Abstract
Previous findings indicate that synaptic facilitation, a cellular mechanism underlying sensitization of the siphon withdrawal response (SWR) in Aplysia, depends on a cascade of postsynaptic events, including activation of inositol triphosphate (IP3) receptors and release of Ca2+ from postsynaptic intracellular stores. These findings suggest that phospholipase C (PLC), the enzyme that catalyzes IP3 formation, may play an important role in postsynaptic signaling during facilitation and learning in Aplysia. Using the PLC inhibitor U73122, we found that PLC activity is required for synaptic facilitation following a 10-min treatment with 5-HT, as measured at 20 min after 5-HT washout. Prior work has indicated that facilitation at this time is supported primarily by postsynaptic processes. To determine whether postsynaptic PLC activity is involved in 5-HT–mediated facilitatory actions, we examined the effect of U73122 on enhancement of the response of motor neurons isolated in cell culture to glutamate, the sensory neuron transmitter. A 10-min application of 5-HT induced persistent (>40 min) enhancement of glutamate-evoked potentials (Glu-EPs) recorded from isolated motor neurons, and this enhancement was blocked by U73122. Finally, we showed that injecting U73122 into intact animals before behavioral training impaired intermediate-term sensitization, indicating that PLC activity contributes to this form of nonassociative learning.
INTRODUCTION
The siphon withdrawal reflex (SWR) of the marine snail Aplysia provides a valuable model system for neurobiological studies of learning and memory (Glanzman 2007; Kandel 2001). In particular, considerable progress has been made in understanding the cellular basis of sensitization, the nonassociative enhancement of the SWR that results from noxious stimulation of the animal's tail (for review, see Glanzman 2006; Hawkins et al. 2006). It is now firmly established that sensitization training induces facilitation of monosynaptic sensorimotor connections within the circuits mediating the SWR reflex (Antonov et al. 1999; Cleary et al. 1998). Facilitation of these synapses occurs through the action of 5-HT, which is the modulatory neurotransmitter released from interneurons activated by sensitizing stimuli (Glanzman et al. 1989; Mackey et al. 1989; Marinesco and Carew 2002; Marinesco et al. 2004). Serotonin liberated in this way produces well-defined presynaptic changes, including increased excitability of sensory neurons and enhanced release of transmitter at sensorimotor synapses (for review, see Byrne and Kandel 1996). However, it is now clear that 5-HT also recruits distinct signaling events in the postsynaptic motor neuron. These learning-related processes have been shown to require an elevation of postsynaptic intracellular Ca2+ and culminate in the functional up-regulation of α-amino-3-hydroxy-5-methylisoxazole-4-propoinoic acid receptor (AMPA)-type glutamate receptors, possibly via the synthesis and subsequent insertion of new receptor subunits into the postsynaptic membrane (for review, see Glanzman 2007). The requirement for postsynaptic modifications during actual learning has also been studied; dishabituation, a form of nonassociative learning related to sensitization (Thompson and Spencer 1966), depends critically on postsynaptic vesicle exocytosis, as shown by the sensitivity of this form of learning to the injection of botulinum toxin into siphon motor neurons (Li et al. 2005). The Ca2+-dependent signaling pathways recruited during the induction of facilitation have been examined in experiments on neurons in dissociated cell culture. Serotonin-dependent enhancement of the response of siphon motor neurons to glutamate, the sensorimotor transmitter (Dale and Kandel 1993; Levenson et al. 2000b) (but see Trudeau and Castellucci 1993), depends on G protein activation (Chitwood et al. 2001), whereas facilitation of the sensorimotor synapse requires postsynaptic activation of inositol triphosphate (IP3) receptors (Li et al. 2005). Taken together, the data from both isolated motor neurons in culture and sensorimotor cocultures suggests a scenario in which G protein–coupled 5-HT receptor activation stimulates the generation of the second messenger IP3 and subsequent Ca2+ release from IP3 receptor–mediated stores via the activation of phospholipase C (PLC).
Several reports have established a role for G protein–activated PLC in the cellular processes regulating learning and memory (Buckley and Caldwell 2004; Nicolle et al. 1999; Sallés et al. 2001). Moreover, activation of the G protein–activated beta subtype of PLC (PLCβ) is necessary for several types of learning-related synaptic plasticity. For example, inhibitory long-term potentiation (LTP) in the visual cortex involves the recruitment of PLCβ via the coordinated activation of GABAB and 5-HT2 receptors (Komatsu 1996), and long-term depression (LTD) in the visual cortex is impaired in transgenic mice lacking the PLCβ gene (Choi et al. 2005). In Aplysia, the long-term (24-h) enhancement of sensorimotor synapses and accompanying increase in sensory neuron varicosities require the activation of PLC in the sensory neuron (Udo et al. 2005). In this situation, PLC is believed to be upstream of the Rho GTPase cdc42, a signaling molecular that plays a key role in regulation of the cytoskeleton. Despite these findings, a number of questions regarding the role of PLC during the induction of synaptic plasticity in Aplysia remain unexplored. What role does PLC play in organizing the signaling required for the induction of earlier forms of facilitation (<1 h)? Is—as suggested by the data from Li et al. (2005)—postsynaptic PLC activation necessary for the induction of facilitation? If so, to what extent does postsynaptic PLC activity contribute to sensitization memory?
Here we describe experiments studying the function of PLC activation during the induction of synaptic facilitation and sensitization in Aplysia. We find that inhibition of PLC activity impairs the induction of facilitation in sensorimotor synapses at a time where facilitation is significantly supported by postsynaptic processes (Li et al. 2005). To determine whether facilitation depends critically on PLC activation in the motor neuron, we examined the effect of PLC inhibition on 5-HT–dependent enhancement of the glutamate response in isolated motor neurons. These experiments showed that the sustained increase in the glutamatergic response following a 10-min application of 5-HT was completely abolished by blockade of PLC activity. Finally, we showed that pharmacological inhibition of PLC before training reduces intermediate-term sensitization of the SWR; this result supports the notion that PLC-dependent signaling, occurring in postsynaptic motor neurons, contributes to persistent behavioral modification of the SWR.
METHODS
Animals
Adult Aplysia californica were obtained from a local supplier (Alacrity Marine Biological, Redondo Beach, CA). Animals were housed in a 50-gal aquarium filled with cooled (12–14°C), aerated seawater (Catalina Water Company, Long Beach, CA).
Cell cultures
Sensorimotor cocultures were fabricated as described by Lin and Glanzman (1994). Briefly, single small siphon (LFS) motor neurons and single presynaptic pleural sensory neurons were individually dissociated from the CNS of Aplysia (60–100 g) before being arranged in pairs in poly-l-lysine coated culture dishes. Cultures were maintained at 18°C for 3–4 days before the start of the experiments. The culture medium contained 50% Aplysia hemolyph and 50% Leibowitz-15 (L-15; Sigma, St. Louis, MO). The cell cultures used in the glutamate puff experiments consisted solely of isolated small siphon (LFS) motor neurons (Chitwood et al. 2001).
