Abstract
Previously, we reported that activation of G protein-coupled receptors (GPCR) in 1321N1 human astrocytoma cells elicits a rapid release of ATP that is partially dependent on a Gq/phophospholipase C (PLC)/Ca2+ mobilization signaling cascade. In this study we assessed the role of Rho-family GTPase signaling as an additional pathway for the regulation of ATP release in response to activation of protease-activated receptor-1 (PAR1), lysophosphatidic acid receptor (LPAR), and M3-muscarinic (M3R) GPCRs. Thrombin (or other PAR1 peptide agonists), LPA, and carbachol triggered quantitatively similar Ca2+ mobilization responses, but only thrombin and LPA caused rapid accumulation of active GTP-bound Rho. The ability to elicit Rho activation correlated with the markedly higher efficacy of thrombin and LPA, relative to carbachol, as ATP secretagogues. Clostridium difficile toxin B and Clostridium botulinum C3 exoenzyme, which inhibit Rho-GTPases, attenuated the thrombin- and LPA-stimulated ATP release but did not decrease carbachol-stimulated release. Thus the ability of certain Gq-coupled receptors to additionally stimulate Rho-GTPases acts to strongly potentiate a Ca2+-activated ATP release pathway. However, pharmacological inhibition of Rho kinase I/II or myosin light chain kinase did not attenuate ATP release. PAR1-induced ATP release was also reduced twofold by brefeldin treatment suggesting the possible mobilization of Golgi-derived, ATP-containing secretory vesicles. ATP release was also markedly repressed by the gap junction channel inhibitor carbenoxolone in the absence of any obvious thrombin-induced change in membrane permeability indicative of hemichannel gating.
Keywords: Rho GTPase, astrocyte, hemichannel
extracellular nucleotides act as autocrine/paracrine signaling molecules by targeting multiple P2 purinergic receptor subtypes that are differentially expressed in most tissues (7). Cells are able to tightly regulate the concentration of ATP and other nucleotides in the extracelluar space through a balance of release and extracellular metabolism of these nucleotides. The four sources of extracellular nucleotides are cell lysis, exocytosis, transport-mediated ATP release, and extracellular nucleotide kinases. In nonexcitable cells, such as astrocytes, unequivocal determination of ATP release mechanisms has remained elusive.
In the brain, ATP can be released by astrocytes, or by other glial cell types, in response to diverse metabolic, mechanical, or inflammatory stimuli (8, 9). Extracellular ATP can target glia and neurons, as well as the smooth muscle cells and endothelial cells that populate cerebrovascular interfaces (1, 21, 32). Although purinergic signaling is an important element of the communication network between astrocytes and surrounding cells, the signaling events upstream of ATP release, as well as the actual conduits or pathways for the export of ATP, have not been clearly established. Studies of regulated ATP release in different astrocyte models have implicated either channel-mediated efflux of cytosolic ATP or exocytosis of vesicles/organelles containing compartmentalized ATP as predominant pathways for the export of intracellular ATP pools. Support for exocytotic models of ATP release has largely been predicated on the inhibitory actions of various reagents, such as brefeldin A, tetanus toxins, or dominant-negative soluble N-ethylmaleimide-sensitive-factor attachment receptor (SNARE) proteins that target particular steps in the standard Golgi → transport vesicle → vesicle/plasma membrane fusion trafficking pathways, as reviewed in Ref. 19. Secretory lysosomes have been recently proposed as a source of releasable ATP from astrocytes based on the ability of glycylphenylalanine 2-napthylamide (GPN), a substrate for lysosomal cathepsin C, to coordinately collapse lysosome integrity and repress the ATP release stimulated by metabolic stress or glutamate receptor activation (63). Studies of conductive pathways have predominantly focused on nonjunctional “gap-junction hemichannels” composed of connexin or pannexin subunits that may act as conduits for stimulated ATP efflux from astrocytes (57) and other cell types (20, 22, 23, 40, 41, 64). Support for this mode of ATP release has been based in part on the inhibitory actions of pharmacological agents, such as glycerrhetinic acid or carbenoxolone (CBX), known to target gap-junction channels. Although CBX was first characterized as an inhibitor of 11-β-hydroxysteroid dehydrogenase, it has also been used extensively to inhibit the activity of intercellular gap-junction channels and gap-junction hemichannels (18).
Regardless of whether channel-mediated efflux or vesicle exocytosis comprises the predominant ATP release mechanism, most (14, 15, 17, 33, 43, 44, 50, 63), but not all (62), studies have identified elevation of cytosolic Ca2+ as an important regulator of nucleotide export in the different astrocyte model systems. In this regard, we have previously reported that elevated cytosolic Ca2+ plays a critical role in the ATP release elicited by stimulation of protease-activated receptor 1 (PAR1) or M3-muscarinic (M3R) GPCR in the 1321N1 human astrocytoma cell line. PAR1 stimulation-induced ATP release was consistently approximately fourfold higher than that induced by M3R stimulation despite equivalent Ca2+ mobilization responses to either receptor. Experiments with BAPTA-loaded cells revealed that M3R-induced ATP release was entirely dependent on elevation of cytosolic Ca2+, whereas the PAR1-triggered ATP accumulation involved an additional Ca2+-independent component (33). Brown and colleagues (3, 39, 49) have demonstrated that while PAR1 and M3R both activate Gq in 1321N1 cells, only PAR1 additionally couples to G12/13 to regulate Rho signaling. Rho activation and other changes in cytoskeletal organization have been implicated in the activation or modulation of ATP release in other model systems (16, 25, 28, 37). Therefore, we hypothesized that Rho activation and subsequent Rho kinase (ROCK) signaling may synergize with Ca2+ mobilization to increase GPCR-dependent ATP release. We used Clostridium difficile Toxin B (ToxB) and Clostridium botulinum Toxin C3 (C3) to demonstrate that Rho family GTPases potentiate Ca2+-dependent ATP release from 1321N1 human astrocytoma cells via a ROCK-independent signaling pathway. Experiments with brefeldin-treated cells suggest that this Rho-sensitive pathway may involve, in part, the mobilization of Golgi-derived, ATP-containing secretory vesicles. We also observed that CBX suppresses GPCR-stimulated ATP release in the absence of any obvious changes in membrane permeability indicative of hemichannel gating.
METHODS
Reagents.
Carbachol, lysophosphatidic acid (LPA), thrombin, βγ-methylene ATP (βγ-meATP), and lyophilized firefly luciferase ATP assay mix (FL-AAM, LUC) containing luciferase, luciferin, MgSO4, dithiothreitol, EDTA, bovine serum albumin (BSA), and Tricine buffer were from Sigma-Aldrich. Thrombin receptor activating peptide (SFLLRD-TRAP) was synthesized by SynPep. The cytosolic [Ca2+]-buffering agent BAPTA-AM was obtained from Molecular Probes. Wild-type 1321N1 human astrocytoma cells were obtained from Drs. Ken Harden and Jose Boyer (University of North Carolina-Chapel Hill). Purified ToxB was obtained from the Tech Laboratory diagnostic test kit. C3 exoenzyme, the RhoA-“G-LISA” kit, and F-Actin Visualization kit were from Cytoskeleton. A cDNA construct of the fusion protein glutathione S-transferase-rhotekin Rho-binding domain (GST-TRBD) was kindly provided by Dr. Martin Schwartz (University of Virginia). Rabbit polyclonal antibody to RhoA (sc-119) was obtained from Santa Cruz.
Cell culture.
1321N1 human astrocytes were maintained in Dulbecco's minimal essential medium (DMEM) containing 10% iron-supplemented bovine calf serum (Hyclone), penicillin (100 U/ml), and streptomycin (100 μg/ml). For all luciferase-based and Rho activation experiments, 1321N1 cells were seeded on 35-mm dishes (Falcon) at 3 × 105 cells per dish, or cells were seeded on 24-well plates at a density of 4 × 104 cells per well. All experiments were conducted using confluent cell monolayers cultured for 5 to 7 days postplating followed by serum starvation for 16 to 24 h before analysis of ATP release. Serum-free DMEM contained 0.1% BSA, penicillin (100 U/ml), and streptomycin (100 μg/ml).
Clostridial toxin loading.
Confluent 1321N1 cell monolayers were treated with a 1:50 dilution of purified ToxB (TechLab) for 3 h at 37°C until significant (>95%) cell rounding was observed (see Fig. 2B). Alternatively, cell monolayers were treated with 2 μg/ml of cell-permeant C3 exoenzyme for 6 h, which did not cause cell rounding (see Fig. 2C).
