Abstract
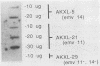
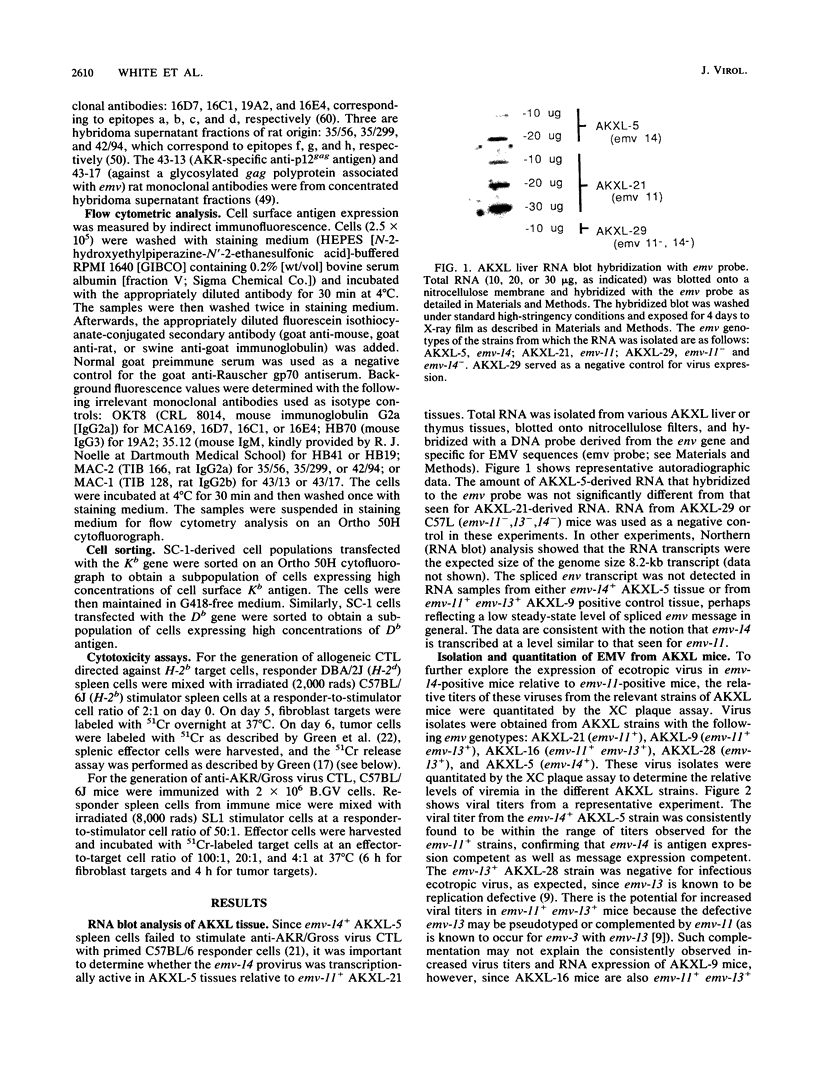