Electrophysiology
The electrophysiological methods used in the experiments on sensorimotor cocultures were as described previously (Li et al. 2005). All electrophysiological tests were performed using custom protocols programmed into Axograph version 9 (Molecular Devices, Union City, CA). Because a computer executed these protocols automatically, the experiments were not performed blind. During electrophysiological recording, cultures were perfused with perfusion medium consisting of 50% sterile artificial seawater (ASW) and 50% L-15. All experiments were performed at room temperature. The presynaptic sensory neuron and postsynaptic motor neuron in each coculture were impaled with sharp microelectrodes (20–30 MΩ). To prevent spontaneous firing of the motor neurons during testing and to minimize stimulus-evoked firing, the motor neuron was held at –80 mV throughout the experiment by passing negative current (typical range, 0.3–0.8 nA) into the cell via the bridge circuit of the microelectrode amplifier. The threshold for eliciting an action potential in the presynaptic sensory neuron was determined 5 min after impaling the neuron by injection of brief (20 ms) depolarizing current pulses, starting at 0.1 nA, and increasing sequentially in steps of 0.1 nA, until a spike was generated in the presynaptic cell. The amount of current required to activate an action potential in the sensory neuron ranged from 0.4 to 1.2 nA. This value was kept constant in the majority of experiments; however, in 33% of experiments, it was necessary to adjust this value between 0.1 and 0.2 nA. The size of the evoked excitatory postsynaptic potential (EPSP) recorded from the postsynaptic motor neuron was also noted. Synaptic responses were determined again 30 min after the initial test, with the resulting EPSP data serving as the pretest values for the ongoing experiment. Cocultures that exhibited >50% depression between the first and second EPSP tests were excluded from the experiment. The input resistances of the sensory and motor neurons were monitored throughout the experiment by injecting 300-ms pulses of negative current (0.1 nA) before each test. Cocultures that exhibited a drop in input resistance by >50% in either cell were excluded from further analysis. Only one coclulture was thereby excluded. A 10-min pulse of 5-HT (20 μM) was applied immediately after the pretest. The synaptic response was retested 20 min after the end of the 5-HT application. The PLC inhibitor U73122 (500 nM in 0.025% DMSO; Sigma and Tocris Bioscience) was applied continuously in the perfusion medium starting 20 min before the pretest and continuing until the end of the 5-HT pulse (30-min total application). To control for the effect of 5-HT and to assess baseline synaptic responses, we performed “test alone” experiments in which synapses were stimulated using the same time intervals described above but in the absence of 5-HT. The effect of U73122 on basal synaptic responses was evaluated in test alone experiments. Application of the drug in these experiments was timed as in the experiments with 5-HT. Input resistance in both sensory and motor neurons tended to increase slightly (<30%) through the course of the experiment. Statistical analysis showed that there were no significant differences among input resistance values in the sensory and motor neurons in the four experimental groups (5-HT vehicle, 5-HT U73122, test alone vehicle, and test alone U73122; data not shown).
For experiments on isolated motor neurons, the soma was impaled with a sharp microelectrode (20–30 MΩ) and held at –80 to –85 mV via negative current injection (–0.2 to –0.6 nA) for the duration of the experiment. Motor neurons were stimulated by brief pulses (puffs) of glutamate (final concentration of 2 mM) pressure ejected (10–20 ms, 1–5 psi) using a Picospritzer (General Valve, Fairfield, NJ); the glutamate puffs were directed toward either the soma or initial segment of the major neurite. Glutamate in the ejected puff was rapidly removed from the motor neuron via a perfusion pipette placed in close proximity to the neuron. This arrangement produced a glutamate-evoked potential (Glu-EP) with rapid kinetics resembling those of synaptically evoked EPSPs. Glutamate was dissolved in perfusion medium containing Fast Green (0.02%), which permitted the puffs to be visualized. Puff duration and location were initially adjusted to produce Glu-EPs of 10–15 mV. Thereafter, these parameters were kept constant for the duration of the experiment. Motor neurons received glutamate puffs once every 10 s throughout the duration of the experiment. The input resistance of the motor neuron was monitored throughout the experiment by injecting 300-ms pulses of negative current (0.1 nA) before each glutamate puff. Motor neurons that exhibited a drop in input resistance by >50% were excluded from further analysis. Three motor neurons treated with 5-HT U73122 and two motor neurons treated with 5-HT plus the vehicle were thereby excluded. No neurons were excluded in either of the test alone groups. Experiments began with a 20-min baseline period during which time the amplitude of the Glu-EP was monitored to confirm that the response was stable. Following 20 min of stable recording, 5-HT (20 μM) was applied to the culture dish via the perfusion medium for 10 min, after which the 5-HT pulse was terminated by perfusion with regular recording medium. The Glu-EP response was recorded for a further 40 min. Application of U73122 (400 nM in 0.02% DMSO) started 10 min before the start of the 5-HT pulse and continued until the washout period began. Input resistance increased slightly in motor neurons treated with U73122 (12.87 ± 5.68% for 5-HT U73122 cells and 3.79 ± 3.62% for test alone-U73122 cells vs. −3.74 ± 3.44% for 5-HT-vehicle cells and −11.57 ± 10.5% for test alone-vehicle cells). However, a statistical comparison of these data did not show significant between-group differences (data not shown). In experiments using the putative PLC activator m-3m3FBS (Calbiochem, EMD Biosciences, San Diego, CA), the Glu-EP was recorded for 20 min after the end of the 5-HT application. In these experiments, a 10-min application of m-3m3FBS (1 μM in 0.01% DMSO) was followed by a 5-min application of 5-HT (20 μM). Thus, m-3m3FBS was applied 10 min into the baseline recording session and was applied for a total of 15 min.