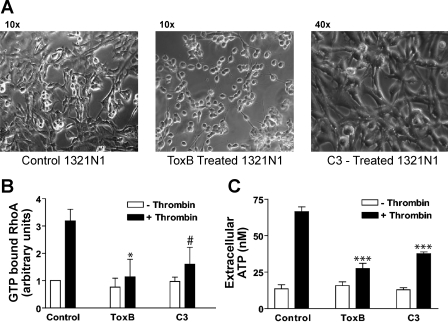
Fig. 2.
Rho-GTPase activity is correlated with thrombin induced ATP release. A: photographs were taken of serum-starved 1321N1 cells maintained treated for 3 h with 1:50 dilution of ToxB or treated for 6 h with 2 μg/ml C3. B: G-LISA was performed as described in methods. Data represent means ± SE of 3 independent experiments each performed in duplicate for control and C3 groups and two independent experiments each performed in duplicate for the ToxB groups. *P < 0.05 vs. thrombin-treated control. #P = 0.06 vs. thrombin-treated control. C: 1321N1 cells were treated with 300 μM βγ-meATP for 15 min. A sample of the reaction media was taken for a baseline [ATP] measurement (openr bars) and then cells were treated with 2 U/ml thrombin for 15 min before removing a second sample of the reaction media (solid bars). Extracellular ATP concentration was measured via an off-line luciferin/luciferase assay as described in methods. Data represent means ± SE of 4 independent experiments each performed in triplicate. ***P < 0.001 vs. thrombin-treated control.
RhoA-GTP-Rhotekin pull-down assay.
GST-TRBD protein was expressed (in E. coli BL21 strain) and purified using previously described protocols except that 200 μg/ml lysozyme was added before sonication of the bacteria (51). The effects of ToxB on Rho signaling were studied in parallel matched samples of untreated versus ToxB-loaded 1321N1 cells and analyzed by RhoA-GTP-Rhotekin pull-down assays. Briefly, a 5 × Mg2+ lysis buffer (MLB) was made containing 125 mM HEPES, pH 7.5, 750 mM NaCl, 5% Nonidet P40, 50 mM MgCl2, 5 mM EDTA, and 10% glycerol. Confluent 35-mm dishes of serum-starved 1321N1 cells pretreated for 3 h with or without ToxB were washed twice and bathed in 1 ml basal saline solution (BSS) containing: 130 mM NaCl, 5 mM KCl, 1.5 mM CaCl2, 1 mM MgCl2, 25 mM HEPES (pH 7.5), 5 mM glucose, and 0.1% BSA. The cells were equilibrated at room temperature (22–25°C) for ∼45 min before being treated with 3 μM TRAP, 100 μM carbachol, or 10 μM LPA for 2 min. The BSS was aspirated, and the cells were lysed and scraped on ice in 1 ml of MLB (plus protease inhibitors). The lysates were then clarified at 14,000 rpm for 5 min at 4°C. Untreated control samples were separated into 2 × 0.5 ml aliquots on ice. For the GTPγS control, one of these aliquots was treated with 10 μl of 0.5 mM EDTA to chelate Mg2+ ions. After addition of 10 μM GTPγS, this lysate sample was subsequently incubated at room temperature for 30 min. The reaction was stopped by addition of 32 μl of 1.0 M MgCl2, and the GTP-loading control was run to verify pull down of activated RhoA (data not shown). Along with this positive control, the rest of the samples were aliquoted in 0.5 ml cleared lysate/tube. To each sample ∼30 μg of freshly thawed GST-TRBD-bead slurry was added, and the reaction mixtures were rotated for 45 min at 4°C. The beads were washed three times with 0.5 ml MLB, and the slurries were resuspended in 40 μl 2 × Laemmli buffer, boiled for 5 min, and then treated with 2 μl of 1.0 M DTT (to ensure dissociation of bound Rho-GTP from the GSH-beads). Standard Western blot analysis techniques were then used to probe for activated RhoA using 1:200 rabbit polyclonal anti-RhoA antibody (Santa Cruz). This antibody also recognizes RhoB in loading controls (whole cell lysates), but only RhoA binds to the Rhotekin protein.
ELISA-based RhoA activation assay.
RhoA activity was determined in whole cell lysates prepared from monolayers of 1321N1 cells using the absorbance based G-LISA RhoA activation assay kit (Cytoskeleton) according to the manufacturer's instructions. After 2 min of stimulation with 2 U/ml thrombin, cells were lysed using the supplied cell lysis buffer. Lysates were clarified by centrifugation at 10,000 rpm at 4°C for 2 min. One portion of the lysate was used for quantification of protein concentration and the other portion was used for Rho G-LISA assay. The lysate used in the Rho G-LISA assay was snap frozen in liquid nitrogen as soon as possible after cell lysis to prevent GTP hydrolysis by the extracted Rho. After protein quantification, the frozen aliquots of cell lysate were rapidly thawed, and 0.75 mg/ml protein was used in each well of the supplied 96-well plate. All subsequent incubation and detection followed the instructions provided by the manufacturer.
Measurement and buffering of cytosolic Ca2+.
Receptor-triggered elevations in cytosolic [Ca2+] were assayed using fura 2 fluoresecence measurements as previously described (33). Briefly, serum-starved 1321N1 cell monolayers on 10-cm plates were trypsinized and resuspended in BSS and loaded with 1 μM fura2-AM at room temperature (20°C) for 1 h. The washed cells were then assayed for fura 2 fluorescence (339-nm excitation and 500-nm emission) in the presence of 300 μM βγ-meATP, 3 μM TRAP, 100 μM carbachol, or 10 μM LPA. The role of cytosolic [Ca2+] in stimulated ATP release was studied using 1321N1 cell monolayers loaded with the cell-permeable Ca2+ chelator 1,2-bis (2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetrakis acetoxymethyl ester (BAPTA-AM) as previously described (33).
On-line luciferase-based ATP assay.
Serum-starved 1321N1 astrocytes were prepared for ATP release assays as described previously (33, 34). Briefly, serum-starved 1321N1 cell monolayers were washed twice and bathed in 1 ml BSS. The washed monolayers were then incubated for ∼45 min at room temperature before experimental manipulation. Soluble FL-AAM (Sigma) was reconstituted with 5 ml of sterile filtered water and stored in frozen 500-μl aliquots. For experiments, aliquots of FLAAM were thawed at room temperature and diluted 1:25 (40 μl) into the 35-mm dishes before start of luminescence recordings. All extracellular ATP measurements were recorded using a Turner Designs (TD 20/20) luminometer that accommodates 35-mm culture dishes. ATP-dependent changes in extracellular luciferase activity were measured as relative luminescence unit (RLU) values integrated over 5-s photon counting periods. For all experiments, the luciferase activity was recorded every 2-min for up to 30 min. Calibration curves were generated for each experiment using cell-free dishes pulsed with increasing concentrations of ATP standards. The limit of ATP detection was 100 fmol per 1 ml assay volume and luminescence was linear with increasing ATP concentration up to 1,000 nM. After luciferase activity reached steady state, 1321N1 monolayers were treated for up to 15 min with 2 U/ml of thrombin, 3 μM of SFLLRD-TRAP, 100 μM carbachol, or 10 μM LPA. The ecto-ATPase inhibitor βγ-meATP (300 μM) was added either simultaneously with agonist or 15 min before agonist addition. Luciferase activity was recorded every 2 min during the stimulation period, and every addition to the 1-ml ATP assay volumes was made using 100- to 1,000-fold concentrated stocks of the various test reagents. At the end of each experiment, cell monolayers were permeabilized using digitonin (50 μg/ml), and the peak concentrations of digitonin-releasable ATP were averaged in matched dishes.
Off-line luciferase-based ATP assay.
Serum-starved 1321N1 cell monolayers in 24-well plates were washed twice and bathed in a final assay volume of 300 μl basal saline solution (BSS) for ∼60 min at room temperature before experimental manipulation. All subsequent additions to the sample resulted in less than 1% total change in volume. After addition of agonist, cells were incubated at room temperature for 15 min. Samples of extracellular media (50 μl) were carefully removed at designated times and boiled immediately for 5 min. After a brief (2 min, 1,000 g) centrifugation step to clarify the samples, ATP content was quantified using a Turner Designs (TD 20/20) luminometer. For all measurements, 25 μl of sample was added to a mix of 4 μl of FLAAM and 71 μl of BSS. The final volume was 100 μl with a 1:25 dilution of FLAAM. The solution was added to a 12 mm × 50 mm disposable plastic cuvette (Promega, Madison WI), and RLU values were integrated over 5-s photon counting periods.
Ethidium influx as assay of hemichannel activity.
