ clearance mechanism in DRG (Lu et al. 2006; Usachev et al. 2002). Our studies, however, indicate that the SERCA also plays a major role in Ca2+ clearance (see Fig. 13) both in Ca2+-free medium and in normal Ca2+-containing medium (data not shown). Although our data appear at variance with that of others (Usachev et al. 2002), the contribution made by SERCA to Ca2+ clearance must depend on the extent to which ER Ca2+ stores are already full. The relative contributions of SERCA versus PMCA to Ca2+ clearance must therefore depend on the conditions in which such measurements are made. We found no evidence for any Na+o-dependent Ca2+ extrusion (unpublished data) and so presume that all CPA-insensitive Ca2+ extrusion is mediated by the PMCA.
Anoxia appeared to inhibit both SERCA- and PMCA-mediated Ca2+ clearance equally. This inhibition of Ca2+ clearance may help explain why the actions of anoxia on [Ca2+]i are relatively sustained. The most likely cause of the inhibition of SERCA and PMCA is impaired cellular energy metabolism. In a parallel study to this we have estimated that cytoplasmic [MgATP] falls by ∼30% in anoxia (Henrich and Buckler 2007). Thus changes in ATP concentration and/or phosphorylation potential could influence the activity of Ca2+ pumps. We note that oligomycin, an inhibitor of mitochondrial ATP synthase, has recently been reported to change the dynamics of caffeine-induced Ca2+ oscillations in sensory neurons. This effect was also attributed to inhibition of SERCA activity by ATP depletion (Jackson and Thayer 2006). At this stage, however, we cannot exclude the possibility that other oxygen dependent signaling pathways might also be involved.
Interactions among the effects of anoxia on Ca2+ clearance, mitochondrial Ca2+ buffering, and ER Ca2+ uptake
The effects of anoxia on Ca2+ clearance lead to some interesting interactions between the various sources and sinks of Ca2+ within the cell. We noted that despite the fact that anoxia appeared to have no major effect on mitochondrial Ca2+ buffering, the time taken for the buffered Ca2+ load to be removed was much increased (see e.g., Fig. 8A). While this could be due to a direct inhibition of mitochondrial Ca2+ release, it is also possible that it is simply an indirect consequence of reduced cytosolic Ca2+ clearance via PMCA and SERCA. In support of this hypothesis, we noted that when SERCA is inhibited with CPA (10 μM) or thapsigargin (100 nM), the duration of the mitochondrial Ca2+ release phase following voltage-gated Ca2+ entry was also increased to 228 ± 95% (n = 5, P < 0.05) compared with control (data not shown).
We also noted that despite anoxia inhibiting SERCA activity, ER store loading was as effective under anoxic conditions as under control conditions (Fig. 10). One possible explanation for this is that store filling takes place under conditions in which PMCA and SERCA compete for the Ca2+ released from the mitochondrion. With both pumps inhibited to an equivalent degree, the dynamic balance between Ca2+ extrusion and ER Ca2+ uptake is probably not significantly altered. The only functional difference therefore is that redistribution of Ca2+ from the mitochondrion to the ER and external milieu takes longer under anoxic conditions. This diversion of Ca2+ entering through voltage-gated Ca2+ channels via the mitochondria seems to be important to achieving efficient ER Ca2+ loading because inhibition of mitochondrial Ca2+ buffering with FCCP substantially reduced caffeine-releasable Ca2+ stores (Fig. 11).
Conclusions
Our studies indicate that anoxia of short- to medium-term duration, sufficient to partially compromise cellular energy metabolism, has a number of effects on Ca2+ homeostasis in DRG neurons. These include a small ψm-dependent release of Ca2+ from either the mitochondrion itself or other internal Ca2+ buffers/stores and a rather more significant Ca2+ release from the ER. Anoxia also significantly reduced Ca2+ clearance via both the SERCA and PMCA and substantially slowed Ca2+ recycling from the mitochondrion (probably as a consequence of inhibition of SERCA and PMCA). Collectively these events cause a sustained rise in [Ca2+]i. We have also investigated, albeit only relatively perfunctorily, the effects of more severe energy depletion on Ca2+ homeostasis. Removal of both oxygen and glucose leads to a biphasic decline in cellular ATP (Henrich and Buckler 2007). This is reflected in a biphasic rise in cytosolic [Ca2+]i and, (after ∼15 min in anoxic aglycemia), profound disturbance of Ca2+ homeostasis with near complete failure of Ca2+ clearance by both SERCA and PMCA and complete depletion of the ER Ca2+ stores.
