Abstract
The present study reports distinct dynamic consequences for the T- and R-states of human normal adult hemoglobin (Hb A) due to the binding of a heterotropic allosteric effector, inositol hexaphosphate (IHP). A nuclear magnetic resonance (NMR) technique based on modified transverse relaxation optimized spectroscopy (TROSY) has been used to investigate the effect of conformational exchange of Hb A in both deoxy and CO forms, in the absence and presence of IHP, at 14.1 and 21.1 T, and at 37 °C. Our results show that the majority of the polypeptide backbone amino acid residues of deoxy- and carbonmonoxy-forms of Hb A in the absence of IHP is not mobile on the ·s-ms time scale, with the exception of several amino acid residues, that is, · 109Val and · 132Lys in deoxy-Hb A, and R40Lys in HbCO A. The mobility of R40Lys in HbCO A can be explained by the crystallographic data showing that the H-bond between R40Lys and · 146His in deoxy-Hb A is absent in HbCO A. However, the conformational exchange of ·109Val, which is located in the intradimer (R1· 1 or R2· 2) interface, is not consistent with the crystallographic observations that show rigid packing at this site. IHP binding appears to rigidify R40Lys in HbCO A, but does not significantly affect the flexibility of · 109Val in deoxy-Hb A. In the presence of IHP, several amino acid residues, especially those at the interdimer (R1 · 2 or R2 · 1) interface of HbCO A, exhibit significant conformational exchange. The affected residues include the proximal ·92His in the ·-heme pocket, as well as some other residues located in the flexible joint (·C helix-RFG corner) and switch (RC helix-·FG corner) regions that play an important role in the dimer-dimer rotation of Hb during the oxygenation process. These findings suggest that, upon IHP binding, HbCO A undergoes a conformational fluctuation near the R-state but biased toward the T-state, apparently along the trajectory of its allosteric transition, accompanied by structural fluctuations in the heme pocket of the ·-chain. In contrast, no significant perturbation of the dynamic features on the ms-·s time scale has been observed upon IHP binding to deoxy-Hb A. We propose that the allosteric effector-induced quaternary structural fluctuation may contribute to the reduced ligand affinity of ligated hemoglobin. Conformational exchange mapping of the ·-chain of HbCO A observed at 21.1 T shows significantly increased scatter in the chemical exchange contribution to the transverse relaxation rate (Rex) values, relative to those at lower fields, due to the enhanced effect of the local chemical shift anisotropy (CSA) fluctuation. A spring-on-scissors model is proposed to interpret the dynamic phenomena induced by the heterotropic effector, IHP.
Characterization of the dynamic features of hemoglobin (Hb1) has been emerging as a new frontier in Hb studies (1-5). During the past decade, multiple conformations have been observed or implied for both the T (tense) and R (relaxed) allosteric states of Hb (6-11). Previous studies in our laboratory using the nuclear magnetic resonance (NMR) residual dipolar coupling (RDC) approach have suggested that the solution structure of HbCO A is a dynamic ensemble of at least two crystal structures, R and R2 (7, 10), and this average can be shifted by various allosteric effectors (10). These new findings call for a dynamic model rather than the static picture of Hb described by the classical two-state/structure stereochemical model for the cooperative oxygenation of Hb, which requires the four subunits of hemoglobin to maintain either an all-T (low-affinity) or all-R (high-affinity) structure until the allosteric transition between T and R is completed (12, 13).
NMR spectroscopy is a powerful tool for investigating protein dynamics (14, 15). Protein mobility spans a broad range of time scales that require different NMR methods for observation. Our laboratory recently carried out a study of the fast (ps-ns time scale) motions of the Hb polypeptide backbone in both the ligated (CO) and unligated (deoxy) forms (5) using the model-free approach (16). We have found that the fast backbone mobility of several amino acid residues of Hb is perturbed upon CO binding. Such perturbations occur at the carboxy-terminal amino acid residues, e.g., ·146His, as a direct consequence of the allosteric structural transition, and also at the intradimer (R1· 1 or R2· 2) interfaces, e.g., R31Arg and ·123Thr, where X-ray crystallographic results indicate rigid packing. These fast (ps-ns) motions represent only one end of the spectrum of protein dynamics. Slower (·s-ms time scale) conformational flexibility, which involves the large-segmental movements in the protein molecule or the ligand-association processes, may be more relevant to the biological functions of a protein molecule than those occurring on the fast time scale (ps-ns). Such slower motions may be detected by their specific effects on the transverse relaxation of reporter nuclei. The contribution of conformational exchange to the transverse relaxation rate (Rex) can, in principle, be derived from the model-free analysis. In our previous study, the results implied possible slow dynamic events at amino acid residues after 100 for both R- and ·-chains, especially ·109Val (5). However, since the Rex values derived from a model-free analysis are mainly introduced for fitting purpose, they are not considered as a solid evidence for conformational exchange. Various NMR methods other than model-free have been developed in order to more accurately characterize the slow motions in proteins (17). However, the applications of many such methods to Hb are difficult because of its large size (MW· 65 kD). When spectral assignments of only certain side-chain signals had been made, we used the CPMG-TROSY relaxation dispersion method to study the ·s-ms time scale motions of the tryptophan side chains (4). The side-chain indole NH of ·37Trp, used as a marker for the T and R states, located in the interdimer (R1 · 2 or R2·1) interface, has been found to show slow conformational mobility under certain experimental conditions, e.g., HbCO A bound with an allosteric effector, inositol hexaphosphate (IHP) (4). More recently, our laboratory has assigned the polypeptide backbone resonances for both HbCO A (18) and deoxy-Hb A (19), making it possible to study the slow conformational exchanges of most backbone N-H bonds. In the present study, we apply a TROSY-based conformational exchange mapping technique that is designed for proteins with MW > 50 kD (20). This approach avoids the problems caused by the typically very fast transverse relaxation rates for large proteins, by detecting those narrower components of the multiplet narrowed by the interaction between the 1H-15N dipole-dipole (DD) coupling and 15N chemical shift anisotropy (CSA).
Our present study explores the slow time scale mobility of Hb in both its T- and R-states on a residue-by-residue basis, in the absence and presence of IHP. Such a study has the potential to correlate the dynamics of the Hb molecule to the effects of organic phosphates that regulate the oxygen affinity under physiological conditions (21, 22). Previous studies have shown that IHP can reduce the oxygen affinity of Hb (23, 24). To explain the mechanism of allosteric effectors, the classical two-state/structure model has proposed that the binding of an allosteric effector to Hb can shift the equilibrium quaternary structure from the R-state to the T-state (12, 13). Recently, Yonetani and co-workers (25) have suggested that a change in the tertiary structure of Hb induced by effector binding could account for the affinity change. Such effector-induced tertiary structural changes have, in fact, been suggested by NMR (26) and X-ray crystallographic studies (27). However, the effect of the effector regulation appears not to be the same for the deoxy- and ligated forms of Hb A. The T- and R-states of Hb A have been found to differ in the effector binding sites (28, 29). IHP was found to bind more strongly to deoxy-Hb A (with an association constant of 5 · 107 M-1) than to HbCO A (with an association constant of 2 · 104 M-1) (30). Crystallographic results have indicated that IHP is held between the two ·-chains of deoxy-Hb A (31), while, for HbCO A, additional or different IHP binding sites in the central cavity have been proposed but not structurally confirmed (32-34). However, there is no clear explanation of why and how the difference between the binding sites of the effectors in the deoxy- and CO forms of Hb is correlated to the functional regulation.