Effects of PAR1 activation on hemichannel activity was assayed using ethidium dye influx as described previously (31). Briefly, trypsinized, suspended 1321N1 cells were assayed in a stirred cuvette at 37°C at a concentration of 5 × 105 cells/ml. Ethidium bromide (20 μM) was added and fluorescence was measured at 360 nm excitation/575 nm emission before and after stimulation with thrombin (2 U/ml); experiments were terminated by addition of digitonin (50 μg/ml) to permeabilize the cells to permit maximum binding of ethidium to cellular nucleic acids.
Data evaluation.
Relative luminescence unit (RLU) recordings were downloaded into Microsoft Excel using the Turner Designs spreadsheet interface software (version 2.0.1, Sunnyvale, CA). RLU values were converted to ATP concentrations using calibration curves generated with each experiment. OD490 absorbance values of GTP-bound RhoA were recorded using a (Molecular devices) SpectraMax 340 96-well plate reader. Measured values were normalized to untreated control cells. (GraphPad) Prism 3.0 software was used to compute the means and SE as well as generate graphs of the calculated ATP levels and relative GTP-bound RhoA from identical, independent experiments. Some figures were also generated using Adobe Illustrator 7.0 and Microsoft PowerPoint software. For statistical analysis of data the two-tailed Student's t-test was used to evaluate differences between means ± SE.
RESULTS
PAR1-activated ATP release is suppressed by BAPTA-buffering of cytosolic [Ca2+] or ToxB inhibition of Rho family GTPases.
PAR1 activation by either 3 μM TRAP or 2 U/ml thrombin elicits a rapid ATP release from 1321N1 astrocytes that is markedly attenuated by BAPTA-buffering of cytosolic Ca2+ increases as described in our previous report33 (Fig. 1, A and B). To detect steady-state increases in extracellular ATP in this model it is necessary to include βγ-MeATP, which suppresses the rapid clearance of released ATP by the predominant ecto-ATPase expressed on 1321N1 astrocytes (34). PAR1-induced ATP release was also reduced (50–75%) when these cells were pretreated with Clostridial difficile ToxB for 3 h. (Figs. 1, A and B, and 2C). ToxB catalyzes the transfer of the glucosyl moiety from UDP-glucose to conserved threonine residues in the effector targeting domains of RhoA, Rac, and Cdc42 and renders all of these Rho family GTPases functionally inactive (2). The observed inhibitory action of ToxB suggests that Rho family GTPases can synergize with elevated Ca2+ to potentiate ATP release in this astrocyte model system.
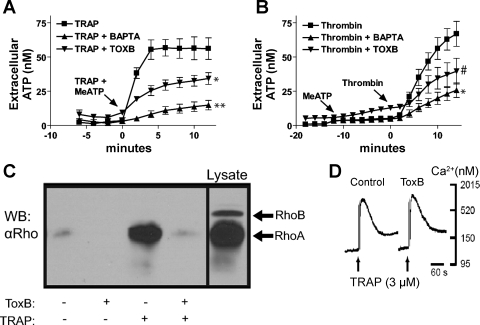
Fig. 1.
Protease-activated receptor (PAR1)-mediated ATP release is sensitive to BAPTA and Clostridium difficile toxin B (ToxB). A: changes in stimulated extracellular [ATP] were recorded in control cells (▪) versus cells pretreated with ToxB (▾), or BAPTA (▴) as described in methods. On-line ATP measurements were made every 2 min after addition of 3 μM thrombin receptor-activating peptide (TRAP) in combination with 300 μM βγ-methylene ATP (meATP) for 12 min and measured extracellular ATP concentration was measured via an on-line luciferin-luciferase assay. Data represent means ± SE of 3 independent experiments each performed in triplicate. *P < 0.05 vs. TRAP-treated control. **P < 0.01 vs. TRAP-treated control. The differences between the control and the treated groups were significant at the indicated P values from the t = 2-min through t = 12-min time points. B: 1321N1 cells were treated with 300 μM βγ-meATP for 12 min followed by 2 U/ml thrombin for 14 min. Extracellular ATP concentration was measured via an on-line luciferin-luciferase assay. Data represent means ± SE of 3 independent experiments performed in triplicate. *P < 0.05 vs. thrombin-treated control. #P < 0.08 vs. thrombin-treated control. The differences between the control and the treated groups were significant (P < 0.05) from the t = 4-min through t = 12-min time points. C: stimulation with 3 μM TRAP for 2 min induces RhoA-GTP accumulation and ToxB disrupts this process. Aliquots of lysate were subjected to Rhotekin (TRBD)-RhoA-GTP pull-down assays as described in methods, and Western blots (WB) were done using anti-RhoA antibody. The data are representative of two separate experiments. D: suspended 1321N1 cells were loaded with fura 2-AM and treated with 3 μM TRAP to determine that ToxB-loading had no observable effect on elevations in cytosolic [Ca2+]. The data are representative of two separate experiments.
To verify that PAR1 stimulation elicits a ToxB-sensitive activation of Rho family GTPases, control or ToxB-treated 1321N1 cells were stimulated with TRAP for 2 min before lysis and analysis by a Rhotekin-based RhoA-GTP pull-down assay. ToxB treatment effectively reduced the basal RhoA-GTP content and strongly suppressed the robust TRAP-activated increase in RhoA-GTP levels observed in control cells (Fig. 1C). Use of a quantitative ELISA-based protocol to quantify RhoA-GTP content similarly indicated that PAR activation triggered a 3.2-fold increase in Rho-GTP, which was reduced by >85% in ToxB-treated cells (Fig. 2B). In contrast, ToxB treatment had no effect on the TRAP-stimulated elevation in cytosolic Ca2+ concentrations (Fig. 1D). Therefore, the PAR1-mediated signal transduction pathways leading to ATP release involve both Rho family GTPase activation and Ca2+ mobilization. Moreover, suppression of Rho family GTPases does not attenuate Ca2+ mobilization, indicating that these responses comprise parallel signaling pathways that converge on the ATP release process.
ToxB-treated monolayers were characterized by elevated basal levels of extracellular ATP (relative to control cells) when assayed by the on-line luciferase assay (Fig. 1, A and B) but not the off-line ATP measurements (Fig. 2C). We speculate that this may be due to the repeated movement of the culture dishes into and out of the luminometer chamber in the former, but not latter, protocol. This repeated movement may induce mechanical stimulation-dependent ATP release due to enhanced effects of fluid shear on the rounded-up cells that characterize the ToxB-treated cultures (Fig. 2A).
Increased Rho-GTP accumulation but not Rho-kinase activity is correlated with thrombin-induced ATP release.
Because ToxB glucosylates and inhibits the Rho, Rac, and Cdc42 members of the Rho-GTPase family, use of this reagent does not reveal which particular member(s) of this small GTPase family regulates the ATP release response elicited by PAR1 agonists. Clostridial botulinum C3 toxin is a mono-ADP-ribosyl transferase that selectively inhibits RhoA, RhoB, and RhoC by covalently modifying the N-41 residue of these proteins, preventing nucleotide exchange (61). We used a membrane-permeable version of C3 toxin to test whether Rho subtype GTPases, in particular, are involved in ATP release. Although ToxB treatment for 3 h caused uniform rounding of adherent 1321N1 human astrocytes, a 6-h preincubation with 2 μg/ml C3 minimally affected cell shape (Fig. 2A). However, this C3 treatment produced a 75% decrease in thrombin-stimulated Rho-GTP accumulation (Fig. 2B), which was comparable to the 85% reduction produced by ToxB. The C3-induced decrease in Rho activation was correlated with a 57% decrease in thrombin-triggered ATP release (Fig. 2C); this compared with the 75% decrease observed in ToxB-treated cells assayed under identical conditions. Thus C3 is only marginally less efficacious than ToxB as an inhibitor of ATP release despite the ability of ToxB to additionally target Rac and Cdc42. This indicates that the inhibitory effects of ToxB on stimulated ATP release predominantly reflect the inactivation of Rho-dependent signals.
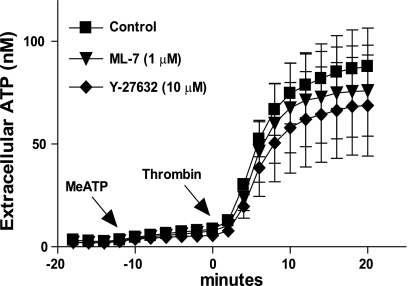
We tested whether this Rho-dependent component of regulated ATP release was related to the well-characterized roles of Rho on cytoskeletal dynamics. These latter actions of Rho are mediated in part by the downstream Rho-dependent kinases I/II (ROCK1/2) coupled to myosin light chain (MLC) phosphorylation. Previous studies have indicated that PAR1 activation of 1321N1 cells triggers rapid ROCK-dependent changes in cell shape and organization of the actin cytoskeleton (29, 36, 42, 56). However, treatment of 1321N1 cells with 10 μM of the Y-27632 ROCK inhibitor for 1 h before thrombin stimulation did not attenuate the rate or peak magnitude of ATP release (Fig. 3A). Likewise, 1321N1 astrocytes treated with 1 μM of the ML-7 MLC-kinase inhibitor exhibited no changes in their ATP release response to thrombin. These data indicate that thrombin-dependent ATP release does not involve an obligatory role for ROCK, MLCK, or major reorganization of actin stress-fibers. The efficacies of Y-27632 and ML-7 were confirmed by their ability to reduce the number of longitudinal stress fibers in 1321N1 cells stained with rhodamine-phalloidin (data not shown).