Behavioral experiments
Sensitization experiments were performed on adult Aplysia housed individually in custom-built Plexiglass chambers continuously perfused with cooled (14°C) seawater. One day before training, each animal was weighed and implanted bilaterally with Teflon-coated platinum wires (0.008-in coated diameter, A-M Systems, Carlsborg, WA). For this procedure, the animal was anesthetized by cooling in cold water (4°C) for 13 min. Wires, prepared by removing the Teflon from the ends with forceps, were threaded through a 20-gauge needle, which was used to insert the wire into the animal's tail. Following this procedure, the animal was placed into the training chamber, where it was given ≥24 h to recover and acclimate to the chamber. The SWR was tested as follows: The siphon was lightly stimulated with a soft, flexible probe (broom bristle), and the duration of the ensuing SWR was timed. Timing of the SWR began once the siphon had retracted completely within the parapodia and ended the moment the siphon became visible again. Responses were given a score of 1.0 s if the siphon did not withdraw completely inside the parapodia. Three pretests were delivered once every 10 min beginning 50 min before the start of training. The experimenter conducting these tests was unaware of the experimental treatments each animal had undergone. Pilot experiments indicated that injection of DMSO at levels >1% produced nonspecific effects on sensitization learning (data not shown). For this reason, and because of the relatively poor solubility of U73122, we were limited to a maximum injected concentration of 20 μM U73122. In our experience (see also Levenson et al. 2000a), the effective concentrations for drugs injected into whole Aplysia are typically within the high micromolar to low millimolar range. Thus, to maximize administration of the drug at low micromolar concentrations, animals were given two injections of drug (final U73122 concentration 20 μM in 1% DMSO in ASW) or vehicle (1% DMSO in ASW). Injections were made at 25 and 5 min before the first training trial. Intrahemocoel injections (1 ml/100 g) were made into the animal's neck. (This site was chosen because it was found to cause the least disturbance to the animal.) Sensitization training consisted of three bouts of electrical shocks at 20-min intervals. During each bout of training, the animal received three trains of shocks spaced once every 2 s. Each train was 1 s in duration and consisted of shocks (10-ms pulse duration, 40 Hz, 120 V) delivered to the animal's tail via a Grass stimulator (S88, Astro-Med, West Warwick, RI) connected to the platinum wires. The SWR was tested 30, 60, 90, and 120 min after the end of the last trial. Repeated testing of the SWR under these conditions did not result in any appreciable habituation of the response.
Statistical analyses
All statistical tests were computed using Prism 4.0 for Macintosh (Graphpad Software, El Camino Real, CA). For electrophysiological experiments on sensorimotor cocultures, the peak amplitude of the evoked EPSP recorded at the posttest was normalized to the amplitude of the pretest EPSP and expressed as the percent mean ± SE. For experiments on isolated motor neurons, the peak amplitude of each Glu-EP was measured and normalized to the mean amplitude of the 60 Glu-EPs observed during the first 10 min of baseline recording. Normalized Glu-EPs were expressed as the percent means ± SE. Frequency histograms of data sets were prepared to determine whether the data exhibited a normal distribution. Because all of the electrophysiological data sets were found to exhibit a normal distribution, the between-group comparisons for these data were analyzed using parametric t-tests. The behavioral data did not exhibit a normal distribution; we therefore used nonparametric statistics to analyze these data. Behavioral change was assessed as follows: First, mean pretest responses were determined for each animal. Next the posttest data obtained for each time point—30, 60, 90, and 120 min—were corrected by subtracting the appropriate mean pretest value. The corrected posttest scores were averaged for each animal, producing a single mean posttest score. Finally, the mean posttest scores were compared using a Mann-Whitney U test. Data from experiments in which the animals received only the test stimuli (test alone experiments) were analyzed the same way as those in which the animals received training with electrical shocks.
RESULTS
Disruption of PLC activity impairs 5-HT–dependent facilitation of sensorimotor synapses in culture
To examine the role of PLC activation in synaptic facilitation, we studied the effect of U73122, a specific inhibitor of G protein–mediated PLC activity (Yule and Williams 1992) on facilitation induced by a brief (10-min) application of 5-HT. The experiments were performed using sensorimotor cocultures (Lin and Glanzman 1994). The cocultures comprised a single sensory neuron monosynaptically connected to a single small siphon (LFS-type) motor neuron (Frost et al. 1988). All cultures were 3–5 days old at the time of experiments.
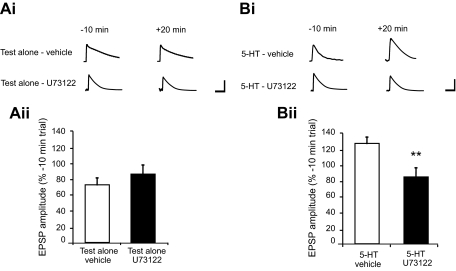
Previous work by Li et al. (2005) has shown that, following a 10-min application of 5-HT, the balance between pre- and postsynaptic processes changes over time, with postsynaptic processes becoming increasingly prominent in facilitation after washout of the drug. We wished to examine the potential connection between PLC activation and IP3 receptor-dependent facilitation in the motor neuron. Accordingly, we examined facilitation 20 min after termination of the 5-HT pulse, a time at which postsynaptic mechanisms seem to predominate in facilitation (Li et al. 2005). An initial experiment was performed to examine the affect of U73122 on basal synaptic responses (Fig. 1 Ai). In this experiment, we compared one group of cocultures (test alone-vehicle) treated with the vehicle solution (0.025% DMSO) for 30 min with a second group of cocultures (test alone-U73122) treated with U73122 (500 nM in vehicle) for 30 min. A t-test conducted on the mean normalized EPSP values recorded 20 min after washout of either the U73122 or vehicle did not show a significant difference (t = 0.95, df = 6, P = 0.38; Fig. 1Aii); therefore application of the PLC inhibitor U73122 for 30 min did not produce a deleterious effect on basal synaptic transmission. We next examined the effect of U73122 on 5-HT–induced facilitation. In this experiment, synaptic responses in one group of cocultures (5-HT-vehicle) treated with the vehicle solution (0.025% DMSO) for 20 min followed by 5-HT (20 μM) in the vehicle for 10 min were compared with responses recorded from a second group (5-HT-U73122) treated with U73122 (500 nM) for 20 min followed by 5-HT plus U73122 for 10 min (Fig. 1Bi). Comparison of the mean normalized EPSP values determined 20 min after 5-HT washout in these groups showed a significant difference (t = 3.22, df = 12, P < 0.01; Fig. 1Bii). Taken together, these data show that synaptic responses in the 5-HT-vehicle group were significantly stronger than those in the 5-HT-U73122 group, indicating that synaptic facilitation was prevented by blockade of PLC activity.
FIG. 1.
Bath application of U73122, a specific inhibitor of phospholipase C (PLC) activity, disrupts 5-HT–dependent facilitation of the sensorimotor synapse in culture. Ai: sample excitatory postsynaptic potentials (EPSPs) recorded from siphon motor neurons for each of the test alone groups. Times shown are relative to the end of the application of either drug or vehicle, i.e., 10 min before and 20 min after washout. Calibration bars represent 10 mV and 50 ms. Aii: mean normalized group data for the 2 test alone groups: synapses treated with DMSO solution (test alone-vehicle, n = 4) and synapses treated with U73122 (test alone-U73122, n = 4). The mean EPSP amplitudes in the test alone-vehicle and test alone-U73122 were not significantly different (73.8 ± 8.8 vs. 85.1 ± 11.77%). Bi: sample EPSPs recorded from siphon motor neurons in the 2 experimental groups. Times shown are relative to the end of the application of either drug or vehicle, i.e., 10 min before and 20 min after washout. Calibration bars represent 10 mV and 50 ms. Bii: mean normalized group data for the 2 experimental groups of synapses: those treated with 5-HT in the absence of U73122 (5-HT-vehicle, n = 7) and those treated with 5-HT in the presence of U73122 (5-HT-U73122, n = 7). Synaptic responses in the 5-HT–treated synapses were significantly larger than responses in the 5-HT U73122–treated synapses (127.4 ± 5.8 vs. 87.72 ± 11.7%), indicating that inhibition of PLC disrupted synaptic facilitation. **Significance at P < 0.001. Data are expressed as means ± SE.