Assuming that the basic mechanisms of Ca2+ regulation in dendrites are similar to those in the soma, we would predict a sequence of changes in Ca2+ metabolism in sensory nerve endings during ischemia. With the initial loss of oxygen, we would expect Ca2+ metabolism to be significantly altered with a sustained elevation in basal [Ca2+]i due to store release and with reduced capacity for Ca2+ clearance possibly enhancing and/or prolonging [Ca2+]i responses to Ca2+ entry. The fundamental components of Ca2+ regulation, however, would probably retain basic functionality. In contrast, once cellular reserves of anaerobic sources of energy are used up, there would be a failure of key regulatory mechanisms and consequent further elevation in [Ca2+]i.
In addition to the preceding direct effects anoxia on [Ca2+]i regulation, we would also anticipate that these events could significantly affect neuronal responses to other ischemic conditions/stimuli. Of particular interest will be to determine the extent to which anoxia influences responses to acidosis because this is thought to be a key stimulus in mediating sensory neuron response to ischemia.
GRANTS
This work was supported by a grant from the Wellcome Trust to K. J. Buckler and DFG research fellowship HE3678/1-1 to M. Henrich.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- Aley et al. 2006.Aley PK, Murray HJ, Boyle JP, Pearson HA, Peers C. Hypoxia stimulates Ca2+ release from intracellular stores in astrocytes via cyclic ADP ribose-mediated activation of ryanodine receptors. Cell Calcium 39: 95–100, 2006. [DOI] [PubMed] [Google Scholar]
- Armour 1999.Armour JA Myocardial ischaemia and the cardiac nervous system. Cardiovasc Res 41: 41–54, 1999. [DOI] [PubMed] [Google Scholar]
- Armour et al. 1994.Armour JA, Huang MH, Pelleg A, Sylven C. Responsiveness of in situ canine nodose ganglion afferent neurones to epicardial mechanical or chemical stimuli. Cardiovasc Res 28: 1218–1225, 1994. [DOI] [PubMed] [Google Scholar]
- Babcock et al. 1997.Babcock DF, Herrington J, Goodwin PC, Park YB, Hille B. Mitochondrial participation in the intracellular Ca2+ network. J Cell Biol 136: 833–844, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow and Chance 1976.Barlow CH, Chance B. Ischemic areas in perfused rat hearts: measurement by NADH fluorescence photography. Science 193: 909–910, 1976. [DOI] [PubMed] [Google Scholar]
- Benham et al. 1992.Benham CD, Evans ML, McBain CJ. Ca2+ efflux mechanisms following depolarization evoked calcium transients in cultured rat sensory neurons. J Physiol 455: 567–583, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson et al. 1999.Benson CJ, Eckert SP, McCleskey EW. Acid-evoked currents in cardiac sensory neurons: a possible mediator of myocardial ischemic sensation. Circ Res 84: 921–928, 1999. [DOI] [PubMed] [Google Scholar]
- Bevan and Geppetti 1994.Bevan S, Geppetti P. Protons: small stimulants of capsaicin-sensitive sensory nerves. Trends Neurosci 17: 509–512, 1994. [DOI] [PubMed] [Google Scholar]
- Bevan and Yeats 1991.Bevan S, Yeats J. Protons activate a cation conductance in a sub-population of rat dorsal root ganglion neurons. J Physiol 433: 145–161, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown 1967.Brown AM Excitation of afferent cardiac sympathetic nerve fibers during myocardial ischemia. J Physiol 190: 35–53, 1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budd and Nicholls 1996a.Budd SL, Nicholls DG. Mitochondria, calcium regulation, and acute glutamate excitotoxicity in cultured cerebellar granule cells. J Neurochem 67: 2282–2291, 1996a. [DOI] [PubMed] [Google Scholar]
- Budd and Nicholls 1996b.Budd SL, Nicholls DG. A reevaluation of the role of mitochondria in neuronal Ca2+ homeostasis. J Neurochem 66: 403–411, 1996b. [DOI] [PubMed] [Google Scholar]
- Caterina et al. 1997.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389: 816–824, 1997. [DOI] [PubMed] [Google Scholar]
- Chalmers and Nicholls 2003.Chalmers S, Nicholls DG. The relationship between free and total calcium concentrations in the matrix of liver and brain mitochondria. J Biol Chem 278: 19062–19070, 2003. [DOI] [PubMed] [Google Scholar]