Fig. 3.
Inhibition of Rho kinase (ROCK)I/II and myosin light chain kinase (MLCK) does not affect thrombin-induced ATP release. Cells were treated with 300 μM βγ-meATP for 10 min and then stimulated with 2 U/ml thrombin (▪) or stimulated with 2 U/ml thrombin after 60 min pretreatment with 10 μM Y-27632 (▾) or 1 μM ML-7 (⧫) as described in methods. Measurements were made every 2 min using an on-line luciferin-luciferase assay. Data represent means ± SE of 3 independent experiments performed in triplicate.
Role for Rho signaling in the ATP release triggered by LPA receptors but not muscarinic receptors.
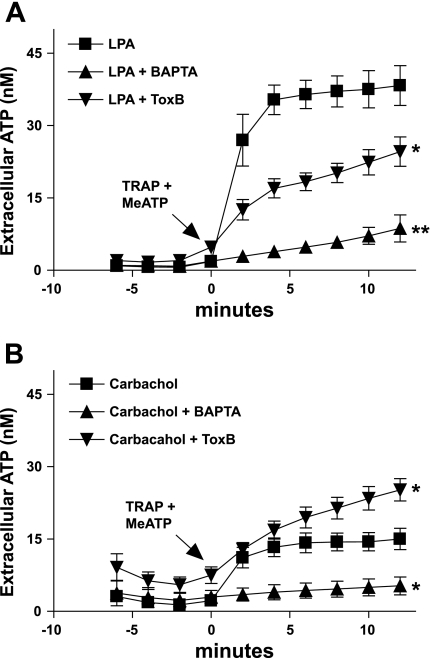
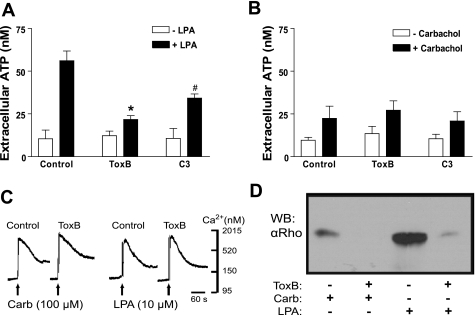
The role of Rho as a positive modulator of Ca2+-dependent ATP release was further analyzed by comparing the responses of 1321N1 cells to LPA versus carbachol. LPA acts on LPA1R, LPA2R, and LPA3R (formerly known as EDG-family receptors 2,4,7) (12), which are expressed in 1321N1 cells and, like PAR1, are able to both mobilize intracellular Ca2+ and activate Rho. Carbachol activates M3R, the predominant muscarinic receptor in 1321N1 cells (4). M3R couple to Gq/phospholiase C (PLC) and mobilize intracellular Ca2+ but do not activate G12/13/Rho signaling pathways (24). Similar to PAR1 activation, lysophosphatidic acid receptor (LPAR) stimulation elicited a robust ATP release that was greater than threefold greater than that triggered by carbachol in either the on-line (Fig. 4, A and B) or off-line (Fig. 4, C and D) luciferase assays. The LPA-induced ATP release was markedly reduced by BAPTA, ToxB, or C3 toxin, whereas the carbachol-stimulated release was eliminated by BAPTA loading but not affected by the Rho-directed toxins. As noted previously, ToxB treatment modestly enhances the basal accumulation of extracellular ATP in the on-line luciferase assays but not the off-line assays (compare Figs. 4B and 5B). Thus the aggregate basal plus carbachol-induced extracellular ATP accumulation in ToxB-treated cells was actually greater than that in the control monolayers (Fig. 4B). Again, this effect of ToxB was not recapitulated in the off-line ATP assays (Fig. 5B).
Fig. 4.
Effects of ToxB and BAPTA-loading on ATP release from 1321N1 astrocytes in response to lysophosphatidic acid (LPA) and carbachol. Changes in stimulated extracellular [ATP] were recorded in control (▪) cells versus ToxB-pretreated (▴), or BAPTA-pretreated (▴) cells as described under methods. A: on-line ATP measurements were made every 2 min after addition of 300 μM βγ-meATP concurrent with 10 μM LPA. *P < 0.05 vs. LPA-treated control. The differences between the control and the treated groups were significant (P < 0.05) from t = 2-min through t = 12-min time points. B: on-line ATP measurements were made every 2 min after addition of 300 μM βγ-meATP concurrent with 100 μM carbachol. *P < 0.05 vs. carbachol-treated control. The differences between the control and the BAPTA-treated groups was significant (P < 0.05) from the t = 2-min through the t = 12-min time points. The differences between the control and the ToxB-treated groups were significant (P < 0.05) at the t = 10 min and t = 12-min time points. Data for both panels represent means ± SE of 3 independent experiments each performed in triplicate.
Fig. 5.
Rho-GTPase activity is correlated with LPA- but not carbachol-induced ATP release. A and B: 1321N1 cells were treated with 300 μM βγ-meATP for 15 min. A sample of the reaction media was taken for a baseline [ATP] measurement (open bars), and then cells were stimulated with 100 μM carbachol or 10 μM LPA for 15 min before removing second sample of the reaction media (solid bars). Extracellular ATP concentration was measured via an off-line luciferin-luciferase assay as described in methods. Data represent means ± SE of 4 independent experiments each performed in triplicate. *P < 0.01 vs. LPA-treated control. #P < 0.05 vs. LPA-treated control. C: suspended 1321N1 cells were loaded with fura 2-AM and stimulated with 100 μM carbachol or 10 μM LPA to determine that ToxB loading had no observable effect on elevations in cytosolic [Ca2+]. This experiment was performed once. D: stimulation with 10 μM LPA for 2 min induced RhoA-GTP accumulation, whereas stimulation with 100 μM carbachol does not. Aliquots of lysate were subjected to Rhotekin (TRBD)-RhoA-GTP pull-down assays as described in methods and WB were done using anti-RhoA antibody. The data are representative of two separate experiments.
ToxB and C3 treatment similarly attenuated ATP release in response to LPA (Fig. 5A) but not carbachol (Fig. 5B). We also confirmed that LPA, but not carbachol, recapitulates the ToxB-sensitive stimulation of RhoA-GTP acccumulation elicited by PAR1 agonists (compare Figs. 5D and 1C). In contrast, ToxB treatment did not attenuate the equivalent Ca2+ mobilization responses to either LPA or carbachol (Fig. 5C). The similar abilities of PAR1 and LPAR, but not M3R, to coordinately stimulate Ca2+ mobilization, RhoGTP accumulation, and robust ATP release further supports the hypothesis that Rho-GTPase activation acts as a strong positive modulator of Ca2+-dependent ATP release.
Clostridial toxins and BAPTA-loading do not affect 1321N1 cell ecto-ATPase activity.
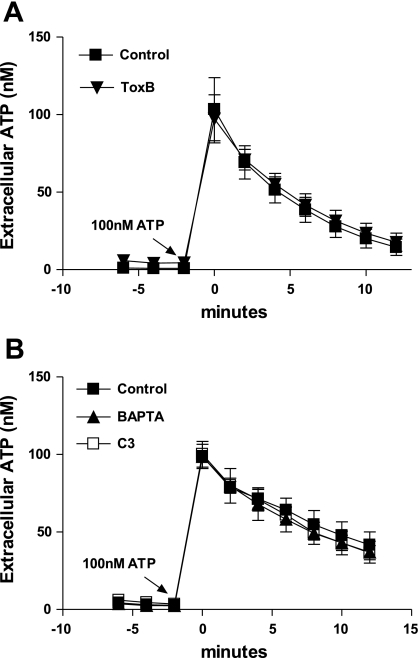
Extracellular ATP concentrations reflect a balance between ATP release and ATP clearance by ectonucleotidases. Thus a decrease in GPCR-induced extracellular ATP accumulation could reflect an increased rate of ATP clearance rather than, or in addition to, a reduced rate of ATP export. Although MeATP was routinely included to suppress ATP clearance, it was important to verify that treatment of 1321N1 cells with Rho-directed toxins or BAPTA-loading did not upregulate a MeATP-insensitive ecto-ATPase. Alternatively, the higher basal (preagonist) level of extracellular ATP observed in ToxB-treated cells assayed by the on-line luciferase protocol could be indicative of a reduced rate of ATP clearance. However, direct comparison of the ecto-ATPase activities in control, ToxB-, C3-, or BAPTA-treated cultures of 1321N1 cells challenged with identical 100 nM pulses of exogenous ATP revealed no differences in nucleotide clearance (Fig. 6, A and B). Modest differences in the control rates of hydrolysis between Fig. 6, A and B, experiments likely reflect differences in passage number and/or cell density.