In our present work, we have investigated the nature of the allosteric effector regulation of Hb from a new angle, namely, that of protein motional properties, which has not been previously addressed. Our results demonstrate dramatically distinct dynamic responses of the T- and R-states of Hb upon IHP binding. Remarkable conformational exchange processes in HbCO A have been found to be induced by IHP, mainly along the interdimer (R1· 2 or R2· 1) interface, and several amino acid residues in the ·-heme pocket. These dynamic events imply the existence of a structural fluctation of Hb along its allosteric structural trajectory, i.e., between the R-state and some intermediate structure near the R-state, but biased toward the T-state. Conformational fluctuation in the ·-heme pocket that accompanies the quaternary structure change may be a direct cause of the affinity modulation by the allosteric effector. In contrast, no extensive conformational instability has been observed for deoxy-Hb A after binding of IHP, implying less of an influence of IHP on the dynamic properties of the T-state than on those of the R-state of Hb. Although no extensive intrinsic dynamic (conformational exchange) process on the ·s-ms time scales has been found for IHP-free Hb, ·109Val and ·132Lys in deoxy-Hb A and R40Lys in HbCO A exhibit mobility in the absence of IHP. Consistent with the result of our previous model-free analysis (5), ·109Val is again an indicator of flexibility of the intradimer (R1· 1 or R2· 2) interface of deoxy-Hb A, not consistent with the crystallographic results (22, 35). The mobility of R40Lys in the IHP-free HbCO A confirms previously proposed major structural consequences, i.e., breaking of strong interactions (36), due to the allosteric transition.
MATERIALS AND METHODS
Hemoglobin Samples
Two types of chain-specifically U-[2H,15N]-labeled recombinant Hb A samples for NMR studies, namely, R-chains labeled and ·-chains unlabeled, and R-chains unlabeled and ·-chains labeled, were prepared as described previously (37). The rHb samples were of· 0.8 mM concentration (per tetramer) in 0.05 M sodium phosphate buffer at pH 7.0 in the presence of 10% D2O. The samples with IHP were prepared by adding IHP stock solution to the Hb samples directly with a final concentration of 3 mM. In our Hb sample preparations for the NMR studies, no chloride ions were present except for those samples with IHP, in which case sufficient amounts of HCl (·0.15 M) were added to adjust the pH of the stock IHP solution to pH 7.0.
Conformational Exchange (Rex) Measurements
In this study, the conformational exchange of an amide N-H bond was detected by measuring Rex, the contribution of the exchange processes to R2R, the transverse relaxation rate of the narrower component of the NMR doublet, due to the interaction between 1H-15N dipole-dipole coupling (DD) and 15N chemical shift anisotropy (CSA). Rex was determined according to the approach introduced by Wang et al. (20) as
| (1) |
where R12HzNz is the relaxation rate constant of the longitudinal two-spin order, ·xy is the rate constant for 1H-15N DD-15N CSA cross-correlation, and R1N is the longitudinal relaxation rate of 15N, which can be neglected for proteins with MW > 30 kD (typical R1N is 0.5 s-1 for Hb at 14.1 T).· ) R20/·xy is the ratio of the rates of autorelaxation for the in-phase 15N magnetization (R20) and the cross-correlation (·xy) from 15N CSA and 1H-15N DD interactions. In practice, the ratio · was obtained by the trimmed mean of 1 + (R2R - R12HzNz/2 + R1N/2)/·xy for all of the amino acid residues. For each residue, R2R - R12HzNz/2 and ·xy were determined by the NMR signal intensities, IR, I·, and I2HzNz, obtained from the three experiments designed to measure R2R, R2· (transverse relaxation rate constant for the broader component in the doublet due to 1H-15N DD-15N CSA interaction), and R1 2HzNz, respectively, as
| (2a) |
| (2b) |
The NMR experiments were carried out at 37 °C on 14.1-T Bruker DRX-600 (Carnegie Mellon University) and 21.1-T Varian Inova-900 (NMRFAM, Madison, WI) NMR spectrometers. The relaxation delay 2· used in this study was 21.6 ms at 14.1 T, and 10.8 ms at 21.1 T. All experiments were repeated three times to estimate the experimental errors in Rex values.
Cancellation of the CSA Contribution to Rex
The baseline for the Rex values obtained by Rex-mapping is not flat, but with fluctuations from residue to residue [Figures 1, 3, and 4, and also refer to ref 20]. The cause of this fluctuation is due to the variation in the magnitude and orientation of the 15N CSA tensor for different amino acid residues (20, 38). The CSA-generated baseline noise can obscure the small Rex values. This problem becomes more serious at very high magnetic fields. Fortunately, for most amino acid residues of Hb without significant Rex values, the baseline fluctuation due to the CSA variation has similar profiles for the IHP-bound and IHP-free forms of the same type of Hb samples. On the basis of this observation, to remove CSA-caused fluctuation, we can simply subtract the Rex values of the IHP-free Hb from those of the IHP-Hb complex. In principle, this subtraction may also remove meaningful Rex values, which are identical for the two samples. Fortunately, few residues in the IHP-free Hb have significant Rex values.
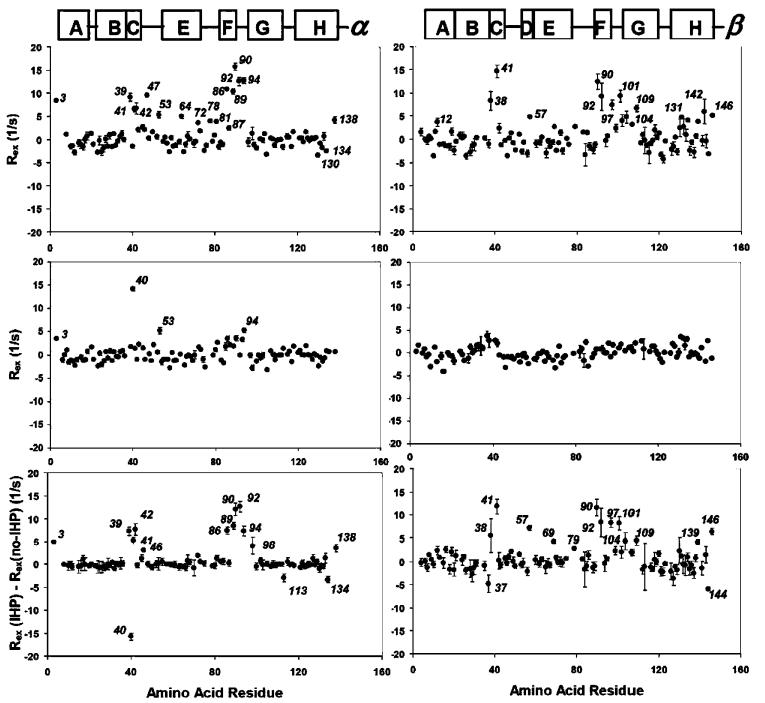
Figure 1.
Conformational exchange on the ·s-ms time scale, as indicated by the Rex values of the amide N-H bonds of HbCO A, at 37 °C and 14.1 T (left column for the R-chain and right column for the ·-chain). Top, Rex in IHP-bound HbCO A; middle, Rex in HbCO A; and bottom, subtraction of the Rex values of HbCO A from those of IHP-bound HbCO A. IHP-induced conformational flexibility can be observed at the C helix (switch region) and the F-G corner (joint region) in the R-chain, and the C helix (joint region), the F-G corner (switch region), and the C-terminus in the ·-chain.