- Cheong et al. 2005.Cheong E, Tumbev V, Stoyanovsky D, Salama G. Effects of pO2 on the activation of skeletal muscle ryanodine receptors by NO: a cautionary note. Cell Calcium 38: 481–488, 2005. [DOI] [PubMed] [Google Scholar]
- Davies et al. 1988.Davies NW, Lux HD, Morad M. Site and mechanism of activation of proton-induced sodium current in chick dorsal root ganglion neurons. J Physiol 400: 159–187, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dipp and Evans 2001.Dipp M, Evans AM. Cyclic ADP-ribose is the primary trigger for hypoxic pulmonary vasoconstriction in the rat lung in situ. Circ Res 89: 77–83, 2001. [DOI] [PubMed] [Google Scholar]
- Dubinsky and Rothman 1991.Dubinsky JM, Rothman SM. Intracellular calcium concentrations during “chemical hypoxia” and excitotoxic neuronal injury. J Neurosci 11: 2545–2551, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchen 1999.Duchen MR Contributions of mitochondria to animal physiology: from homeostatic sensor to calcium signalling and cell death. J Physiol 516: 1–17, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchen et al. 1990.Duchen MR, Valdeolmillos M, O'Neill SC, Eisner DA. Effects of metabolic blockade on the regulation of intracellular calcium in dissociated mouse sensory neurones. J Physiol 424: 411–426, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott et al. 1992.Elliott AC, Smith GL, Eisner DA, Allen DG. Metabolic changes during ischemia and their role in contractile failure in isolated ferret hearts. J Physiol 454: 467–490, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erecinska and Silver 1994.Erecinska M, Silver IA. Ions and energy in mammalian brain. Prog Neurobiol 43: 37–71, 1994. [DOI] [PubMed] [Google Scholar]
- Eu et al. 2000.Eu JP, Sun J, Xu L, Stamler JS, Meissner G. The skeletal muscle calcium release channel: coupled O2 sensor and NO signalling functions. Cell 102: 499–509, 2000. [DOI] [PubMed] [Google Scholar]
- Evans and Dipp 2002.Evans AM, Dipp M. Hypoxic pulmonary vasoconstriction: cyclic adenosine diphosphate-ribose, smooth muscle Ca2+ stores and the endothelium. Respir Physiolo Neurobiol 132: 3–15, 2002. [DOI] [PubMed] [Google Scholar]
- Friel and Tsien 1994.Friel DD, Tsien RW. An FCCP-sensitive Ca2+ store in bullfrog sympathetic neurons and its participation in stimulus-evoked changes in [Ca2+]i. J Neurosci 14: 4007–4024, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galione 1993.Galione A Cyclic ADP-ribose: a new way to control calcium. Science 259: 325–326, 1993. [DOI] [PubMed] [Google Scholar]
- Grondahl et al. 1998.Grondahl TO, Hablitz JJ, Langmoen IA. Depletion of intracellular Ca2+ stores or lowering extracellular calcium alters intracellular Ca2+ changes during cerebral energy deprivation. Brain Res 796: 125–131, 1998. [DOI] [PubMed] [Google Scholar]
- Gruss et al. 2006.Gruss M, Ettorre G, Stehr AJ, Henrich M, Hempelmann G, Scholz A. Moderate hypoxia influences excitability and blocks dendrotoxin sensitive K+ currents in rat primary sensory neurons. Mol Pain 2: 12, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz et al. 1985.Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem 260: 3440–3450, 1985. [PubMed] [Google Scholar]
- Hansen 1985.Hansen AJ Effect of anoxia on ion distribution in the brain. Physiol Rev 65: 101–148, 1985. [DOI] [PubMed] [Google Scholar]
- Henrich and Buckler 2007.Henrich M, Buckler KJ. Effects of anoxia, aglycemia and acidosis on cytosolic Mg2+, ATP, and pH in rat sensory neurons. Am J Physiol Cell Physiol 294: C280–294, 2007. [DOI] [PubMed] [Google Scholar]
- Hilgemann 1997.Hilgemann DW Cytoplasmic ATP-dependent regulation of ion transporters and channels: mechanisms and messengers. Annu Rev Physiol 59: 193–220, 1997. [DOI] [PubMed] [Google Scholar]
- Huang et al. 1996.Huang MH, Horackova M, Negoescu RM, Wolf S, Armour JA. Polysensory response characteristics of dorsal root ganglion neurons that may serve sensory functions during myocardial ischaemia. Cardiovasc Res 32: 503–515, 1996. [PubMed] [Google Scholar]
- Immke and McCleskey 2001a.Immke DC, McCleskey EW. ASIC3: a lactic acid sensor for cardiac pain. Sci World J 1: 510–512, 2001a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Immke and McCleskey 2001b.Immke DC, McCleskey EW. Lactate enhances the acid-sensing Na+ channel on ischemia-sensing neurons. Nat Neurosci 4: 869–870, 2001b. [DOI] [PubMed] [Google Scholar]