5-HT–dependent enhancement of the glutamate response of isolated motor neurons depends on activation of PLC
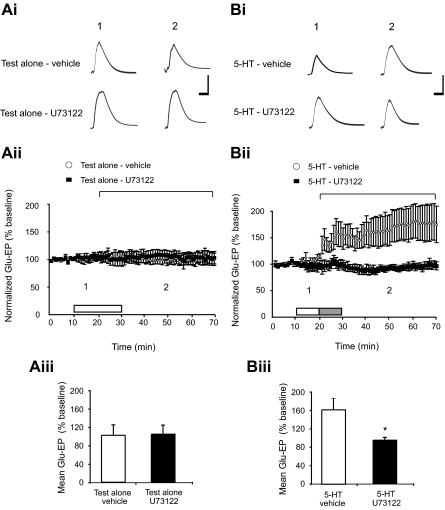
The above data provide strong evidence that PLC activation is required for the induction of synaptic facilitation in Aplysia. However, the data do not allow us to determine whether the necessary PLC-dependent pathways reside in the sensory neuron or motor neuron because the drug was applied in the perfusion medium and thus could potentially have affected PLC activity in both pre- and postsynaptic cells. Currently, cell membrane impermeant PLC inhibitors are unavailable; therefore it is not possible to examine this idea directly in sensorimotor cocultures. To address the question of whether synaptic facilitation in Aplysia requires the activation of a PLC-dependent pathway within the motor neuron, we turned to experiments on isolated motor neurons. Application of 5-HT for 10 min to isolated siphon motor neurons in dissociated cell culture produces an enhancement of the response to glutamate that persists for >50 min (Chitwood et al. 2001; Villareal et al. 2007), and, like facilitation of the sensorimotor synapse (Li et al. 2005), requires an increase in intracellular Ca2+ within the motor neuron (Chitwood et al. 2001). The single motor neuron cell culture system therefore provides a valuable tool for isolating the postsynaptic mechanisms that regulate plasticity of the Aplysia sensorimotor synapse. Accordingly, we examined the effect of U73122 on 5-HT–induced enhancement of the Glu-EP in isolated motor neurons. An initial experiment was conducted to determine if U73122 produced a deleterious effect on the basal Glu-EP response of the isolated motor neuron. Two groups of motor neurons were examined in this experiment (Fig. 2 Ai). The first group (test alone-vehicle) received the vehicle solution (0.02% DMSO) for 20 min, whereas the second group (test alone-U73122) received U73122 (400 nM) in the vehicle for 20 min. Glu-EP responses in the test alone-U73122 group remained stable throughout the duration of the experiment (Fig. 2Aii). Comparison of the averaged postbaseline responses in the test alone-vehicle and test alone-U73122 groups did not show a significant difference (t = 0.14, df = 10, P = 0.89), indicating that application of the PLC inhibitor for 20 min did not produce a measurable effect on the Glu-EP response (Fig. 2Aiii). We next examined the effect of U73122 on 5-HT–induced enhancement of the Glu-EP. This experiment involved two groups of motor neurons (Fig. 2Bi). The first group (5-HT-vehicle) was subjected to an application of vehicle (0.02% DMSO, 10 min) followed by 5-HT plus vehicle (20 μM 5-HT, 10 min), whereas the second group (5-HT-U73122) received treatment with U73122 (400 nM, 10 min) in the vehicle followed by 5-HT plus U73122 in vehicle (10 min). Application of 5-HT produced a sustained increase in the amplitude of the Glu-EP that was not observed in the U73122-treated group (Fig. 2Bii). A comparison of the averaged postbaseline responses in the two groups showed a significant difference (t = 2.38, df = 9, P < 0.05), indicating that the mean Glu-EP response in the 5-HT-vehicle group was significantly stronger than the mean response recorded in the 5-HT-U73122 group (Fig. 2Biii). Overall, these data show that stimulation with 5-HT for 10 min recruits a PLC-dependent pathway within the motor neuron that contributes to the persistent enhancement of the neuron's response to glutamate.
FIG. 2.
5-HT–induced facilitation in the isolated siphon motor neuron depends on PLC activation. Ai: sample glutamate-evoked potential (Glu-EP) records for 2 time points from each test alone experiment. The numbers above each trace correspond to the time points indicated in the graph in Aii. Calibration bars represent 10 mV and 50 ms. Aii: summary data for the 2 test alone groups: motor neurons treated only with the vehicle (test alone-vehicle, n = 8), and motor neurons exposed to U73122 in the vehicle (test alone-U73122, n = 4). Each data point represents the normalized mean of 6 Glu-EPs. The horizontal bracket identifies the portion of the data summarized in the histogram in Aiii. The white bar indicates an application of either U73122 in the vehicle or the vehicle alone. Aiii: histogram showing the mean Glu-EP values recorded in the test alone experiments. There was no significant difference between the mean responses in test alone-vehicle and test alone-U73122 groups recorded during the 50-min period starting 10 min into the application of the drug and ending 40 min after washout (101.9 ± 22.85 vs. 103.7 ± 19.72%). Bi: sample Glu-EP records for 2 time points in the 2 5-HT–treated groups. The numbers above each trace correspond to the time points indicated in the graph in Bii. Calibration bars represent 10 mV and 50 ms. Bii: summary data for the 2 5-HT–treated groups: motor neurons treated with 5-HT in DMSO (5-HT-vehicle, n = 6) and motor neurons given a 10-min pretreatment with U73122 in the vehicle followed by 5-HT plus U73122 (5-HT-U73122, n = 5). Each data point represents the normalized mean of 6 Glu-EPs. The white bar indicates an application of either U73122 in the vehicle or the vehicle alone. The gray bar indicates an application of 5-HT plus either U73122 or the vehicle. The horizontal bracket identifies the portion of the data displayed in Biii. Biii: histogram displaying the mean Glu-EP data recorded during the 50-min period from the start of 5-HT application through the subsequent 40-min washout period. The average Glu-EP values in the 5-HT-vehicle group were significantly larger than those in the 5-HT-U73122 group (161.6 ± 25.18 vs. 94.2 ± 6.22%). *Significance at P < 0.05. Data are expressed as means ± SE.