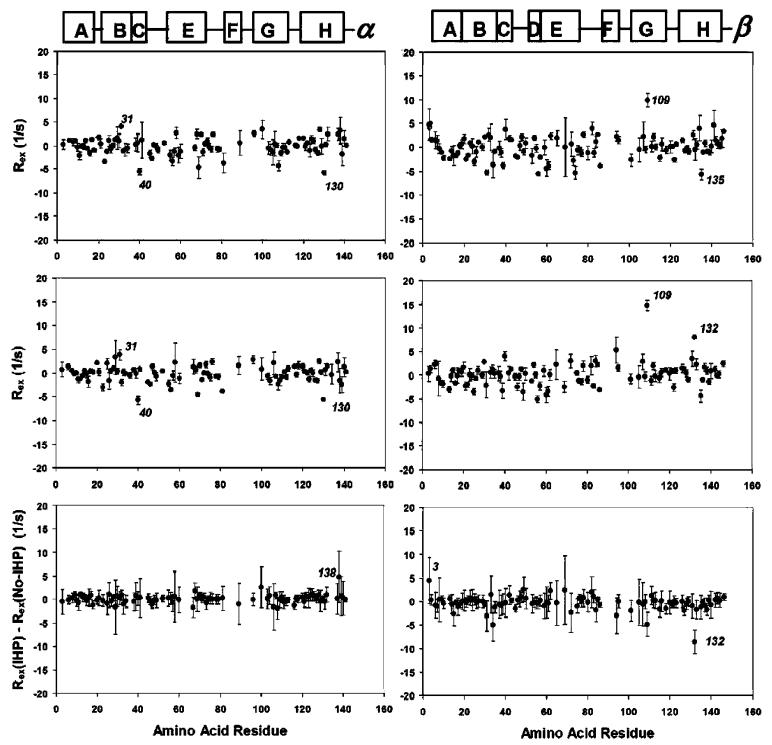
Figure 3.
Conformational exchange on the ·s-ms time scale, as indicated by the Rex values of the amide N-H bonds of deoxy-Hb A, at 37 °C and 14.1 T (left column for the R-chain and right column for the ·-chain). Top, Rex in IHP-bound deoxy-Hb A; middle, Rex in deoxy-Hb A; and bottom, subtraction of the Rex values of deoxy-Hb A from those of IHP-bound deoxy-Hb A. No significant conformational exchange processes are observed in the R-chain. ·109Val and ·132Lys exhibit significant Rex values. ·109Val retains its conformational flexibility upon IHP binding.
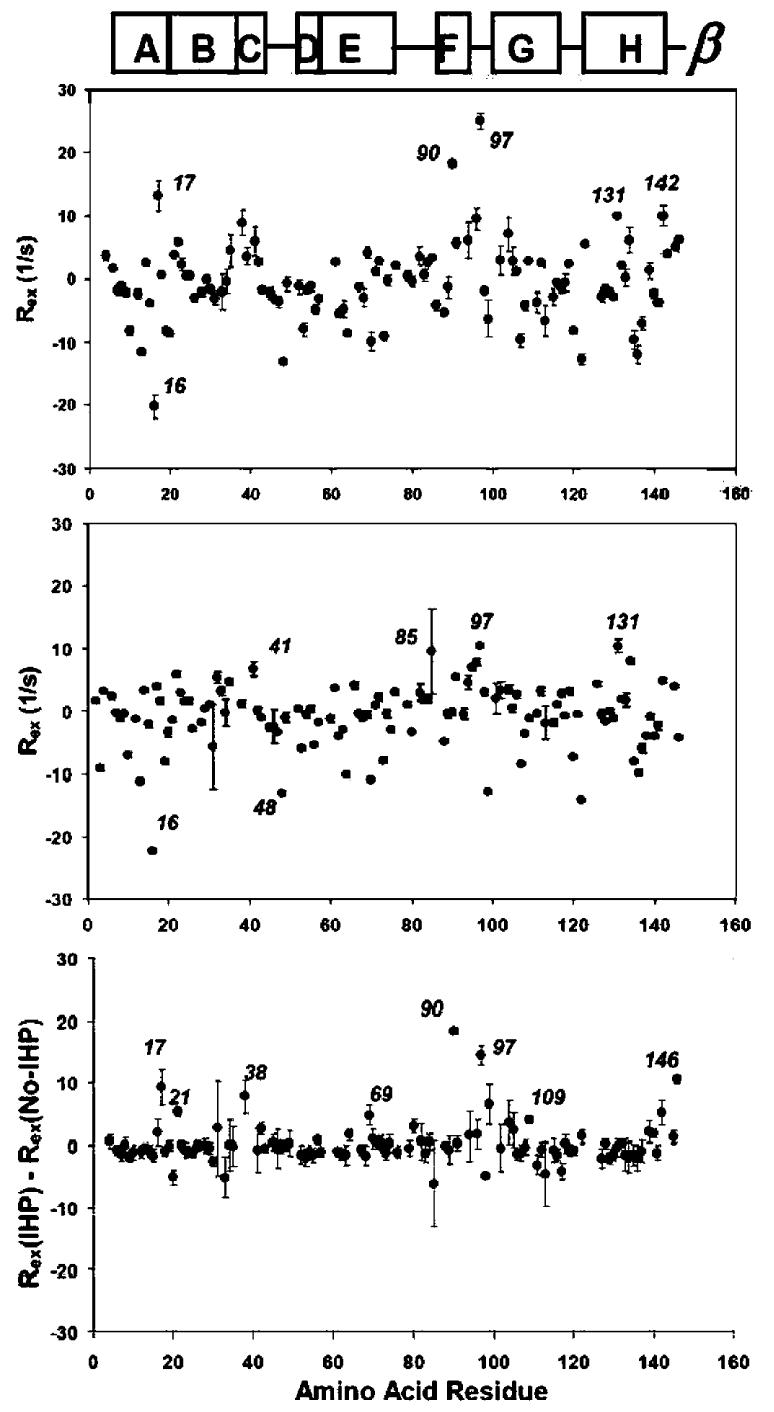
Figure 4.
Conformational exchange on the ·s-ms time scale, as indicated by the Rex values of the amide N-H bonds of the ·-chain in HbCO A, at 37 °C and 21.1 T. Top, Rex in IHP-bound HbCO A; middle, Rex in HbCO A; and bottom, subtraction of the Rex values of the ·-chain of HbCO A from those of the ·-chain of IHP-bound HbCO A. Errors due to fluctuations in local CSA are much larger than those at l4.1 T.