- Jabr et al. 1997.Jabr RI, Toland H, Gelband CH, Wang XX, Hume JR. Prominent role of intracellular Ca2+ release in hypoxic vasoconstriction of canine pulmonary artery. Br J Pharmacol 122: 21–30, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson and Thayer 2006.Jackson JG, Thayer SA. Mitochondrial modulation of Ca2+ -induced Ca2+ -release in rat sensory neurons. J Neurophysiol 96: 1093–1104, 2006. [DOI] [PubMed] [Google Scholar]
- Kang et al. 2002.Kang TM, Park MK, Uhm DY. Characterization of hypoxia-induced [Ca2+]i rise in rabbit pulmonary arterial smooth muscle cells. Life Sci 70: 2321–2333, 2002. [DOI] [PubMed] [Google Scholar]
- Konnerth et al. 1987.Konnerth A, Lux HD, Morad M. Proton-induced transformation of calcium channel in chick dorsal root ganglion cells. J Physiol 386: 603–633, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishtal and Pidoplichko 1980.Krishtal OA, Pidoplichko VI. A receptor for protons in the nerve cell membrane. Neuroscience 5: 2325–2327, 1980. [DOI] [PubMed] [Google Scholar]
- Krishtal and Pidoplichko 1981.Krishtal OA, Pidoplichko VI. A receptor for protons in the membrane of sensory neurons may participate in nociception. Neuroscience 6: 2599–2601, 1981. [DOI] [PubMed] [Google Scholar]
- Lattanzio and Bartschat 1991.Lattanzio FA, Bartschat DK. The effect of pH on rate constants, ion selectivity and thermodynamic properties of fluorescent calcium and magnesium indicators. Biochem Biophys Res Commun 177: 184–191, 1991. [DOI] [PubMed] [Google Scholar]
- Ledda et al. 1993.Ledda F, Amerini S, Filippi S, Mantelli L, Morbidelli L, Rubino A, Ziche M. Cardiovascular effects of capsaicin-sensitive neurons. Cardioscience 4: 1–7, 1993. [PubMed] [Google Scholar]
- Li and Peng 2002.Li YJ, Peng J. The cardioprotection of calcitonin gene-related peptide-mediated preconditioning. Eur J Pharmacol 442: 173–177, 2002. [DOI] [PubMed] [Google Scholar]
- Liu et al. 2004.Liu M, Willmott NJ, Michael GJ, Priestley JV. Differential pH and capsaicin responses of Griffonia simplicifolia IB4 (IB4)-positive and IB4-negative small sensory neurons. Neuroscience 127: 659–672, 2004. [DOI] [PubMed] [Google Scholar]
- Longhurst et al. 2001.Longhurst JC, Tjen ALSC, Fu LW. Cardiac sympathetic afferent activation provoked by myocardial ischemia and reperfusion. Mechanisms and reflexes. Ann NY Acad Sci 940: 74–95, 2001. [DOI] [PubMed] [Google Scholar]
- Lopez Barneo 1994.Lopez Barneo J Oxygen-sensitive ion channels: how ubiquitous are they? Trends Neurosci 17: 133–135, 1994. [DOI] [PubMed] [Google Scholar]
- Lu et al. 2006.Lu SG, Zhang X, Gold MS. Intracellular calcium regulation among subpopulations of rat dorsal root ganglion neurons. J Physiol 577: 169–190, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukyanetz et al. 2003.Lukyanetz EA, Stanika RI, Koval LM, Kostyuk PG. Intracellular mechanisms of hypoxia-induced calcium increase in rat sensory neurons. Arch Biochem Biophys 410: 212–221, 2003. [DOI] [PubMed] [Google Scholar]
- Malliani 1990.Malliani A Cardiocardiac excitatory reflexes during myocardial ischemia. Basic Res Cardiol 85, Suppl 1: 243–252, 1990. [DOI] [PubMed] [Google Scholar]
- Nicholls 1978.Nicholls DG The regulation of extramitochondrial free calcium ion concentration by rat liver mitochondria. Biochem J 176: 463–474, 1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls 2005.Nicholls DG Mitochondria and calcium signaling. Cell Calcium 38: 311–317, 2005. [DOI] [PubMed] [Google Scholar]
- Nicholls and Akerman 1982.Nicholls D, Akerman K. Mitochondrial calcium transport. Biochim Biophys Acta 683: 57–88, 1982. [DOI] [PubMed] [Google Scholar]
- Nicholls and Budd 2000.Nicholls DG, Budd SL. Mitochondria and neuronal survival. Physiol Rev 80: 315–360, 2000. [DOI] [PubMed] [Google Scholar]
- Nicholls and Scott 1980.Nicholls DG, Scott ID. The regulation of brain mitochondrial calcium-ion transport. The role of ATP in the discrimination between kinetic and membrane-potential-dependent calcium-ion efflux mechanisms. Biochem J 186: 833–839, 1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opie 1991.Opie L The Heart: Physiology and Metabolism. New York: Raven, 1991.