As a further test for the role of PLC signaling in the postsynaptic motor neuron, we examined the effect of the PLC activator m-3m3FBS (Bae et al. 2003) on the glutamatergic response of isolated motor neurons. Using human lymphoma cells, Bae et al. (2003) reported significant PLC activation with m-3m3FBS at 25–50 μM. However, at this concentration, we observed deleterious effects on both the input resistance and the Glu-EP (data not shown). At lower concentrations (1–2 μM) we observed a slight depression in the Glu-EP response that persisted after washout of the drug (data not shown). Although low concentrations of m-3m3FBS alone did not induce prolonged enhancement of the Glu-EP, we found that a 10-min pretreatment with m-3m3FBS (1 μM), when followed by a brief (5 min) application of 5-HT, led to enhancement of the Glu-EP that persisted for significantly longer (>20 min) than in a control group of motor neurons treated with 5 min of 5-HT alone (t = 3.39, df = 8, P < 0.01; data not shown). Interpretation of these data is complicated by the fact that the specificity of m-3m3FBS as a stimulator of PLC has been questioned (Krjukova et al. 2004). In particular, this compound may stimulate the release of Ca2+ from intracellular stores in a PLC-independent fashion. Given that facilitation requires the release of Ca2+ from postsynaptic stores (Li et al. 2005), the application of m-3m3FBS might, if it acted to increase Ca2+ release in the motor neuron, lead to enhanced facilitation following brief applications of 5-HT. Consequently, whether the enhancement in response observed in this experiment is caused by effects on PLC activation is unclear at present.
Behavioral sensitization memory requires the activation of PLC
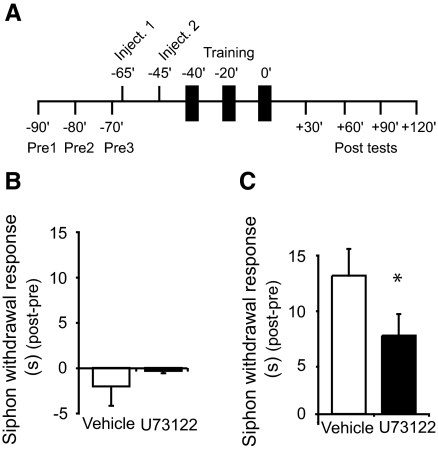
Sensitization of the SWR is mediated, in part, by facilitation of the monosynaptic connections between central sensory and motor neurons (Antonov et al. 1999; Cleary et al. 1998). Consequently, one would expect intracellular signaling pathways, such as G protein–mediated PLC activation, that play crucial roles in synaptic facilitation to also be important in sensitization. Accordingly, we examined the necessity of PLC activity for behavioral modification of the SWR by injecting U73122 into animals before training. We first sought to explore the effect of U73122 injection on basal SWR responses. In this experiment, the SWR was monitored in two groups of animals: one that received injections of vehicle solution (1% DMSO in ASW) and another that received injections of U73122 (20 μM) in the vehicle. Analysis of the mean corrected posttest values from these two groups indicated that the mean group responses did not differ (u = 29, P > 0.80; Fig. 3 B). Thus, injection of the PLC inhibitor did not produce a deleterious effect on the SWR. Having confirmed that U73122 did not affect the normal expression of the SWR, we next examined the effect of this drug on sensitization learning. Two groups of animals were examined in this study: the first group received injections of vehicle solution (1% DMSO in ASW) before training, whereas the second received pretraining injections of U73122 (20 μM) in vehicle (Fig. 3A). Injection of U73122 disrupted sensitization learning (Fig. 3C). Comparison of the averaged corrected posttest (post-pre) responses for the two groups showed that the SWR in the U73122-injected animals was significantly weaker than in the vehicle-injected controls (u = 38, P < 0.05).
FIG. 3.
Intermediate-term sensitization memory requires PLC activity. A: schematic showing the procedures used for the behavioral experiments. The timing of the pretests, drug/vehicle injections, training, and posttests are shown relative to the end of the last training session. Vertical black bars represent bouts of sensitization training. An identical protocol was used for the test alone experiments (summarized in B), except that the sensitization training was omitted. B: data from behavioral experiments in which sensitization training was withheld. Mean corrected siphon withdrawal response (SWR; posttest –pretest) for the 4 tests taken after injection of either vehicle (test alone-vehicle, n = 4) or U73122 (test alone-U73122, n = 4). The withdrawal responses did not differ between vehicle- and U73122-injected groups (–2.06 ± 2.12 vs. –0.35 ± 0.27 s). C: graph presenting the mean corrected posttest data for animals that received injections of either the vehicle (n = 12) or U73122 (n = 14) before sensitization training. A statistical comparison of the mean corrected SWR, averaged across the 4 posttests, showed that responses in the vehicle-injected group were of a significantly longer duration than those observed in the U73122-injected group (12.44 ± 2.4 vs. 7.05 ± 1.94 s). *Significance at P < 0.05. Data are expressed as the mean corrected siphon withdrawal duration (s) ±SE.
DISCUSSION
Potential role of postsynaptic PLC activity in learning-related synaptic facilitation in Aplysia
Our data provide evidence that postsynaptic PLC signaling contributes to synaptic facilitation in Aplysia. Although in our experiments on in vitro sensorimotor synapses, the PLC inhibitor U73122 was bath-applied and therefore might have disrupted presynaptic PLC activity, rather than or in addition to, postsynaptic PLC activity, our experiments on isolated motor neurons in dissociated cell culture provide strong support for the notion that at least part of the effect of U73122 in our synaptic experiments was caused by the drug's disruption of postsynaptic PLC activity. Previous evidence from our laboratory (Chitwood et al. 2001;Li et al. 2005; Villareal et al. 2007) indicates that a 10-min application of 5-HT to isolated motor neurons recruits the postsynaptic component of intermediate-term facilitation (ITF) in Aplysia (see Sutton and Carew 2002). In this study, we observed that a 10-min pulse of 5-HT produced enhancement of the Glu-EP in isolated motor neurons that persisted for >40 min and that depended on PLC activity because it was blocked by U73122. These results, together with the previous work from our laboratory (Chitwood et al. 2001; Li et al. 2005; Villareal et al. 2007), support the notion that postsynaptic PLC activity is critical for ITF. Note that a defining property of ITF is that it persists for 30 min to 3 h (Sutton and Carew 2002). Admittedly, in our experiments on in vitro sensorimotor synapses, we tested facilitation only at 20 min after the end of a 10-min pulse of 5-HT; however, we have previously shown that such a treatment induces synaptic facilitation that persists for >40 min (Li et al. 2005) and therefore satisfies the temporal criterion for ITF.