RESULTS
Figure 1 shows the Rex values determined for the backbone amide N-H bonds in HbCO A in the absence and presence of IHP at 37 °C and 14.1 T. Except for several amino acid residues in the R-chain, i.e., R3Ser, R40Lys, R53Ala, and R94Asp, the backbone of HbCO A, as represented by the amide N-H bonds, does not appear to be flexible on the · s-ms time scale covered by the Rex mapping method used in this study. However, upon binding of IHP, significant conformational exchanges are induced in both R- and · -subunits. The areas most significantly perturbed in their dynamic properties by IHP are apparently along the interdimer (R1· 2 or R2· 1) interface, i.e., the C helix (R39Thr and R41Thr), C-E loop (R47Asp), and F-G corner (R81Ser, R89His, R90Lys, R92Arg, R94Asp, and R98Phe) of the R-chain, the C helix (·38Thr and ·41Phe), and F-G corner (·90Glu, ·92His, ·97His, ·101Glu, ·104Arg, and ·109Val) of the ·-chain, and the terminal residues, such as R3Ser, R138Ser, and ·146His. Interestingly, most of these residues are amino acids with charged side chains. The net Rex contribution attributed to the effect of IHP was determined for each amide N-H bond by subtracting its Rex value in the HbCO A from that in the IHP-HbCO A complex. This baseline correction is intended to cancel the unwanted Rex component due to the CSA difference for each residue. This cancelation is more effective for the R-chain than for the ·-chain. The net Rex values of most amide N-H bonds that are not along the interdimer (R1· 2 or R2· 1) interface are close to null. The amino acid residues of HbCO A with significant conformational exchange as reflected by their amide N-H bonds are displayed by the space-filled model shown in Figure 2.
Figure 2.
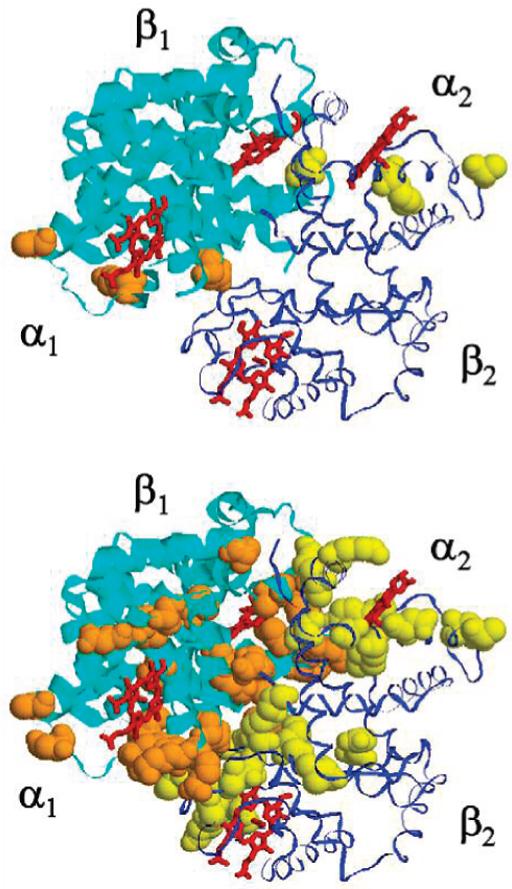
Conformational exchange on the ·s-ms time scale induced along the interdimer interface of HbCO A, at 37 °C and 14.1 T. Top, HbCO A; and bottom, IHP-bound HbCO A. The R1· 1 dimer is shown with a ribbon thicker than that of the R2· 2 dimer. The amino acid residues in space-filled style, orange for R1· 1 and yellow for R2· 2, are those with Rex > 5.
Figures 3 shows the Rex values determined for the backbone amide N-H bonds in deoxy-Hb A in the absence and presence of IHP at 37 °C and 14.1 T. Similar to HbCO A, deoxy-Hb A exhibits little flexibility on the ms-·s time scale, except for two residues in the ·-chain, ·109Val and ·132Lys. However, in great contrast with HbCO A, deoxy-Hb A shows almost no observable conformational exchanges along its backbone induced by IHP binding. The residue ·109Val remains flexible, while ·132Lys becomes rigid upon IHP binding.
Figure 4 shows the Rex values measured for HbCO A ·-chain at 37 °C and 21.1 T. The scatter in the Rex values due to local CSA fluctuations is approximately doubled compared to that at 14.1 T. Baseline correction by subtracting Rex values for HbCO A from those for the HbCO A-IHP complex needs to be applied in order to detect any IHP-induced Rex values at this high field.
DISCUSSION
Distinct Dynamic BehaVior of Deoxy-Hb A and HbCO A upon IHP Binding
Backbone Rigidity of Deoxy-Hb A Is Not Affected by IHP Binding. Except for ·132Lys that loses its intrinsic conformational exchange after IHP binding, the rigidity of deoxy-Hb A, on the ·s-ms time scale studied in this research, is found to be insensitive to the IHP binding. In other words, IHP does not induce slow motions of the polypeptide backbone of deoxy-Hb A.
Conformational Fluctuation of HbCO A upon IHP Binding
A number of amino acid residues in both the R- and ·-chains of HbCO A exhibit greatly increased mobility on the ·s-ms time scale upon IHP binding (Figures 1, 2, and 3). The mobility enhancement is responsible for exchanging the chemical environments of several backbone amide N-H bonds in Hb A. A careful mapping analysis of the Rex values shows that the increased conformational exchange occurs mainly along the interdimer (R1· 2 or R2· 1) interface, especially in the switch region (RC helix-·FG corner), the joint region (·C helix-RFG corner), and the carboxy-terminals of both R- and ·-chains. These areas are called sliding contacts, which undergo the major conformational changes during the Hb allosteric transition. In the areas other than the interdimer interfaces, the dynamic perturbation due to IHP binding is less significant. These dynamic phenomena suggest a dynamic motional mode for the quaternary structure of IHP-bound HbCO A between the solution R state and an intermediate state near R, but biased toward T, possibly along the allosteric trajectory, although not at an amplitude large enough to complete an allosteric transition. This is consistent with the suggestion by Zuiderweg et al. (30) based on 31P NMR and pH-Stat techniques that the IHP-HbCO A complex could adopt two conformations. The fluctuation phenomenon can also explain the slight shifting of the averaged solution quaternary structure of IHP-bound HbCO A toward the R structure from the midpoint between the R and R2 structures, as suggested by RDC experiments (10). Previous studies in our laboratory (4) have also found that the side chain of ·37Trp at the interdimer (R1· 2 or R2· 1) interface shows slow conformational fluctuation of thisinterface only for IHP-bound HbCO A, not for deoxy-Hb A and not for IHP-free HbCO A, which is consistent with our current observation.
In addition, among the amino acid residues involved in conformational exchanges, we have proximal ·92His, which is the key element of the ·-heme pocket (the residue bound to the heme-iron atom), and the perturbation of its dynamic properties implies another mechanism for the IHP effects on ligand binding affinity (26). This observation may be correlated with the effector-induced structural changes found by X-ray crystallography, i.e., shortened distance between the heme-Fe and the distal histidine (27). It should be noted that the structural variation at the ·-heme pocket is also one of the key features associated with the T-R allosteric transition, according to Perutz’ stereochemistry model (13). What is interesting is how a slight quaternary structural change, while still in an R state (25, 39), could induce tertiary structure changes in the ·-heme pocket, possibly large enough to change the ligand affinity.
Also interestingly, amino acid residues in HbCO A with significant Rex values are amino acids with charged side chains. This could be simply due to the nature of the amino acids along the interdimer (R1· 2 or R2· 1) interface or due to the high sensitivity of these charged residues to the slight conformational variation. It should be mentioned that the binding of IHP (a highly charged molecule) to Hb A could also affect the pK values as well as the NH exchange rates of those amino acids that exhibit changes in the dynamics. Additional work is needed to elucidate this interesting observation.