- Owman 1990.Owman C Peptidergic vasodilator nerves in the peripheral circulation and in the vascular beds of the heart and brain. Blood Vessels 27: 73–93, 1990. [DOI] [PubMed] [Google Scholar]
- Pan et al. 1999.Pan HL, Longhurst JC, Eisenach JC, Chen SR. Role of protons in activation of cardiac sympathetic C-fiber afferents during ischaemia in cats. J Physiol 518: 857–866, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel and Honoré 2001.Patel AJ, Honoré E. Molecular physiology of oxygen-sensitive potassium channels. Eur Resp J I18: 221–227, 2001. [DOI] [PubMed] [Google Scholar]
- Peers 1997.Peers C Oxygen-sensitive ion channels. Trends Pharmacol Sci 18: 405–408, 1997. [DOI] [PubMed] [Google Scholar]
- Rumsey et al. 1994.Rumsey WL, Pawlowski M, Lejavardi N, Wilson DF. Oxygen pressure distribution in the heart in vivo and evaluation of the ischemic “border zone.” Am J Physiol Heart Circ Physiol 266: H1676–1680, 1994. [DOI] [PubMed] [Google Scholar]
- Sato et al. 1991.Sato M, Ikeda K, Yoshizaki K, Koyano H. Response of cytosolic calcium to anoxia and cyanide in cultured glomus cells of newborn rabbit carotid body. Brain Res 551: 327–330, 1991. [DOI] [PubMed] [Google Scholar]
- Shishkin et al. 2002.Shishkin V, Potapenko E, Kostyuk E, Girnyk O, Voitenko N, Kostyuk P. Role of mitochondria in intracellular calcium signaling in primary and secondary sensory neurons of rats. Cell Calcium 32: 121–130, 2002. [DOI] [PubMed] [Google Scholar]
- Sims 1995.Sims NR Calcium, energy metabolism and the development of selective neuronal loss following short-term cerebral ischemia. Metab Brain Dis 10: 191–217, 1995. [DOI] [PubMed] [Google Scholar]
- Solovyova and Verkhratsky 2003.Solovyova N, Verkhratsky A. Neuronal endoplasmic reticulum acts as a single functional Ca2+ store shared by ryanodine and inositol-1,4,5-trisphosphate receptors as revealed by intra-ER [Ca2+] recordings in single rat sensory neurones. Pfluegers 446: 447–454, 2003. [DOI] [PubMed] [Google Scholar]
- Somlyo et al. 1985.Somlyo AP, Bond M, Somlyo AV. Calcium content of mitochondria and endoplasmic reticulum in liver frozen rapidly in vivo. Nature 314: 622–625, 1985. [DOI] [PubMed] [Google Scholar]
- Tanaka and Tashjian 1995.Tanaka Y, Tashjian AH Jr. Calmodulin is a selective mediator of Ca2+-induced Ca2+ release via the ryanodine receptor-like Ca2+ channel triggered by cyclic ADP-ribose. Proc Natl Acad Sci USA 92: 3244–3248, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer and Miller 1990.Thayer SA, Miller RJ. Regulation of the intracellular free calcium concentration in single rat dorsal root ganglion neurons in vitro. J Physiol 425: 85–115, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer and Wang 1995.Thayer SA, Wang GJ. Glutamate-induced calcium loads: effects on energy metabolism and neuronal viability. Clin Exp Pharmacol Physiol 22: 303–304, 1995. [DOI] [PubMed] [Google Scholar]
- Thorn et al. 1994.Thorn P, Gerasimenko O, Petersen OH. Cyclic ADP-ribose regulation of ryanodine receptors involved in agonist evoked cytosolic Ca2+ oscillations in pancreatic acinar cells. Embo J 13: 2038–2043, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toescu and Verkhratsky 2000.Toescu EC, Verkhratsky A. Assessment of mitochondrial polarization status in living cells based on analysis of the spatial heterogeneity of rhodamine 123 fluorescence staining. Pfluegers 440: 941–947, 2000. [DOI] [PubMed] [Google Scholar]