Fig. 6.
Neither toxin treatment nor BAPTA affect extracellular ATPase activity. A and B: exogenous ATP (100 nM) was added at time = 0 min to control (▪), or 1321N1 cells pretreated with toxB (▴), C3 (□) or BAPTA (▴). Extracellular [ATP] was recorded every two min using an on-line luciferin-luciferase assay. Data represent means ± SE of 3 independent experiments each performed in triplicate.
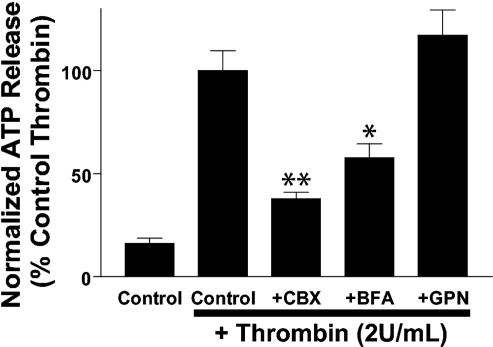
PAR1-stimulated ATP release is reduced by CBX and brefeldin-A but not GPN.
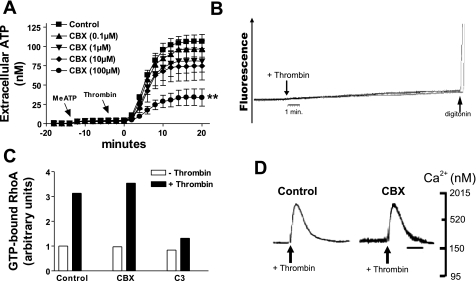
We tested the involvement of Golgi-derived secretory vesicles, secretory lysosomes, or gap junction hemichannels as possible ATP release mechanisms by pretreating 1321N1 monolayers with brefeldin A (BFA, 5 μg/ml × 90 min), CBX (100 μM × 30 min), or GPN (200 μM × 15 min) before stimulation with thrombin for 15 min (Fig. 7). ATP release was reduced by 80% with CBX and by 50% with BFA but not suppressed in GPN-treated cells. Given its marked inhibitory actions, we further characterized the actions of CBX on PAR1-stimulated signaling, ATP release, and possible hemichannel activity. CBX caused a concentration-dependent inhibition of thrombin-induced ATP release from 1321N1 cells with ∼35% and 80% inhibition by 10 and 100 μM CBX, respectively (Fig. 8A). Significantly, activation of PAR receptors with thrombin did not elicit ethidium bromide uptake, an indicator of nonselective pore activity (Fig. 8B). Permeabilization with digitonin verified that maximal ethidium-dependent fluorescence increases were equivalent in all assays. Despite its marked suppression of ATP release, CBX did not affect either the Rho-GTP activation (Fig. 8C) or the Ca2+ mobilization signals that mediate thrombin-stimulated ATP release (Fig. 8D).
Fig. 7.
ATP release is attenuated by brefeldin A (BFA) and carbenoxolone (CBX) but not glycylphenylalanine-2-napthylamide (GPN). Extracellular [ATP] was measured in cell monolayers treated with βγ-MeATP (300 μM) alone or βγ-MeATP added concurrently with thrombin (2 U/ml) for 15 min. Where indicated, cells were pretreated with the following inhibitors before the ATP release assay: CBX (100 μM) for 30 min, BFA (5 μg/ml) for 2 h, and GPN (200 μM) for 15 min. Off-line ATP measurements were made using a luciferin-luciferase assay as described in methods. ATP release values were normalized to thrombin-stimulated ATP export measured in the absence of pharmacological inhibitors. Data represent means ± SE of 3 independent experiments each performed in triplicate. *P < 0.05 vs. thrombin-treated control. **P < 0.01 vs. thrombin-treated control.
Fig. 8.
CBX inhibition of thrombin-stimulated ATP release is not correlated with changes in hemichannel activity or PAR1 signaling. A: changes in stimulated extracellular [ATP] were recorded in control cells (▪) versus cells pretreated with 0.1 μM CBX (▴), 1 μM CBX (▾), 10 μM CBX (⧫), or 100 μM CBX (•) for 30 min. βγ-MeATP (300 μM) was added 12 min before thrombin (2 U/ml). On-line ATP measurements were made every 2 min as described in methods via an on-line luciferin-luciferase assay as described in methods. **P < 0.01 vs. thrombin-treated control. The differences between the control and the CBX (100 μM)-treated groups were significant (P < 0.05) from the t = 4-min through the t = 12-min time points. Data represent means ± SE of 3 independent experiments each performed in triplicate. B: suspended 1321N1 cells were incubated in basal saline solution (BSS) supplemented with ethidium bromide (20 μM) as described in methods before the addition of thrombin (2 U/ml). Experiments were terminated by the addition of digitonin to permeabilize cells. The data are representative of two separate experiments. C: G-LISA was performed as described in methods. Data represent one independent experiment in duplicate. D: suspended 1321N1 cells were loaded with fura 2-AM as described in methods. 100 μM CBX was added 30 min before thrombin (2 U/ml) addition. The data are representative of two separate experiments.
DISCUSSION
We used 1321N1 astrocytoma cells as a model system to investigate ATP release in response to GPCR activation. The principal finding is that activation of Rho signaling markedly potentiates Ca2+-dependent ATP release in these cells. This extends and clarifies our previous observation that PAR1 activation in 1321N1 cells leads to greater release of ATP than is induced by M3R activation even though stimulation of either receptor leads to equivalent Ca2+ mobilization. However, other findings dissociated this effect of Rho activation on ATP release from its known actions on ROCK effector enzymes, actin cytoskeleton dynamics, or cell shape change. We additionally identified LPA as an efficacious ATP secretagogue in 1321N1 astrocytes. LPAR activation, similar to PAR1 activation, triggered both Ca2+ mobilization and Rho activation, thus inducing greater ATP release than that was observed with M3R activation. Finally, ATP release in response to thrombin was markedly repressed by the gap junction inhibitor CBX and by BFA, which disrupts the Golgi-derived exocytotic secretory pathway.
Signaling mechanisms that regulate ATP release.
Our observations suggest that the efficacy of a particular GPCR in inducing ATP release from nonexcitable cells will be limited by its capacity to coordinately couple to both PLC→Ca2+ mobilization and RhoGEF→Rho activation pathways. Although this will generally involve coupling to parallel Gq→ PLCβ→ and G12/13→ RhoGEF cascades, Gq has been implicated as an upstream inducer of Rho activation in some cell types, and G12/13 may regulate Ca2+ mobilization via Rho-dependent PLCɛ activation in other cellular contexts (30, 54, 55). Additionally, lymphoid blast crisis (lbc) Rho-GEF activity can augment Gq signaling via interactions independent of accumulated active RhoA (53). Thus cellular responses, such as ATP release, which require Gq→ PLC→ Ca2+ mobilization as necessary signals may be modulated by Rho signaling via multiple networks. Although PAR1 activation triggers markedly less inositol phosphate accumulation than M3R stimulation in 1321N1 astocytes, both receptors couple to PLC in these cells via pertussis toxin-insensitive and presumably Gq-mediated pathways (36, 38, 39). In contrast, LPAR has been reported to elicit inositol lipid turnover in 1321N1 cells via a PTX-sensitive pathway likely involving Giβγ regulation of other PLC isoforms (27). Moreover, Citro et al. (13) have recently reported that inositol phosphate generation in response to thrombin, but not LPA or carbachol, depends on primary Rho activation in cultures of primary rat astrocytes. Despite these differences in GPCR-induced inositol phosphate generation pathways in various astrocyte models, we observed no differences in maximal Ca2+ mobilization in response to thrombin, LPA, or carbachol in our 1321N1 model (Fig. 1D, 4C). In contrast, activation of PAR1 and LPAR, but not M3R, triggered robust accumulation of active Rho in these astrocytes. Similar divergent effects of PAR1 versus M3R on Rho activation, as well as Rho-dependent rounding of 1321N1 cells, have been previously described (56).