Activation of PLC catalyzes the generation of the second messengers IP3 and diacylglycerol (DAG) through the hydrolysis of membrane-bound inositol precursors (Katan 2005). Both of these signaling molecules may contribute to postsynaptic induction of facilitation via distinct pathways. First, IP3, generated through the activation of PLC, could contribute to the induction of facilitation through its action as a stimulator of Ca2+ release from IP3 receptor-operated stores (Berridge 1993). In fact, IP3 receptor-dependent Ca2+ release in the motor neuron plays an essential role in the induction of ITF of the sensorimotor synapse (Li et al. 2005). Accordingly, the data presented here are consistent with a scheme in which G protein–activated PLCβ-type enzymes (Katan 2005), stimulated by the activation of postsynaptic 5-HT receptors, generate an IP3-dependent Ca2+ signal; this signal, in turn, leads to the recruitment of postsynaptic signaling pathways necessary for the expression of facilitation. Additional support for this idea is provided by experiments that show an increase in IP3 production in the motor neuron during a 10-min application of 5-HT (Jin et al. 2007). Taken together, these data provide strong evidence that 5-HT–induced facilitation requires the postsynaptic activation of a PLC-IP3 signaling system and that this IP3-dependent pathway leads to the functional up-regulation of AMPA-type glutamate receptors (Chitwood et al. 2001; Li et al. 2005). Second, PLC-stimulated DAG would be expected to activate the typical isoforms of protein kinase C (PKC), which have been implicated in several forms of synaptic plasticity in Aplysia (for review, see Sossin 2007). Contrary to this expectation, however, a recent study by Zhao et al. (2006)—which examined the translocation from the cytoplasm to the cell membrane of fluorescently tagged Aplysia PKC in both sensory and motor neurons of sensorimotor cocultures—found that a brief, 5-min application of 5-HT did not activate postsynaptic PKC. However, Zhao et al. (2006) did not test whether postsynaptic PKC activation occurs following a longer (10 min) application of 5-HT, such as that used in our experiments. Our laboratory has found that bath application of either bisindolylmaleimide (Bis I), a specific inhibitor of typical PKCs, or chelerythrine, an inhibitor of both typical and atypical PKCs, disrupts induction of the enhancement of the glutamate response in isolated siphon motor neurons following a 10-min application of 5-HT (Villareal et al. 2006) (but see Hawkins et al. 2006).
Furthermore, we have recently identified a role for PKC during the expression of this form of plasticity. Chelerythrine, applied after the establishment of stable 5-HT–induced enhancement of the Glu-EP, rapidly reversed the enhancement; in contrast, application of Bis I did not reverse the effect of 5-HT on the Glu-EP (Villareal et al. 2006). Because chelerythrine is significantly more potent at inhibiting atypical PKCs than is Bis I (Laudanna et al. 1998; Ling et al. 2002; Martiny-Baron et al. 1993), these data point to a specific requirement for atypical PKC during the expression of 5-HT–dependent enhancement of the Glu-EP. PLC activity would therefore seem not to be needed for expression in this form of plasticity, because activation of the atypical PKCs occurs independently of the generation of DAG (Sossin 2007). Overall, the pharmacological data suggest that the PLC/DAG pathway in the motor neuron is recruited during the induction of 5-HT–dependent facilitation of the sensorimotor synapse but does not participate in the maintenance of facilitation. Interestingly, a study by Ghirardi et al. (1992) found that PKC activity was specifically required for facilitation of depressed synapses but not for facilitation of rested synapses. On first reading this would seem to suggest that the effect of PLC blockade in these experiments, which involved rested synapses, must not be mediated through disruption of the DAG-PKC pathway. However, Ghirardi et al. explicitly examined short-term facilitation (≤5 min) resulting from only a 1-min application of 5-HT. In contrast, these experiments studied ITF (at 20 min), which is produced by a 10-min treatment with 5-HT (Li et al. 2005). As discussed above, recent results from our laboratory indicate that the induction of ITF depends on postsynaptic PKC (Villareal et al. 2006). Taken together, these findings suggest that the effects of PLC inhibition observed in our synaptic experiments reflect an influence on both the IP3- and DAG-PKC–dependent component of the PLC signaling pathway.
Whereas these data indicate that postsynaptic PLC is required for ITF in Aplysia, other data support a role for presynaptic PLC in facilitation of the sensorimotor synapse. For example, PKC has been implicated in presynaptic processes, including spike broadening and mobilization of presynaptic vesicles, that contribute to short-term facilitation (STF) in Aplysia (for review, see Byrne and Kandel 1996). Furthermore, Zhao et al. (2006) have found that a 5-min pulse of 5-HT translocates Apl II, the Ca2+-independent (novel) isoform of PKC from the cytoplasm to the cell membrane of the sensory neuron, and that this effect can be reproduced by application of PDBu alone, a phorbolester known to activate DAG. These results implicate presynaptic PLC activity in STF. Other evidence indicates that presynaptic PLC activity is required for both long-term facilitation (LTF), as well as the increase in presynaptic varicosities that accompanies LTF (Udo et al. 2005; also see Glanzman et al. 1990). Similarly, recent experiments have provided evidence that presynaptic PKC activity, presumably downstream from PLC activation, is important for ITF of the sensorimotor synapse caused by a 10-min application of 5-HT (for review, see Hawkins et al. 2006) such as was used in this study (see also Sutton and Carew 2000). In summary, there is significant evidence for roles for presynaptic PLC activity in various forms of synaptic facilitation in Aplysia, in addition to the role for postsynaptic PLC activity indicated by these results.
Behavioral sensitization requires PLC activity
Sensitization training involving repeated spaced applications of tail shocks produces several distinct phases of memory that can be distinguished along temporal and pharmacological lines (Sutton et al. 2001). In these experiments, the SWR was sampled between 30 and 120 min after spaced training. During this time, the memory depends on new protein synthesis, but not transcription; it is therefore distinct from long-term memory for sensitization, which requires both protein and mRNA synthesis (Castellucci et al. 1989; Sutton et al. 2001) and from short-term memory, which requires neither (Sutton et al. 2001) (also see Montarolo et al. 1986). This form of sensitization memory, referred to as intermediate-term memory (ITM), has been reported to require protein kinase A (PKA) and calcium/calmodulin-dependent protein kinase II (CaMKII), but not PKC (for review, see Hawkins et al. 2006). However, the requirement for PLC activity in ITM, observed in our experiments, suggests that PKC, IP3, or both, contribute to the induction of ITM, contrary to a previous report (Antonov et al. 2005). It should be pointed out that another form of ITM has been described that does require persistent PKC activity. This form of ITM, “site-specific” ITM, is shown by testing the trained site on the tail following a single tail shock (Sutton et al. 2004). Site-specific ITM differs from repeated trial ITM in its lack of dependence on PKA and protein synthesis (also see Sutton and Carew 2000). Clearly, however, the type of ITM shown here is not site-specific ITM, because the test stimuli were applied to a site (the siphon) that was not trained. It is possible that methodological differences between our experiments and those of Antonov et al. (2005) can account for the apparent discrepancy regarding the possible involvement of PKC in ITM caused by spaced training. For example, we used fewer training trials (3 vs. 4), and sampled sensitization over a longer time period (30–120 min posttraining vs. 60 min). It is also possible that PKC was not one of the learning-related signaling pathways recruited downstream of PLC activation by the sensitization training in this study. However, the fact that cellular evidence from our laboratory implicates PKC in activity-independent ITF (Villareal et al. 2006) means that the question of whether or not activity-independent ITM involves (postsynaptic) PKC activity remains open. Finally, our behavioral results are consistent with previous data from cellular studies that implicate postsynaptic IP3 receptor activity in intermediate-term synaptic facilitation in Aplysia (Li et al. 2005).