Spring-on-Scissors Model
According to Yonetani et al. (25), in the presence of heterotropic effectors, the liganded R-state of Hb has T constraints within a quaternary R-like frame. Coletta et al. (40), Scott et al. (41), and Chen et al. (42) have described the liganded Hb with bound organic phosphate as “an altered R-state Hb” or a tertiary T-like conformation within a R-like quaternary structure. A frustrated, unstable R structure (4) has been proposed for HbCO A with IHP bound. Our present results suggest that the perturbation of HbCO A by IHP is very likely a dynamic phenomenon, i.e., a fluctuation starting from the average solution structure possibly biased toward the T-structure. We interpret this finding by a spring analogy. The allosteric transition from deoxy-Hb to liganded Hb is believed to close the central cavity of the protein molecule (13, 22). However, the presence of a small molecule effector, e.g., IHP, may generate an additional barrier to this closing process by either spatial hindering or electrostatic interactions. Because of the flexibility of the small molecule, such a potential barrier is not sufficient to prevent the central cavity from reaching the size of an R-structure. The IHP molecule serves more like a spring such that a change of the cavity size can occur accompanied by a conformational exchange between the R-structure and a structure slightly toward T-structure as suggested by Gong et al. (10).
What is the structural basis of such a spring? Recent computational simulation suggests that in the ligated R-state Hb, effectors like IHP or 2,3-BPG bind between the R1- and R2-subunits (34), different from the reported IHP and 2,3-BPG binding sites between the ·1- and ·2-subunits in the unligated, deoxy T-state (31, 43) (Figure 5). This change of effector-binding site is consistent with the proposed description that the effector molecule can be squeezed out of its ·1-·2 binding pocket when the two ·-chains come closer together in liganded Hb (22). According to Laberge et al. (34), the effector binding site in the R-state mainly involves R99Lys and R141Arg. In Perutz’ allosteric model, R141Arg is one of the amino acid residues whose salt bridge network is ruptured upon the T-to-R transition of Hb. The effector molecule rebridges the two R-subunits, possibly in such way providing a resistance to the complete formation of the quaternary R-structure. The molecular spring mentioned here is located not at the exact center of the cavity, but instead between the two R-subunits with a tendency to reopen the central cavity. If we see the quaternary structure of liganded Hb during its T-to-R allosteric transition as a pair of scissors, the effector molecule (IHP, etc.) serves as a spring between its two handles.
Figure 5.
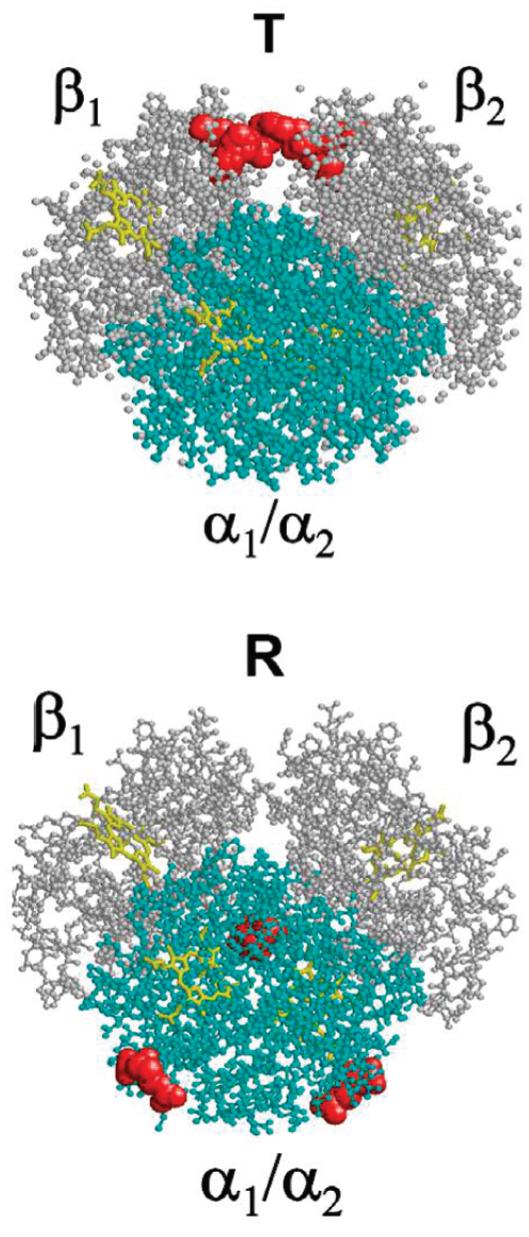
Different IHP-binding sites in the T and R forms of Hb A. Top, IHP bound to deoxy-Hb A between the two ·-chains (in white) at residues ·2His, ·143His, and ·82Lys; and bottom, the proposed IHP-binding site in HbCO A involves R141Arg and R99Lys, between the two R-chains (in cyan). All of the amino acid residues involved in the binding sites are shown in space-filled style and in red. Binding of IHP to HbCO A may generate greater resistance than binding to deoxy-Hb A, leading to structural fluctuations, as observed in this study. This description is being described as a spring-on-scissors model.
We believe that the heterotropic effectors do not induce structural oscillations when they are seated between the two ·-chains in the T-state. This may be partially explained by the fact that deoxy-Hb favors the same tendency as the effectors to open the central cavity (36, 44). In addition, in the structural transition from the T-state to the R-state, the effector binding site moves from the entrance of the central cavity to somewhere deeper into the cavity; therefore, the small molecules may exert a greater perturbation on the quaternary structure and structural stability of Hb in the R-state than in the T-state.
Intrinsic Conformational Flexibility of Hemoglobin on the ·s-ms Timescale
Intrinsic Conformational Flexibility of ·109Val and ·132Lys in Deoxy-Hb A. Residues ·109Val and ·132Lys are the only two amino acid residues that exhibit conformational exchange in deoxy-Hb A. Upon IHP binding, ·132Lys loses its flexibility, but ·109Val retains its large Rex value. Previous backbone dynamics studies using the model-free formalism (5) also indicate significant slow exchange motions at the ·109Val site. However, the Rex terms derived from the model-free analysis are mainly for fitting necessity, and thus often considered questionable. Derived from the more reliable Rex-mapping approach, the present result further confirms the ms-·s time scale mobility of ·109Val, which is located at the intradimer interfaces. These interfaces have been inferred to be flexible and possibly involved in the cross-subunit communication during Hb allostery (2, 3, 45-48). Several other residues along the intradimer interface, such as R31Arg and ·123Thr, according to the model-free analysis (5), and R103His and R122His, according to studies using solvent exchange (1), have been found to be more mobile in deoxy-Hb A than in HbCO A. X-ray crystallographic results describe a rigid packing interface between the two same-dimer (R1· 1 and R2· 2) subunits of Hb (22, 35, 36). The flexibility of the abovementioned residues leads us to a dynamic picture of the intradimer interface in the deoxy- (or T) state of Hb. With ample evidence from the dynamics studies on hand, we may now conclude that the T state of Hb has a very flexible intradimer interface, which possibly serves to transmit allosteric signals between the two same-dimer subunits. As a consequence of the allosteric structural transition, the ligand binding rigidifies the intradimer interface.
Intrinsic Conformational Flexibility of R40Lys in HbCO A
According to the crystal structure, the end of the side chain of this lysine residue forms a H-bond or salt-bridge to the carboxylic oxygen of ·146His in deoxy-Hb (22, 39). Apparently, the interaction between these two amino acids is absent in the CO-bound form, leading to the most significant intrinsic conformational exchange observed in the IHP-free HbCO A. This is important dynamic evidence for the breaking of the C-terminal H-bond (salt-bridge) of the ·-chain in liganded hemoglobin, a key consequence of its allosteric transition from T to R state (13, 36).