C3 toxin selectively inactivates RhoA, RhoB, and RhoC, whereas ToxB nonselectively inactivates all Rho-family GTPases (61). Because both C3 toxin and ToxB inhibited GPCR-activated ATP release from 1321N1 cells to a similar extent (Figs. 2C, 5, A and B), RhoA is the most likely Rho-family GTPase to potentiate Ca2+-dependent ATP release. Rho activation and regulated ATP release have been linked in previous studies using other model systems. Inactivation of Rho with C3 toxin attenuates the ATP release stimulated by hypotonic stress in bovine aortic endothelial cells (37). Moreover, Hirakawa et al. (28) noted that treatment of human endothelial cells (HUVEC) with LPA elicited cotemporal RhoA activation, Ca2+ mobilization, and rapid ATP release similar to our observations with human astrocytes (Figs. 4A and 5, C and D). However, an important difference between these studies was that GPCR-induced ATP release from HUVEC was completely suppressed by the ROCK inhibitor Y-27632, whereas we observed no effect of Y-27632 on PAR1-triggered ATP release from 1321N1 cells (Fig. 3). Several factors may underlie this divergent effect of ROCK inhibition of ATP release in these two cell types. Interestingly, hypotonic stress-induced Ca2+ mobilization in these HUVEC was also inhibited by Y-27632, whereas LPA-induced Ca2+ transients were suppressed by suramin, a nonselective P2Y receptor antagonist. Attenuation of regulated ATP release by Y-27632 in these endothelial cells may reflect, in part, autocrine activation of P2 receptors with consequent ATP-induced ATP release. 1321N1 astrocytes are notable because they lack endogenous P2 receptor expression (45). Signaling reactions that affect accumulation of extracellular ATP release in these cells are not complicated by ATP-induced ATP release.
Previous studies have demonstrated that inhibition of either Rho-kinases by Y-27632 or myosin light chain kinase by ML-7 will suppress thrombin-stimulated rounding of 1321N1 cells, as well as remodeling of actin stress fibers (29, 35, 53). The inability of Y-27632 or ML-7 to attenuate ATP release (Fig. 3) dissociates the well-characterized actions of thrombin on actin cytoskeletal reorganization from its effects on ATP release in 1321N1 cells. Moreover, neither LPA, a potent ATP secretagogue, nor carbachol, a weak ATP secretagogue, mimic the ability of thombin to induce 1321N1 cell rounding (56). This suggests that the cytoskeletal reorganization that underlies cell rounding involves a network of GPCR signals distinct from those that elicit ATP release.
Because inhibition of ROCKs with Y-27632 did not affect ATP release, Rho signaling must potentiate Ca2+-dependent ATP release via another effector protein. Significantly, Kreda et al. (36) also found that the thrombin-stimulated, BAPTA-sensitive release of another nucleotide, UDP-glucose, from 1321N1 cells was unaffected by concentrations of Y-27632 that suppressed cell rounding and actin reorganization. Although the ROCKs are the best-characterized downstream targets of active Rho, several other signaling proteins including other serine-threonine kinases, protein phosphatases, lipid kinases, lipases, and scaffold proteins, have been implicated as Rho effectors (5). Several Rho effectors, other than ROCK, provide clear functional intersections of Ca2+ and Rho signaling that might be involved in GPCR-regulated ATP release. For example, Rho-sensitive phosphatidylinositol-4-phosphate 5-kinase (PI-4-P5K) is required to prime exocytotic vesicles of the Ca2+ regulated secretory pathway (26). Other studies have indicated a role for Rho signaling in the regulation of LPA- and GTPγS-stimulated glucose transport that involves rapid translocation of GLUT4 transporters in intracellular membrane pools to the surface membrane. This regulated mobilization of GLUT4 transporters can be inhibited by C3 toxin or expression of dominant-negative PKN (protein kinase N), a RhoA-regulated serine-threonine kinase (59).
ATP release mechanisms.
Regardless of the GPCR-dependent signals that induce ATP release from 1321N1 cells and other astrocyte models, the actual mechanism(s) by which intracellular ATP is transferred to the extracellular compartment remains poorly understood. Some studies have indicated that exocytosis of ATP within secretory vesicles or atypical organelles is the predominant route for ATP release from astrocytes. For example, Zhang et al. (63) recently reported that stimulation of primary rat astrocytes with ionomycin, glutamate receptor agonists, or metabolic inhibitors triggered an ATP release that involved exocytosis of secretory lysosomes containing compartmentalized ATP. In that system, stimulated ATP export was abolished by GPN, an agent that permeabilizes lysosomes. However, we found that thrombin-stimulated ATP release was not suppressed in GPN-treated 1321N1 cells (Fig. 7A). This is consistent with other reports indicating that ATP release from astrocytes is better correlated with the Ca2+-dependent exocytosis of nonlysosomal vesicles (44, 50). Haydon and colleagues (46) used an inducible transgenic mouse model selectively expressing dominant-negative SNARE protein within astrocytes to demonstrate the requirement of an exocytotic pathway for ATP release and subsequent extracellular adenosine accumulation that mediates activity-dependent heterosynaptic depression. Similarly, in mixed astrocyte/neuron cocultures, astrocyte Ca2+ wave propagation, which depends on paracrine activation of P2 receptors by released ATP, was found to be sensitive to BAPTA and bafilomycin but not to gap junction hemichannel inhibitors (10, 14).
Of particular relevance to our studies, Kreda et al. (36) recently described the GPCR-regulated release of UDP-glucose, a nucleotide-sugar that is the selective agonist of P2Y14 receptors, from 1321N1 astrocytes (11). Those investigators observed that thrombin, but not carbachol, triggered a rapid export of UDP-glucose that was inhibited by BAPTA-loading but was insensitive to the Y-27632 ROCK inhibitor. They also noted that thrombin-stimulated UDP-glucose release was almost completely suppressed (>95%) by BFA, which inhibits the generation of the Golgi-derived transport vesicles used for constitutive export of new proteins and lipids to the cell surface. Because of its role as a substrate for protein glycosylation, UDP-glucose is accumulated within the Golgi and Golgi-derived vesicles. ATP is also compartmentalized within the Golgi for use by the ATP-dependent chaperone proteins that mediate protein folding. Similarly, we observed that BFA treatment (5 μg/ml, 2 h) reduced PAR-1-activated ATP release (Fig. 7) but to a lesser extent (50% inhibition) than the UDP-glucose release. Taken together, our results and those of Kreda et al. indicate that ATP is likely costored and coreleased with UDP-glucose in mobilizable Golgi-derived vesicles.
The ability of BFA to completely suppress PAR1-activated UDP-glucose release while only partially attenuating ATP release from 1321N1 cells suggests that ATP is exported by an additional pathway(s) in this model. In this regard, multiple reports have described strong correlations between stimulated ATP release and the activation of gap junction hemichannels. For example, Ca2+-dependent ATP release from C6 glioma cells is markedly increased by connexin overexpression (15, 17, 58). Multiple studies have used various pharmacological blockers of connexin-based gap junctions and nonjunctional hemichannels to probe the possible role of such channels in ATP export. CBX is one such widely used inhibitor of gap junction channels, hemichannels, and ATP release, Although CBX blocks gap junction channels and nonjunctional hemichannels formed by both pannexins and connexins, hemichannels formed by pannexins have been reported to be more sensitive to CBX blockade (6). Pelegrin and Surprenant (47) recently reported that pannexin-1 is endogenously expressed in 1321N1 astrocytes and that CBX-sensitive hemichannel activity (as assayed by fluorescent dye fluxes) can be stimulated by extracellular ATP in 1321N1 cells engineered to express heterologous P2X7 receptors. Although CBX markedly inhibited PAR1-stimulated ATP release from 1321N1 cells (Figs. 7 and 8A), we were unable to correlate these effects with any pannexin-like hemichannel activity as detected by thrombin-stimulated or CBX-inhibited ethidium bromide uptake (Fig. 8B). This does not unequivocally exclude the possibility that hemichannels mediate ATP efflux because the difference in charge between ethidium (+1) and ATP (−4) or MgATP (−2) could impact movement via such channels. Another possibility is that the pore-forming ability of hemichannels is not required for ATP release but rather that pannexin or connexin proteins modulate release by other mechanisms (48). CBX is also known to affect voltage-gated Ca2+ channels and membrane potential via gap junction channel-independent mechanisms (52, 60). Similarly, CBX may exert connexin/pannexin-independent actions on the signal transduction pathways or membrane dynamics that regulate nonconductive, exocytotic ATP release pathways. However, we did verify that CBX treatment did not attenuate PAR1-stimulated Ca2+ mobilization or Rho activation in 1321N1 astrocytes. (Fig. 8, C and D).