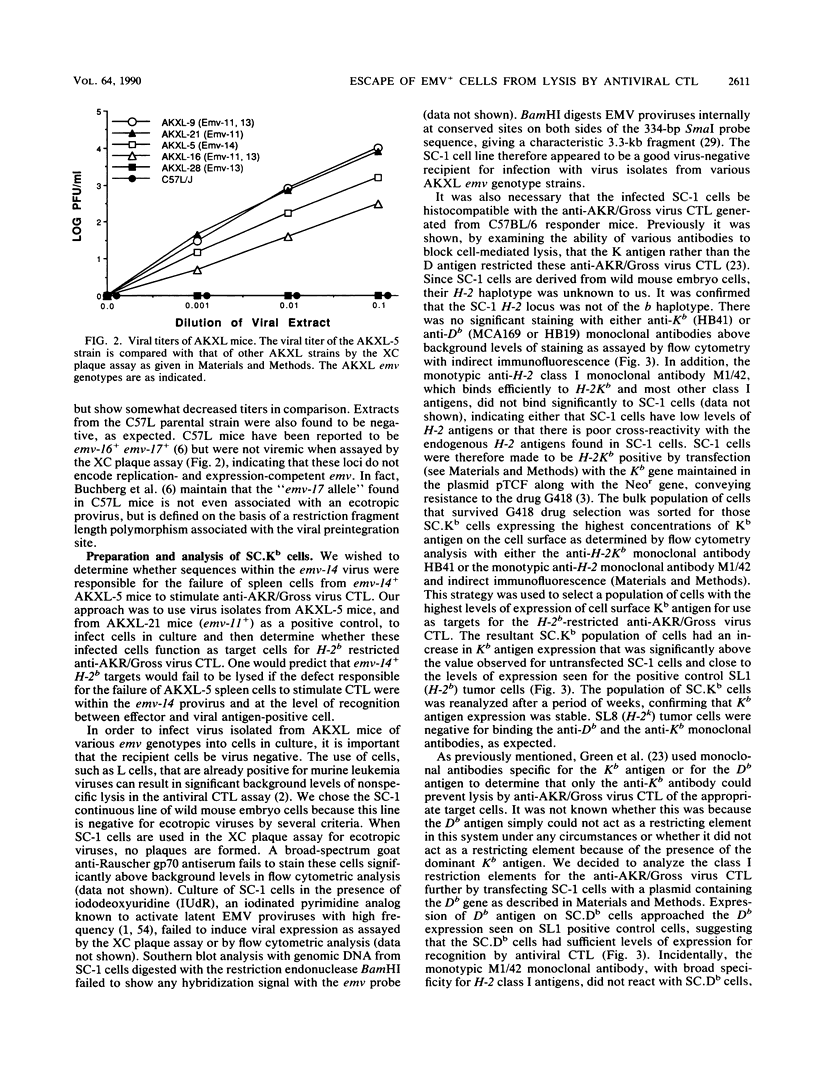