- Usachev et al. 2002.Usachev YM, DeMarco SJ, Campbell C, Strehler EE, Thayer SA. Bradykinin and ATP accelerate Ca2+ efflux from rat sensory neurons via protein kinase C and the plasma membrane Ca2+ pump isoform 4. Neuron 33: 113–122, 2002. [DOI] [PubMed] [Google Scholar]
- Usachev et al. 1993.Usachev Y, Shmigol A, Pronchuk N, Kostyuk P, Verkhratsky A. Caffeine-induced calcium release from internal stores in cultured rat sensory neurons. Neuroscience 57: 845–859, 1993. [DOI] [PubMed] [Google Scholar]
- Verkhratsky 2005.Verkhratsky A Physiology and pathophysiology of the calcium store in the endoplasmic reticulum of neurons. Physiol Rev 85: 201–279, 2005. [DOI] [PubMed] [Google Scholar]
- Waldmann and Lazdunski 1998.Waldmann R, Lazdunski M. H+-gated cation channels: neuronal acid sensors in the NaC/DEG family of ion channels. Curr Opin Neurobiol 8: 418–424, 1998. [DOI] [PubMed] [Google Scholar]
- Walfridsson and Lewis 1987.Walfridsson H, Lewis DH. Myocardial surface oxygen pressure across the border zone in the pig during acute coronary artery occlusion. A comparison of pretreatment with metoprolol or isoprenaline. Basic Res Cardiol 82: 465–472, 1987. [DOI] [PubMed] [Google Scholar]
- Weir et al. 2005.Weir EK, Lopez-Barneo J, Buckler KJ, Archer SL. Acute oxygen-sensing mechanisms. N Engl J Med 353: 2042–2055, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werth and Thayer 1994.Werth JL, Thayer SA. Mitochondria buffer physiological calcium loads in cultured rat dorsal root ganglion neurons. J Neurosci 14: 348–356, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White 1957.White JC Cardiac pain: anatomic pathways and physiologic mechanisms. Circulation 16: 644–655, 1957. [DOI] [PubMed] [Google Scholar]
- Wyatt and Buckler 2004.Wyatt CN, Buckler KJ. The effect of mitochondrial inhibitors on membrane currents in isolated neonatal rat carotid body type I cells. J Physiol 556: 175–191, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto et al. 2003.Yamamoto Y, Henrich M, Snipes RL, Kummer W. Altered production of nitric oxide and reactive oxygen species in rat nodose ganglion neurons during acute hypoxia. Brain Res 961: 1–9, 2003. [DOI] [PubMed] [Google Scholar]
- Yao and Haddad 2004.Yao H, Haddad GG. Calcium and pH homeostasis in neurons during hypoxia and ischemia. Cell Calcium 36: 247–255, 2004. [DOI] [PubMed] [Google Scholar]
- Yuan 2001.Yuan JX Oxygen-sensitive K+ channel(s): where and what? Am J Physiol Lung Cell Mol Physiol 281: L1345–1349, 2001. [DOI] [PubMed] [Google Scholar]
- Zhang et al. 2001.Zhang J, Ugurbil K, From AH, Bache RJ. Myocardial oxygenation and high-energy phosphate levels during graded coronary hypoperfusion. Am J Physiol Heart Circ Physiol 280: H318–326, 2001. [DOI] [PubMed] [Google Scholar]
- Zoccarato and Nicholls 1982.Zoccarato F, Nicholls D. The role of phosphate in the regulation of the independent calcium-efflux pathway of liver mitochondria. Eur J Biochem 127: 333–338, 1982. [DOI] [PubMed] [Google Scholar]