In summary, our studies indicate that the coordinate induction of Ca2+- and Rho-GTPase signals are required for maximal ATP release from astrocytes and that this ATP export reflects in part the mobilization of Golgi-derived transport vesicles. However, defining the mechanisms that underlie the brefeldin-insensitive and CBX-sensitive components of ATP release remains a challenging area of investigation.
GRANTS
This work was supported by National Institutes of Health Grants PO1-HL-18708 and RO1-GM-36387 (to G. R. Dubyak) American Heart Association Grant-in-Aid 9950305N (to G. R. Dubyak). A. E. Blum was supported by T32-HL-07653 “Cleveland Training Program in Cardiovascular Research” and T32-GM-07250 “Medical Scientist Training Program.”
Acknowledgments
We thank Sylvia Kertesy for technical assistance in tissue culture and Domenick Prosdocimo for useful discussions. We also are grateful to Mark Estacion, William Schilling, Jordan Beach, and Thomas Egelhoff for assistance with microscopy.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Abbracchio MP, Verderio C. Pathophysiological roles of P2 receptors in glial cells. Novartis Found Symp 276: 91–103, 2006. [PubMed] [Google Scholar]
- 2.Aktories K, Schmidt G, Just I. Rho GTPases as targets of bacterial protein toxins. Biol Chem 381: 421–426, 2000. [DOI] [PubMed] [Google Scholar]
- 3.Aragay AM, Collins LR, Post GR, Watson AJ, Feramisco JR, Brown JH, Simon MI. G12 requirement for thrombin-stimulated gene expression and DNA synthesis in 1321N1 astrocytoma cells. J Biol Chem 270: 20073–20077, 1995. [DOI] [PubMed] [Google Scholar]
- 4.Ashkenazi A, Peralta EG, Winslow JW, Ramachandran J, Capon DJ. Functional role of muscarinic acetylcholine receptor subtype diversity. Cold Spring Harb Symp Quant Biol 53: 263–272, 1988. [DOI] [PubMed] [Google Scholar]
- 5.Bishop AL, Hall A. Rho GTPases and their effector proteins. Biochem J 348: 241–255, 2000. [PMC free article] [PubMed] [Google Scholar]
- 6.Bruzzone R, Barbe MT, Jakob NJ, Monyer H. Pharmacological properties of homomeric and heteromeric pannexin hemichannels expressed in Xenopus oocytes. J Neurochem 92: 1033–1043, 2005. [DOI] [PubMed] [Google Scholar]
- 7.Burnstock G Purine and pyrimidine receptors. Cell Mol Life Sci 64: 1471–1483, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burnstock G Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev 87: 659–797, 2007. [DOI] [PubMed] [Google Scholar]
- 9.Burnstock G Purinergic signaling-an overview. In: Novartis Foundation Symposium, edited by Chadwick DJ, Goode J. 2006, vol. 276, p. 26–53. [PubMed]
- 10.Bowser DN, Khakh BS. Vesicular ATP is the predominant cause of intercellular calcium waves in astrocytes. J Gen Physiol 129: 485–491, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chambers JK, Macdonald LE, Sarau HM, Ames RS, Freeman K, Foley JJ, Zhu Y, McLaughlin MM, Murdock P, McMillan L, Trill J, Swift A, Aiyar N, Taylor P, Vawter L, Naheed S, Szekeres P, Hervieu G, Scott C, Watson JM, Murphy AJ, Duzic E, Klein C, Bergsma DJ, Wilson S, Livi GP. A G protein-coupled receptor for UDP-glucose. J Biol Chem 275: 10767–10771, 2000. [DOI] [PubMed] [Google Scholar]
- 12.Chun J, Goetzl EJ, Hla T, Igarashi Y, Lynch KR, Moolenaar W, Pyne S, Tigyi G. International Union of Pharmacology. XXXIV Lysophospholipid receptor nomenclature. Pharmacol Rev 54: 265–269, 2002. [DOI] [PubMed] [Google Scholar]
- 13.Citro S, Malik S, Oestreich EA, Radeff-Huang J, Kelley GG, Smrcka AV, Brown JH. Phospholipase Cepsilon is a nexus for Rho and Rap-mediated G protein-coupled receptor-induced astrocyte proliferation. Proc Natl Acad Sci USA 104: 15543–15548, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coco S, Calegari F, Pravettoni E, Pozzi D, Taverna E, Rosa P, Matteoli M, Verderio C. Storage and release of ATP from astrocytes in culture. J Biol Chem 278: 1354–1362, 2003. [DOI] [PubMed] [Google Scholar]
- 15.Cotrina ML, Lin JH, Alves-Rodrigues A, Liu S, Li J, Azmi-Ghadimi H, Kang J, Naus CC, Nedergaard M. Connexins regulate calcium signaling by controlling ATP release. Proc Natl Acad Sci USA 95: 15735–15740, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cotrina ML, Lin JH, Nedergaard M. Cytoskeletal assembly and ATP release regulate astrocytic calcium signaling. J Neurosci 18: 8794–8804, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cotrina ML, Lin JH, López-García JC, Naus CC, Nedergaard M. ATP-mediated glia signaling. J Neurosci 20: 2835–2844, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duax WL, Ghosh D, Pletnev V. Steroid dehydrogenase structures, mechanism of action, and disease. Vitam Horm 58: 121–148, 2000. [DOI] [PubMed] [Google Scholar]
- 19.Dubyak GR ATP Release Mechanisms. In: Progress in Pharmacology and Toxicology: The Roles of Nucleotides in the Regulation of Bone Formation and Resorption, edited by G. Burnstock and T. Arnett. CRC Press, 2006.
- 20.Eltzschig HK, Eckle T, Mager A, Küper N, Karcher C, Weissmüller T, Boengler K, Schulz R, Robson SC, Colgan SP. ATP release from activated neutrophils occurs via connexin 43 and modulates adenosine-dependent endothelial cell function. Circ Res 99: 1100–1108, 2006. [DOI] [PubMed] [Google Scholar]
- 21.Fields RD, Burnstock G. Purinergic signalling in neuron-glia interactions. Nat Rev Neurosci 7: 423–436, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Genetos DC, Kephart CJ, Zhang Y, Yellowley CE, Donahue HJ. Oscillating fluid flow activation of gap junction hemichannels induces ATP release from MLO-Y4 osteocytes. J Cell Physiol 212: 207–214, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gomes P, Srinivas SP, Van Driessche W, Vereecke J, Himpens B. ATP release through connexin hemichannels in corneal endothelial cells. Invest Ophthalmol Vis Sci 46: 1208–1218, 2005. [DOI] [PubMed] [Google Scholar]
- 24.Guizzetti M, Costa P, Peters J, Costa LG. Acetylcholine as a mitogen: muscarinic receptor-mediated proliferation of rat astrocytes and human astrocytoma cells. Eur J Pharmacol 297: 265–273, 1996. [DOI] [PubMed] [Google Scholar]
- 25.Guo Y, Satpathy M, Wilson G, Srinivas SP. Benzalkonium chloride induces dephosphorylation of Myosin light chain in cultured corneal epithelial cells. Invest Ophthalmol Vis Sci 48: 2001–2008, 2007. [DOI] [PubMed] [Google Scholar]
- 26.Hay JC, Fisette PL, Jenkins GH, Fukami K, Takenawa T, Anderson RA, Martin TF. ATP-dependent inositide phosphorylation required for Ca(2+)-activated secretion. Nature 374: 173–177, 1995. [DOI] [PubMed] [Google Scholar]
- 27.Hernández M, Barrero MJ, Crespo MS, Nieto ML. Lysophosphatidic acid inhibits Ca2+ signaling in response to epidermal growth factor receptor stimulation in human astrocytoma cells by a mechanism involving phospholipase C(gamma) and a G(alphai) protein. J Neurochem 75: 1575–1582, 2000. [DOI] [PubMed] [Google Scholar]
- 28.Hirakawa M, Oike M, Karashima Y, Ito Y. Sequential activation of RhoA and FAK/paxillin leads to ATP release and actin reorganization in human endothelium. J Physiol 558: 479–488, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Honma S, Saika M, Ohkubo S, Kurose H, Nakahata N. Thromboxane A2 receptor-mediated G12/13-dependent glial morphological change. Eur J Pharmacol 545: 100–108, 2006. [DOI] [PubMed] [Google Scholar]
- 30.Hubbard KB, Hepler JR. Cell signalling diversity of the Gqalpha family of heterotrimeric G proteins. Cell Signal 18: 135–150, 2006. [DOI] [PubMed] [Google Scholar]