In summary, we showed that the induction of synaptic facilitation in Aplysia depends on activation of PLC. We also showed that the sustained, 5-HT–dependent increase in the response of isolated motor neurons to glutamate, the neurotransmitter of Aplysia sensory neurons, similarly depends on PLC activity. Taken together, these data point to a key role for postsynaptic PLC in 5-HT–dependent facilitation of the sensorimotor synapse. Additionally, we found that intermediate-term sensitization of the SWR is reduced in animals following injection of an inhibitor of PLC, consistent with the involvement of PLC-dependent synaptic facilitation in this form of nonassociative learning.
GRANTS
This work was supported by National Institutes of Health Grants R37 NS-029563 and K02 MH-067062 to D. L. Glanzman.
Acknowledgments
We thank Drs. Greg Villareal and Adam Roberts for helpful comments on the manuscript.
Present address of D. Fulton: Semel Institute for Neuroscience and Human Behavior, David Geffen School of Medicine at UCLA, University of California, Los Angeles, CA 90095-1761.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- Antonov 1999.Antonov I, Kandel ER, Hawkins RD. The contribution of facilitation of monosynaptic PSPs to dishabituation and sensitization of the Aplysia siphon withdrawal reflex. J Neurosci 19: 10438–10450, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonov 2005.Antonov I, Kandel ER, Hawkins RD. Roles of PKA, PKC, and CaMKII in dishabituation and sensitization of the Aplysia siphon-withdrawal reflex. Soc Neurosci Abstr 540.6, 2005. [DOI] [PMC free article] [PubMed]
- Bae 2003.Bae YS, Lee TG, Park JC, Hur JH, Kim Y, Heo K, Kwak JY, Suh PG, Ryu SH. Identification of a compound that directly stimulates phospholipase C activity. Mol Pharmacol 63: 1043–1050, 2003. [DOI] [PubMed] [Google Scholar]
- Berridge 1993.Berridge MJ Inositol trisphosphate and calcium signalling. Nature 361: 315–325, 1993. [DOI] [PubMed] [Google Scholar]
- Buckley 2004.Buckley CT, Caldwell KK. Fear conditioning is associated with altered integration of PLC and ERK signaling in the hippocampus. Pharmacol Biochem Behav 79: 633–640, 2004. [DOI] [PubMed] [Google Scholar]
- Byrne 1996.Byrne JH, Kandel ER. Presynaptic facilitation revisited: state and time dependence. J Neurosci 16: 425–435, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellucci 1989.Castellucci VF, Blumenfeld H, Goelet P, Kandel ER. Inhibitor of protein synthesis blocks long-term behavioral sensitization in the isolated gill-withdrawal reflex of Aplysia. J Neurobiol 20: 1–9, 1989. [DOI] [PubMed] [Google Scholar]
- Chitwood 2001.Chitwood RA, Li Q, Glanzman DL. Serotonin facilitates AMPA-type responses in isolated siphon motor neurons of Aplysia in culture. J Physiol 534: 501–510, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi 2005.Choi S-Y, Chang J, Jiang B, Seol GH, Min SS, Han JS, Shin HS, Gallagher M, Kirkwood A. Multiple receptors coupled to phospholipase C gate long-term depression in visual cortex. J Neurosci 25: 11433–11443, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary 1998.Cleary LJ, Lee WL, Byrne JH. Cellular correlates of long-term sensitization in Aplysia. J Neurosci 18: 5988–5998, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale 1993.Dale N, Kandel ER. L-glutamate may be the fast excitatory transmitter of Aplysia sensory neurons. Proc Natl Acad Sci USA 90: 7163–7167, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost 1988.Frost WN, Clark GA, Kandel ER. Parallel processing of short-term memory for sensitization in Aplysia. J Neurobiol 19: 297–334, 1988. [DOI] [PubMed] [Google Scholar]
- Ghirardi 1992.Ghirardi M, Braha O, Hochner B, Montarolo PG, Kandel ER, Dale N. Roles of PKA and PKC in facilitation of evoked and spontaneous transmitter release at depressed and nondepressed synapses in Aplysia sensory neurons. Neuron 9: 479–489, 1992. [DOI] [PubMed] [Google Scholar]
- Glanzman 2006.Glanzman DL The cellular mechanisms of learning in Aplysia: of blind men and elephants. Biol Bull 210: 271–279, 2006. [DOI] [PubMed] [Google Scholar]
- Glanzman 2007.Glanzman DL Simple minds: the neurobiology of invertebrate learning and memory. In: Invertebrate Neurobiology, edited by North G, Greenspan RJ. New York: Cold Spring Harbor Laboratory Press, 2007, p. 347–380.
- Glanzman 1990.Glanzman DL, Kandel ER, Schacher S. Target-dependent structural changes accompanying long-term synaptic facilitation in Aplysia neurons. Science 249: 799–802, 1990. [DOI] [PubMed] [Google Scholar]
- Glanzman 1989.Glanzman DL, Mackey SL, Hawkins RD, Dyke AM, Lloyd PE, Kandel ER. Depletion of serotonin in the nervous system of Aplysia reduces the behavioral enhancement of gill withdrawal as well as the heterosynaptic facilitation produced by tail shock. J Neurosci 9: 4200–4213, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins 2006.Hawkins RD, Kandel ER, Bailey CH. Molecular mechanisms of memory storage in Aplysia. Biol Bull 210: 174–191, 2006. [DOI] [PubMed] [Google Scholar]
- Jin 2007.Jin I, Rayman JB, Puthanveettil S, Vishwasrao HD, Kandel ER. Spontaneous transmitter release from the presynaptic sensory neuron recruits IP3 production in the postsynaptic motor neuron during the induction of intermediate-term facilitation in Aplysia. Soc Neurosci Abstr 429.13, 2007. [Google Scholar]
- Kandel 2001.Kandel ER The molecular biology of memory storage: a dialogue between genes and synapses. Science 294: 1030–1038, 2001. [DOI] [PubMed] [Google Scholar]
- Katan 2005.Katan M New insights into the families of PLC enzymes: looking back and going forward. Biochem J 391: e7–e9, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu 1996.Komatsu Y GABAB receptors, monoamine receptors, and postsynaptic inositol trisphosphate-induced Ca2+ release are involved in the induction of long-term potentiation at visual cortical inhibitory synapses. J Neurosci 16: 6342–6352, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krjukova 2004.Krjukova J, Holmqvist T, Danis AS, Åkerman KEO, Kukkonen JP. Phospholipase C activator m-3m3FBS affects Ca2+ homeostasis independently of phospholipase C activation. Br J Pharmacol 143: 3–7, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laudanna 1998.Laudanna C, Mochly-Rosen D, Liron T, Constantin G, Butcher EC. Evidence of zeta protein kinase C involvement in polymorphonuclear neutrophil integrin- dependent adhesion and chemotaxis. J Biol Chem 273: 30306–30315, 1998. [DOI] [PubMed] [Google Scholar]