IHP Affects R- and ·-Subunits of HbCO A Differently
Subtraction of the Rex values for the IHP-free Hb from those for the IHP-Hb complex gives an almost null baseline for both R- and ·-chains of deoxy-Hb A as well as the R-chain of HbCO A, indicating that the noise in the Rex values measured for these samples is mainly due to the fluctuation of the CSA of individual amino acid residues. However, the net IHP-induced Rex values for the ·-chain residues of HbCO A remain fluctuating, implying that the dynamic response of the ·-chain HbCO A is more complicated than that of the R-chain upon perturbation by IHP. Gray and Gibson (24) observed a kinetic difference between the R- and ·-chains of Hb induced by IHP binding. Recently, a study from our laboratory based on RDC measurements has shown that IHP causes changes mainly in the tertiary structure of deoxy-Hb and mostly in the ·-subunit (11). These observations show that in general, IHP can affect the two types of subunits of Hb A differently.
Side-Chain Conformational Exchange
The present study is focused on the conformational exchange in the polypeptide backbone of Hb A, using the amide N-H bonds as the probes. The dynamic properties of protein side chains are often different from those of the backbone amide N-H bonds (49, 50). Further dynamic studies of Hb side chains are likely to provide useful information on how effectors, such as IHP, can affect the mobility of Hb.
Conformational Exchange Mapping at 21.1 T
We have also studied the IHP effect on the backbone mobility on the ·s-ms time scale at 21.1 T for the ·-chain of HbCO A (Figure 4). The unwanted components in the determined Rex values due to fluctuations of the local CSA for different amino acid residues significantly increased at 21.1 T, compared to those observed at 14.1 T. This observation needs to be considered when using the Rex mapping approach at high magnetic fields, although in the present study of the IHP effect, the CSA noise can be effectively canceled out by subtracting the results for IHP-free Hb from those for the IHP-Hb complex (Figure 4).
The magnetic field dependence of Rex values can be used to calculate a scaling factor, R, as introduced by Millet et al. (51):
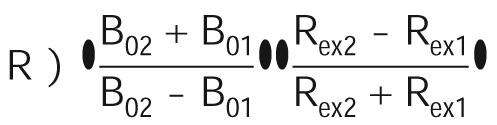 |
(3) |
in which Bon and Rexn (n) 1 for 14.1 T and 2 for 21.1 T) are the magnetic field strength and exchange contribution, respectively. The factor R is related to the time scale of the exchange process, i.e., 0 e R < 1 for slow exchange; R) 1 for intermediate exchange; and 1 < R e 2 for fast exchange. If we let B01 ) 14.1 T and B02 ) 21.1 T, eq 3 can be further expressed as
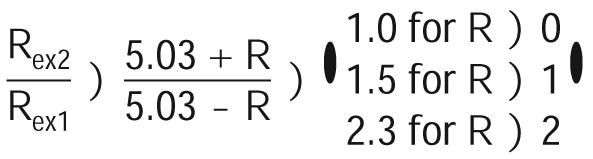 |
(4) |
Comparing the Rex values obtained at 14.1 and 21.1 T for the ·-chain of IHP-bound HbCO A (Figures 1 and 4), most of the IHP-induced exchange processes exhibited for this sample apparently are on the slow to intermediate time scale.
Effector-Induced Structural Fluctuation
According to the above discussions, the allosteric transition of hemoglobin cannot be simply between only two-end states, but through a shifting equilibrium of several states with conventional R and T quaternary structures (7, 8, 10, 11, 52). The global allostery model proposed by Yonetani and co-workers also implies that both conventional R and T states of Hb are not necessarily the structures with high or low affinity (25, 53). On the basis of the above evidence, the observed affinity of Hb can be more complicated than a two-structure average of a T-state with low affinity and an R state with high affinity value, but possibly an ensemble average of all possible T and R conformations in the solution. The extensive conformational exchange upon effector binding, as demonstrated by the present study, must induce a redistribution of the populations of the coexisting R conformations, probably favoring the more low-affinity components. Such a structural fluctuation, combined with tertiary structure modifications, may contribute to the reduced affinity of HbCO A in the presence of IHP. Structural flexibility is believed to be important to protein functions. One important concept of enzyme activation is the pre-existing equilibrium of multiple allosteric conformations. Binding of the substrate or a heterotropic ligand, as well as other protein modifications, such as phosphorylation, can selectively shift the pre-existing equilibrium of populations toward the active state or so-called fit structure (54). Such process was originally proposed by Koshland as the “induced fit” (55, 56), which usually means an acquired optimal conformation and/or stability of the system upon binding of regulating factors or substrates to facilitate enzyme reactions. The results in our present study apparently suggest another type of induced fit, perhaps induced unfit or instability. According to such induced instability or fluctuation, the heterotropic effector, IHP, acts as a ligand affinity regulator for one allosteric end state by generating a structural fluctuation with various populations of conformations with different affinities. Our present study offers a new look at the effect of an allosteric effector on the structure, dynamics, and function of an allosteric protein, hemoglobin.
ACKNOWLEDGMENT
We thank Dr. Chunyu Wang (Rensselaer Polytechnic Institute, Troy, NY) for providing us with his NMR pulse program for Rex mapping, Dr. Klaas Hallenga and Dr. Marco Tonelli (NMRFAM, Madison, WI) for assistance with the measurements at 21.1 T, Dr. Jayashree Soman (Rice University, Houston, TX) for valuable discussions about the X-ray structures of IHP-bound deoxy-Hb, and Dr. David H. Maillet for constructive comments.
Footnotes
This work is supported by research grants from the NIH (grants R01GM-084614 and S10RR-017815); the NMR facilities at NMRFAM are supported by NIH grants P41RR02301 and P41GM66326.
- 2,3-BPG
- 2,3-bis-phosphoglycerate
- CSA
- chemical shift anisotropy
- DD coupling
- dipole-dipole coupling
- Hb A
- human normal adult hemoglobin
- HbCO A
- human normal adult carbonmonoxyhemoglobin
- IHP
- inositol hexaphosphate
- NMR
- nuclear magnetic resonance
- RDC
- residual dipolar coupling
- Rex
- chemical exchange contribution to transverse relaxation rate
- TROSY
- transverse relaxation optimized spectroscopy.