- 31.Humphreys BD, Dubyak GR. Induction of the P2z/P2X7 nucleotide receptor and associated phospholipase D activity by lipopolysaccharide and IFN-gamma in the human THP-1 monocytic cell line. J Immunol 157: 5627–5637, 1996. [PubMed] [Google Scholar]
- 32.Inoue K, Koizumi S, Tsuda M. The role of nucleotides in the neuron–glia communication responsible for the brain functions. J Neurochem 102: 1447–1458, 2007. [DOI] [PubMed] [Google Scholar]
- 33.Joseph SM, Buchakjian MR, Dubyak GR. Colocalization of ATP release sites and ecto-ATPase activity at the extracellular surface of human astrocytes. J Biol Chem 278: 23331–23342, 2003. [DOI] [PubMed] [Google Scholar]
- 34.Joseph SM, Pifer MA, Przybylski RJ, Dubyak GR. Methylene ATP analogs as modulators of extracellular ATP metabolism and accumulation. Br J Pharmacol 142: 1002–1014, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Katoh K, Kano Y, Amano M, Kaibuchi K, Fujiwara K. Stress fiber organization regulated by MLCK and Rho-kinase in cultured human fibroblasts. Am J Physiol Cell Physiol 280: C1669–C1679, 2001. [DOI] [PubMed] [Google Scholar]
- 36.Kreda SM, Seminario-Vidal L, Heusden CV, Lazarowski ER. Thrombin-promoted release of UDP-glucose from human astrocytoma cells. Br J Pharmacol (Jan 21, 2008), doi: 10.1038/sj. bjp.0707692. [DOI] [PMC free article] [PubMed]
- 37.Koyama T, Oike M, Ito Y. Involvement of Rho-kinase and tyrosine kinase in hypotonic stress-induced ATP release in bovine aortic endothelial cells. J Physiol 532: 759–769, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.LaMorte VJ, Harootunian AT, Spiegel AM, Tsien RY, Feramisco JR. Mediation of growth factor induced DNA synthesis and calcium mobilization by Gq and Gi2. J Cell Biol 121: 91–99, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.LaMorte VJ, Kennedy ED, Collins LR, Goldstein D, Harootunian AT, Brown JH, Feramisco JR. A requirement for Ras protein function in thrombin-stimulated mitogenesis in astrocytoma cells. J Biol Chem 268: 19411–19415, 1993. [PubMed] [Google Scholar]
- 40.Litvin O, Tiunova A, Connell-Alberts Y, Panchin Y, Baranova Litvin O A, Tiunova A, Connell-Alberts Y, Panchin Y, Baranova A. What is hidden in the pannexin treasure trove: the sneak peek and the guesswork. J Cell Mol Med 10: 613–634, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Locovei S, Bao L, Dahl G. Pannexin 1 in erythrocytes: function without a gap. Proc Natl Acad Sci USA 103: 7655–7659, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Majumdar M, Seasholtz TM, Goldstein D, de Lanerolle P, Brown JH. Requirement for Rho-mediated myosin light chain phosphorylation in thrombin-stimulated cell rounding and its dissociation from mitogenesis. J Biol Chem 273: 10099–10106, 1998. [DOI] [PubMed] [Google Scholar]
- 43.Montana V, Malarkey EB, Verderio C, Matteoli M, Parpura V. Vesicular transmitter release from astrocytes. Glia 54: 700–715, 2006. [DOI] [PubMed] [Google Scholar]
- 44.Pangrsic T, Potokar M, Stenovec M, Kreft M, Fabbretti E, Nistri A, Pryazhnikov E, Khiroug L, Giniatullin R, Zorec R. Exocytotic release of ATP from cultured astrocytes. J Biol Chem 282: 28749–28758, 2007. [DOI] [PubMed] [Google Scholar]
- 45.Parr CE, Sullivan DM, Paradiso AM, Lazarowski ER, Burch LH, Olsen JC, Erb L, Weisman GA, Boucher RC, Turner JT. Cloning and expression of a human P2U nucleotide receptor, a target for cystic fibrosis pharmacotherapy. Proc Natl Acad Sci USA 91: 3275–3279, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pascual O, Casper KB, Kubera C, Zhang J, Revilla-Sanchez R, Sul JY, Takano H, Moss SJ, McCarthy K, Haydon PG. Astrocytic purinergic signaling coordinates synaptic networks. Science 310: 113–116, 2005. [DOI] [PubMed] [Google Scholar]
- 47.Pelegrin P, Surprenant A. Pannexin-1 mediates large pore formation and interleukin-1beta release by the ATP-gated P2X7 receptor. EMBO J 25: 5071–5082, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pelegrin P, Surprenant A. Pannexin-1 couples to maitotoxin- and nigericin-induced interleukin-1beta release through a dye uptake-independent pathway. J Biol Chem 282: 2386–2394, 2007. [DOI] [PubMed] [Google Scholar]
- 49.Post GR, Collins LR, Kennedy ED, Moskowitz SA, Aragay AM, Goldstein D, Brown JH. Coupling of the thrombin receptor to G12 may account for selective effects of thrombin on gene expression and DNA synthesis in 1321N1 astrocytoma cells. Mol Biol Cell 7: 1679–1690, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pryazhnikov E, Khiroug L. Sub-micromolar increase in [Ca(2+)](i) triggers delayed exocytosis of ATP in cultured astrocytes. Glia 56: 38–49, 2008. [DOI] [PubMed] [Google Scholar]
- 51.Ren XD, Schwartz MA. Determination of GTP loading on Rho. Methods Enzymol 325: 264–272, 2000. [DOI] [PubMed] [Google Scholar]
- 52.Rouach N, Segal M, Koulakoff A, Giaume C, Avignone E. Carbenoxolone blockade of neuronal network activity in culture is not mediated by an action on gap junctions. J Physiol 553: 729–745, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sagi SA, Seasholtz TM, Kobiashvili M, Wilson BA, Toksoz D, Brown JH. Physical and functional interactions of Galphaq with Rho and its exchange factors. J Biol Chem 276: 15445–15452, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sah VP, Seasholtz TM, Sagi SA, Brown JH. The role of Rho in G protein-coupled receptor signal transduction. Annu Rev Pharmacol Toxicol 40: 459–489, 2000. [DOI] [PubMed] [Google Scholar]
- 55.Scemes E Components of astrocytic intercellular calcium signaling. Mol Neurobiol 22: 167–179, 2000. [DOI] [PubMed] [Google Scholar]
- 56.Seasholtz TM, Radeff-Huang J, Sagi SA, Matteo R, Weems JM, Cohen AS, Feramisco JR, Brown JH. Rho-mediated cytoskeletal rearrangement in response to LPA is functionally antagonized by Rac1 and PIP2. J Neurochem 91: 501–512, 2004. [DOI] [PubMed] [Google Scholar]
- 57.Spray DC, Ye ZC, Ransom BR. Functional connexin “hemichannels”: a critical appraisal. Glia 54: 758–773, 2006. [DOI] [PubMed] [Google Scholar]
- 58.Stout CE, Costantin JL, Naus CC, Charles AC. Intercellular calcium signaling in astrocytes via ATP release through connexin hemichannels. J Biol Chem 277: 10482–10488, 2002. [DOI] [PubMed] [Google Scholar]
- 59.Standaert M, Bandyopadhyay G, Galloway L, Ono Y, Mukai H, Farese R. Comparative effects of GTPgammaS and insulin on the activation of Rho, phosphatidylinositol 3-kinase, and protein kinase N in rat adipocytes. Relationship to glucose transport. J Biol Chem 273: 7470–7477, 1998. [DOI] [PubMed] [Google Scholar]
- 60.Vessey JP, Lalonde MR, Mizan HA, Welch NC, Kelly ME, Barnes S. Carbenoxolone inhibition of voltage-gated Ca channels and synaptic transmission in the retina. J Neurophysiol 92: 1252–1256, 2004. [DOI] [PubMed] [Google Scholar]
- 61.Vogelsgesang M, Pautsch A, Aktories K. C3 exoenzymes, novel insights into structure and action of Rho-ADP-ribosylating toxins. Naunyn Schmiedebergs Arch Pharmacol 374: 347–360, 2007. [DOI] [PubMed] [Google Scholar]
- 62.Wang Z, Haydon PG, Yeung ES. Direct observation of calcium-independent intercellular ATP signaling in astrocytes. Anal Chem 72: 2001–2007, 2000. [DOI] [PubMed] [Google Scholar]
- 63.Zhang Z, Chen G, Zhou W, Song A, Xu T, Luo Q, Wang W, Gu XS, Duan S. Regulated ATP release from astrocytes through lysosome exocytosis. Nat Cell Biol 9: 945–953, 2007. [DOI] [PubMed] [Google Scholar]
- 64.Zhao HB, Yu N, Fleming CR. Gap junctional hemichannel-mediated ATP release and hearing controls in the inner ear. Proc Natl Acad Sci USA 102: 18724–18729, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]