- Levenson 2000b.Levenson J, Endo S, Kategaya LS, Fernandez RI, Brabham DG, Chin J, Byrne JH, Eskin A. Long-term regulation of neuronal high-affinity glutamate and glutamine uptake in Aplysia. Proc Natl Acad Sci 97: 12858–12863, 2000a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levenson 2000b.Levenson J, Sherry DM, Dryer L, Chin J, Byrne JH, Eskin A. Localization of glutamate and glutamate transporters in the sensory neurons of Aplysia. J Comp Neurol 423: 121–131, 2000b. [DOI] [PubMed] [Google Scholar]
- Li 2005.Li Q, Roberts AC, Glanzman DL. Synaptic facilitation and behavioral dishabituation in Aplysia: dependence upon release of Ca2+ from postsynaptic intracellular stores, postsynaptic exocytosis and modulation of postsynaptic AMPA receptor efficacy. J Neurosci 25: 5623–5637, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin 1994.Lin XY, Glanzman DL. Long-term potentiation of Aplysia sensorimotor synapses in cell culture: regulation by postsynaptic voltage. Proc Biol Sci 255: 113–118, 1994. [DOI] [PubMed] [Google Scholar]
- Ling 2002.Ling DS, Benardo LS, Serrano PA, Blace N, Kelly MT, Crary JF, Sacktor TC. Protein kinase Mζ is necessary and sufficient for LTP maintenance. Nat Neurosci 5: 295–296, 2002. [DOI] [PubMed] [Google Scholar]
- Mackey 1989.Mackey SL, Kandel ER, Hawkins RD. Identified serotonergic neurons LCB1 and RCB1 in the cerebral ganglia of Aplysia produce presynaptic facilitation of siphon sensory neurons. J Neurosci 9: 4227–4235, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinesco 2002.Marinesco S, Carew TJ. Serotonin release evoked by tail nerve stimulation in the CNS of Aplysia: characterization and relationship to heterosynaptic plasticity. J Neurosci 22: 2299–2312, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinesco 2004.Marinesco S, Kolkman KE, Carew TJ. Serotonergic modulation in Aplysia. I. Distributed serotonergic network persistently activated by sensitizing stimuli. J Neurophysiol 92: 2468–2486, 2004. [DOI] [PubMed] [Google Scholar]
- Martiny-Baron 1993.Martiny-Baron G, Kazanietz MG, Mischak H, Blumberg PM, Kochs G, Hug H, Marme D, Schachtele C. Selective inhibition of protein kinase C isozymes by the indolocarbazole Go 6976. J Biol Chem 268: 9194–9197, 1993. [PubMed] [Google Scholar]
- Montarolo 1999.Montarolo PG, Goelet P, Castellucci VF, Morgan J, Kandel ER, Schacher S. A critical period for macromolecular synthesis in long-term heterosynaptic facilitation in Aplysia. Science 234: 1249–1254, 1986. [DOI] [PubMed] [Google Scholar]
- Nicolle 1999.Nicolle MM, Colombo PlJ, Gallagher M, McKinney M. Metabotropic glutamate receptor-mediated hippocampal phosphoinositide turnover is blunted in spatial learning-impaired aged rats. J Neurosci 19: 9604–9610, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallés 2001.Sallés J, López de Jesús M, Goñi O, Fernández-Teruel A, Driscoll P, Tobeña A, Escorihuela RM. Transmembrane signaling through phospholipase C in cortical and hippocampal membranes of psychogenetically selected rat lines. Psychopharmacology 154: 115–125, 2001. [DOI] [PubMed] [Google Scholar]
- Sossin 2007.Sossin WS Isoform specificity of protein kinase Cs in synaptic plasticity. Learn Mem 14: 236–246, 2007. [DOI] [PubMed] [Google Scholar]
- Sutton 2004.Sutton MA, Bagnall MW, Sharma SK, Shobe J, Carew TJ. Intermediate-term memory for site-specific sensitization in Aplysia is maintained by persistent activation of protein kinase C. J Neurosci 24: 3600–3609, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton 2000.Sutton MA, Carew TJ. Parallel molecular pathways mediate expression of distinct forms of intermediate-term facilitation at tail sensory-motor synapses in Aplysia. Neuron 26: 219–231, 2000. [DOI] [PubMed] [Google Scholar]
- Sutton 2002.Sutton MA, Carew TJ. Behavioral, cellular, and molecular analysis of memory in Aplysia. I. Intemediate-term memory. Integr Comp Biol 42: 725–735, 2002. [DOI] [PubMed] [Google Scholar]
- Sutton 1966.Sutton MA, Masters SE, Bagnall MW, Carew TJ. Molecular mechanisms underlying a unique intermediate phase of memory in Aplysia. Neuron 31: 143–154, 2001. [DOI] [PubMed] [Google Scholar]
- Thompson 1966.Thompson RF, Spencer WA. Habituation: a model phenomenon for the study of neuronal substrates of behavior. Psychol Rev 73: 16–43, 1966. [DOI] [PubMed] [Google Scholar]
- Trudeau 1993.Trudeau LE, Castellucci VF. Excitatory amino acid neurotransmission at sensory- motor and interneuronal synapses of Aplysia californica. J Neurophysiol 70: 1221–1230, 1993. [DOI] [PubMed] [Google Scholar]
- Udo 2005.Udo H, Jin I, Kim JH, Li HL, Youn T, Hawkins RD, Kandel ER, Bailey CH. Serotonin-induced regulation of the actin network for learning-related synaptic growth requires Cdc42, N-WASP, and PAK in Aplysia sensory neurons. Neuron 45: 887–901, 2005. [DOI] [PubMed] [Google Scholar]
- Villareal 2007.Villareal G, Li Q, Cai D, Glanzman DL. The role of rapid, local postsynaptic protein synthesis in learning-related synaptic facilitation in Aplysia. Curr Biol 17: 2073–2080, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villareal 1992.Villareal GJ, Cai D, Fink A, Glanzman D. Serotonin-dependent enhancement of the glutamate-evoked response in isolated siphon motor neurons requires temporal and isoform specific activation of protein kinase C in Aplysia. FENS Abstr 3: AO48.28, 2006. [Google Scholar]
- Yule 1992.Yule DI, Williams JA. U73122 inhibits Ca2+ oscillations in response to cholecystokinin and carbachol but not to JMV-180 in rat pancreatic acinar cells. J Biol Chem 267: 13830–13835, 1992. [PubMed] [Google Scholar]
- Zhao 2006.Zhao Y, Leal K, Abi-Farah C, Martin KC, Sossin WS, Klein M. Isoform specificity of PKC translocation in living Aplysia sensory neurons and a role for Ca2+-dependent PKC APL I in the induction of intermediate-term facilitation. J Neurosci 26: 8847–8856, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]