REFERENCES
- 1.Mihailescu M-R, Russu IM. A signature of the T)>R transition in human hemoglobin. Proc. Natl. Acad. Sci. U.S.A. 2001;98:3773–3777. doi: 10.1073/pnas.071493598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chang C.-k., Simplaceanu V, Ho C. Effects of amino acid substitutions at ·131 on the structure and properties of hemoglobin: evidence for communication between R1· 1 and R1 · 2 subunit interfaces. Biochemistry. 2002;41:5644–5655. doi: 10.1021/bi011919d. [DOI] [PubMed] [Google Scholar]
- 3.Balakrishnan G, Tsai C-H, Wu Q, Case MA, Pevsner A, McLendon GL, Ho C, Spiro TG. Hemoglobin site-mutants reveal dynamical role of interhelical H-bonds in the allosteric pathway: time-resolved UV resonance Raman evidence for intra-dimer coupling. J. Mol. Biol. 2004;340:857–868. doi: 10.1016/j.jmb.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 4.Yuan Y, Simplaceanu V, Lukin JA, Ho C. NMR investigation of the dynamics of tryptophan side-chains in hemoglobins. J. Mol. Biol. 2002;321:863–878. doi: 10.1016/s0022-2836(02)00704-0. [DOI] [PubMed] [Google Scholar]
- 5.Song X.-j., Yuan Y, Simplaceanu V, Sahu SC, Ho NT, Ho C. A comparative NMR study of the polypeptide backbone dynamics of hemoglobin in the deoxy and carbonmonoxy forms. Biochemistry. 2007;46:6795–6803. doi: 10.1021/bi602654u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Silva MM, Rogers PH, Arnone A. A third quaternary structure of human hemoglobin A at 1.7-A resolution. J. Biol. Chem. 1992;267:17248–17256. [PubMed] [Google Scholar]
- 7.Lukin JA, Kontaxis G, Simplaceanu V, Yuan Y, Bax A, Ho C. Quaternary structure of hemoglobin in solution. Proc. Natl. Acad. Sci. U.S.A. 2003;100:517–520. doi: 10.1073/pnas.232715799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kavanaugh JS, Rogers PH, Arnone A. Crystallographic evidence for a new ensemble of ligand-induced allosteric transitions in hemoglobin: the T-to-Thigh quaternary transitions. Biochemistry. 2005;44:6101–6121. doi: 10.1021/bi047813a. [DOI] [PubMed] [Google Scholar]
- 9.Safo MK, Abraham DJ. The enigma of the liganded hemoglobin end state: a novel quaternary structure of human carbonmonoxy hemoglobin. Biochemistry. 2005;44:8347–8359. doi: 10.1021/bi050412q. [DOI] [PubMed] [Google Scholar]
- 10.Gong Q, Simplaceanu V, Lukin JA, Giovannelli JL, Ho NT, Ho C. Quaternary structure of carbonmonoxyhemoglobins in solution: structural changes induced by the allosteric effector inositol hexaphosphate. Biochemistry. 2006;45:5140–5148. doi: 10.1021/bi052424h. [DOI] [PubMed] [Google Scholar]
- 11.Sahu SC, Simplaceanu V, Gong Q, Ho NT, Tian F, Prestegard JH, Ho C. Insights into the solution structure of human deoxyhemoglobin in the absence and presence of an allosteric effector. Biochemistry. 2007;46:9973–9980. doi: 10.1021/bi700935z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Monod J, Wyman J, Changeux J-P. On the nature of allosteric transitions: a plausible model. J. Mol. Biol. 1965;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- 13.Perutz MF. Stereochemistry of cooperative effects in haemoglobin. Nature. 1970;228:726–739. doi: 10.1038/228726a0. [DOI] [PubMed] [Google Scholar]
- 14.Cavanagh J, Fairbrother WJ, Palmer AG., III . Protein NMR Spectroscopy: Principles and Practice. Academic Press; San Diego, CA: 1996. [Google Scholar]
- 15.Palmer AG., III NMR characterization of the dynamics of biomacromolecules. Chem. ReV. 2004;104:3623–3640. doi: 10.1021/cr030413t. [DOI] [PubMed] [Google Scholar]
- 16.Mandel AM, Akke M, Palmer AG., III Backbone dynamics of Escherichia coli ribonuclease HI: correlations with structure and function in an active enzyme. J. Mol. Biol. 1995;246:144–163. doi: 10.1006/jmbi.1994.0073. [DOI] [PubMed] [Google Scholar]
- 17.Palmer AG, III, Grey MJ, Wang CY. Solution NMR spin relaxation methods for characterizing chemical exchange in high-molecular-weight systems. Methods Enzymol. 2005;394:430–465. doi: 10.1016/S0076-6879(05)94018-4. [DOI] [PubMed] [Google Scholar]
- 18.Lukin JA, Kontaxis G, Simplaceanu V, Yuan Y, Bax A, Ho C. Backbone resonance assignments of human adult hemoglobin in the carbonmonoxy form. J. Biomol. NMR. 2004;28:203–204. doi: 10.1023/B:JNMR.0000013816.64039.6f. [DOI] [PubMed] [Google Scholar]
- 19.Sahu SC, Simplaceanu V, Ho NT, Giovannelli JL, Ho C. Backbone resonance assignment of human adult hemoglobin in the deoxy form. J. Biomol. NMR. 2006;36:1. doi: 10.1007/s10858-005-2455-z. [DOI] [PubMed] [Google Scholar]
- 20.Wang C, Rance M, Palmer AG., III Mapping chemical exchange in proteins with MW > 50 KD. J. Am. Chem. Soc. 2003;125:8968–8969. doi: 10.1021/ja035139z. [DOI] [PubMed] [Google Scholar]
- 21.Voet D, Voet JG. Biochemistry. John Wiley & Sons; New York: 1995. [DOI] [PubMed] [Google Scholar]
- 22.Dickerson RE, Geis I. Hemoglobin: Structure, Function, EVolution, and Pathology. Benjamin-Cummings; Menlo Park, CA: 1983. [Google Scholar]
- 23.Janig G-R, Ruckpaul K, Jung F. Interaction of haemoglobin with ions binding of inositol hexaphosphate to human haemoglobin A. FEBS Lett. 1971;17:173–176. doi: 10.1016/0014-5793(71)80141-2. [DOI] [PubMed] [Google Scholar]
- 24.Gray RD, Gibson QH. The effect of inositol hexaphosphate on the kinetics of CO and O2 binding by human hemoglobin. J. Biol. Chem. 1971;246:7168–7174. [PubMed] [Google Scholar]
- 25.Yonetani T, Park S, Tsuneshige A, Imai K, Kanaori K. Global allostery model of hemoglobin. J. Biol. Chem. 2002;277:34508–34520. doi: 10.1074/jbc.M203135200. [DOI] [PubMed] [Google Scholar]
- 26.Lindstrom TR, Ho C. Effects of anions and ligands on the tertiary structure around ligand binding site in human adult hemoglobin. Biochemistry. 1973;12:134–139. doi: 10.1021/bi00725a022. [DOI] [PubMed] [Google Scholar]
- 27.Shibayama N, Miura S, Tame JRH, Yonetani T, Park S-Y. Crystal structure of horse carbonmonoxyhemoglobin-bezafibrate complex at 1.55 A resolution. J. Biol. Chem. 2002;277:38791–38796. doi: 10.1074/jbc.M205461200. [DOI] [PubMed] [Google Scholar]
- 28.Bucci E, Salahuddin A, Bonaventura J, Bonaventura C. Characterization of the ionizable groups interacting with anionic allosteric effectors of human hemoglobin. J. Biol. Chem. 1977;253:821–827. [PubMed] [Google Scholar]
- 29.Nelson DP, Miller WD, Kiesow LA. Calorimetric studies of hemoglobin function, the binding of 2,3-diphosphspho-glycerate and inositol hexaphosphate to human hemoglobin A. J. Biol. Chem. 1974;249:4770–4775. [PubMed] [Google Scholar]
- 30.Zuiderweg ERP, Hamers LF, Bruin S. H. d., Hilbers CW. Equilibrium aspects of the binding of myo-Inositol hexakisphosphate to human hemoglobin as studied by 31P NMR and pH-Stat techniques. Eur. J. Biochem. 1981;118:85–94. doi: 10.1111/j.1432-1033.1981.tb05489.x. [DOI] [PubMed] [Google Scholar]
- 31.Arnone A, Perutz MF. Structure of inositol hexaphosphate-human deoxyhaemoglobin complex. Nature. 1974;249:34–36. doi: 10.1038/249034a0. [DOI] [PubMed] [Google Scholar]
- 32.Zuiderweg ERP, Hamers LF, Rollema HS, Bruin S. H. d., Hilbers CW. 31P NMR study of the kinetics of binding of myo-inositol hexakisphosphate to human hemoglobin: observation of fast-exchange kinetics in high-affinity systems. Eur. J. Biochem. 1981;118:95–104. doi: 10.1111/j.1432-1033.1981.tb05490.x. [DOI] [PubMed] [Google Scholar]
- 33.Riccio A, Tamburrini M, Giardina B, Prisco G. d. Molecular dynamics analysis of a second phosphate site in the hemoglobins of the seabird, south polar skua. Is there a site-site migratory mechnism along the central cavity. Biophys. J. 2001;81:1938–1946. doi: 10.1016/S0006-3495(01)75845-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laberge M, Kovesi I, Yonetani T, Fidy J. R-state hemoglobin bound to heterotropic effectors: models of the DPG, IHP, and RSR13 binding sites. FEBS Lett. 2005;579:627–632. doi: 10.1016/j.febslet.2004.12.033. [DOI] [PubMed] [Google Scholar]
- 35.Fermi G, Perutz MF, Shaanan B. The crystal structure of human deoxyhaemoglobin at 1.74 A resolution. J. Mol. Biol. 1984;175:159–174. doi: 10.1016/0022-2836(84)90472-8. [DOI] [PubMed] [Google Scholar]
- 36.Baldwin J, Chothia C. Haemoglobin: the structural changes related to ligand binding and its allosteric mechanism. J. Mol. Biol. 1979;129:175–220. doi: 10.1016/0022-2836(79)90277-8. [DOI] [PubMed] [Google Scholar]
- 37.Simplaceanu V, Lukin JA, Fang T-Y, Zou M, Ho NT, Ho C. Chain-selective isotopic labeling for NMR studies of large multimeric proteins: application to hemoglobin. Biophys. J. 2000;79:1146–1154. doi: 10.1016/S0006-3495(00)76368-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fushman D, Tjandra N, Cowburn D. Direct measurement of 15N chemical shift anisotropy in solution. J. Am. Chem. Soc. 1998;120:10947–10952. [Google Scholar]
- 39.Perutz MF, Fermi G. Haemoglobin and Myoglobin. Vol. 2. Oxford University Press; New York: 1981. [Google Scholar]
- 40.Coletta M, Angeletti M, Ascenzi P, Bertollini A, Della LS, Sanctis GD, Priori AM, Santucci R, Amiconi G. Coupling of the oxygen-linked interaction energy for inositol hexakisphosphate and bezafibrate binding to human HbA. J. Biol. Chem. 1999;274:6865–6874. doi: 10.1074/jbc.274.11.6865. [DOI] [PubMed] [Google Scholar]
- 41.Scott TW, Friedman JM, Ikeda-Saito M, Yonetani T. Subunit heterogeneity in the structure and dynamics of hemoglobin. A transient Raman study. FEBS Lett. 1983;158:68–72. doi: 10.1016/0014-5793(83)80678-4. [DOI] [PubMed] [Google Scholar]
- 42.Chen Q, Lalezari I, Nagel RL, Hirsch RE. Liganded hemoglobin structural perturbations by allosteric effector L35. Biophys. J. 2005;88:2057–2067. doi: 10.1529/biophysj.104.046136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Luisi B, Liddington B, Fermi G, Shibayama N. Structure of deoxy-quaternary haemoglobin with ligated beta subunits. J. Mol. Biol. 1990;214:7–14. doi: 10.1016/0022-2836(90)90139-d. [DOI] [PubMed] [Google Scholar]
- 44.Perutz MF. Mechanisms of cooperativity and allosteric regulation in proteins. Q. ReV. Biophys. 1989;22:139–236. doi: 10.1017/s0033583500003826. [DOI] [PubMed] [Google Scholar]
- 45.Ramadas N, Rifkin JM. Molecular dynamics of human methemoglobin: the transmission of conformational information between subunits in an R· dimer. Biophys. J. 1999;76:1796–1811. doi: 10.1016/S0006-3495(99)77340-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tsai C-H, Fang TY, Ho NT, Ho C. Novel recombinant hemoglobin, rHb(beta N108Q), with low oxygen affinity, high cooperativity, and stability against autoxidation. Biochemistry. 2000;39:13719–13729. doi: 10.1021/bi001116a. [DOI] [PubMed] [Google Scholar]
- 47.Tsai C-H, Shen T.-j., Ho NT, Ho C. Effects of substitutions of lysine and aspartic acid for asparagine at ·108 and of tryptophan for valine at R96 on the structural and functional properties of human normal adult hemoglobin: roles of R1· 1 and R1 · 2 subunit interfaces in the cooperative oxygenation process. Biochemistry. 1999;38:8751–8761. doi: 10.1021/bi990286o. [DOI] [PubMed] [Google Scholar]
- 48.Mouawad L, Perahia D, Robert CH, Guilbert C. New insights into the allosteric mechanism of human hemoglobin from molecular dynamics simulations. Biophys. J. 2002;82:3224–3245. doi: 10.1016/S0006-3495(02)75665-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee AL, Kinnear SA, Wand AJ. Redistribution and loss of side chain entropy upon formation of a calmodulin-peptide complex. Nat. Struct. Biol. 2000;7:72–77. doi: 10.1038/71280. [DOI] [PubMed] [Google Scholar]
- 50.Song X.-j., Flynn PF, Sharp K, Wand AJ. Temperature dependence of fast dynamics in protein. Biophys. J. 2007;92:L43–L45. doi: 10.1529/biophysj.106.102160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Millet O, Loria JP, Kroenke CD, Pons M, Palmer AG., III. The static magnetic field dependence of chemical exchange linebroadening defines the NMR chemical shift time scale. J. Am. Chem. Soc. 2000;122:2867–2877. [Google Scholar]
- 52.Mueser TC, Rogers PH, Arnone A. Interface sliding as illustrated by the multiple quaternary structures of liganded hemoglobin. Biochemistry. 2000;39:15353–15364. doi: 10.1021/bi0012944. [DOI] [PubMed] [Google Scholar]
- 53.Tsuneshige A, Park S, Yonetani T. Heterotropic effectors control the hemoglobin function by interacting with its T and R states - a new view on the principle of allostery. Biophys. Chem. 2002;98:49–63. doi: 10.1016/s0301-4622(02)00084-4. [DOI] [PubMed] [Google Scholar]
- 54.Kern D, Zuiderweg ER. The role of dynamics in allosteric regulation. Curr. Opin. Struct. Biol. 2003;13:748–757. doi: 10.1016/j.sbi.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 55.Koshland DE. Application of a theory of enzyme specificity to protein synthesis. Proc. Natl. Acad. Sci. U.S.A. 1958;44:98–104. doi: 10.1073/pnas.44.2.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Koshland DE. Evolution of catalytic function. Cold Spring Harbor Symp. Quant. Biol. 1987;52:1–7. [PubMed] [Google Scholar]