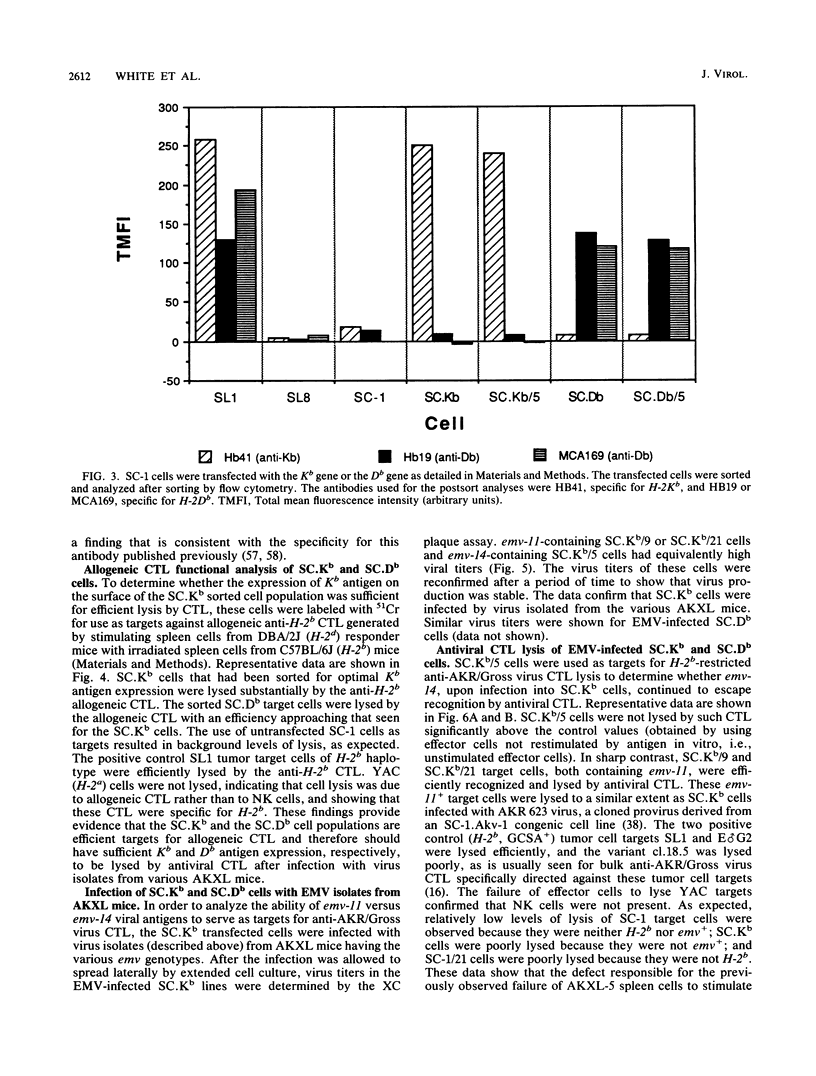
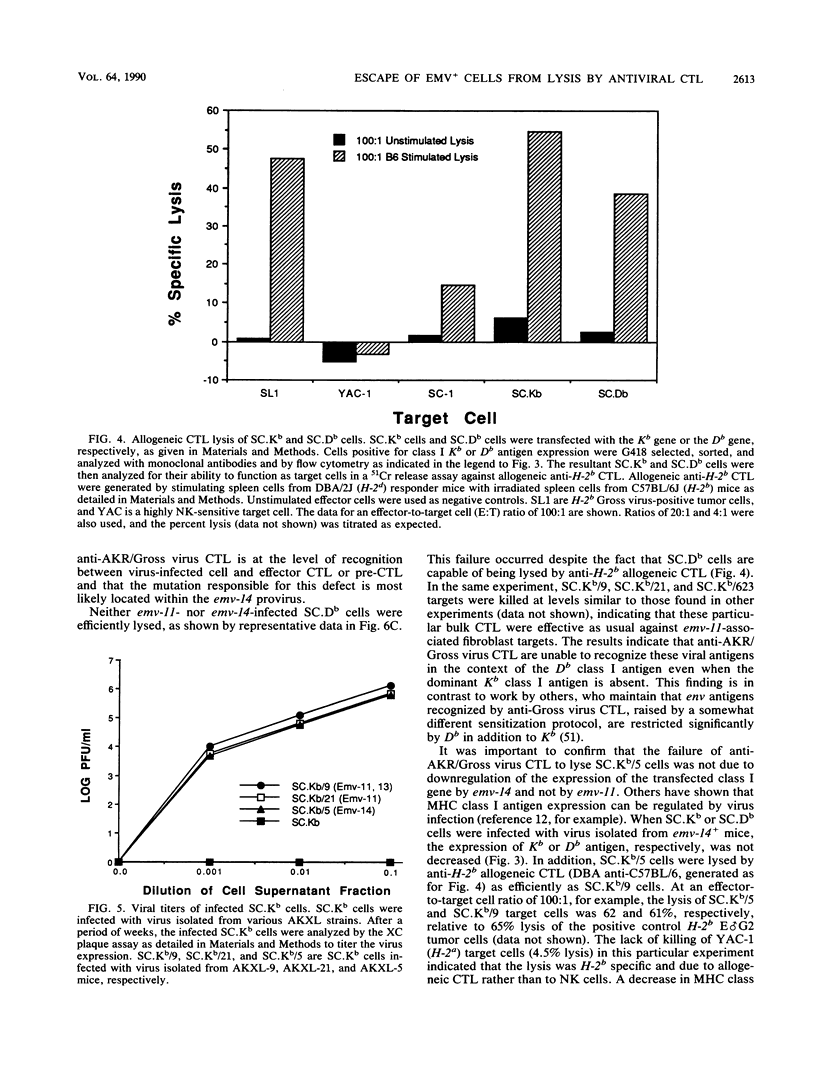
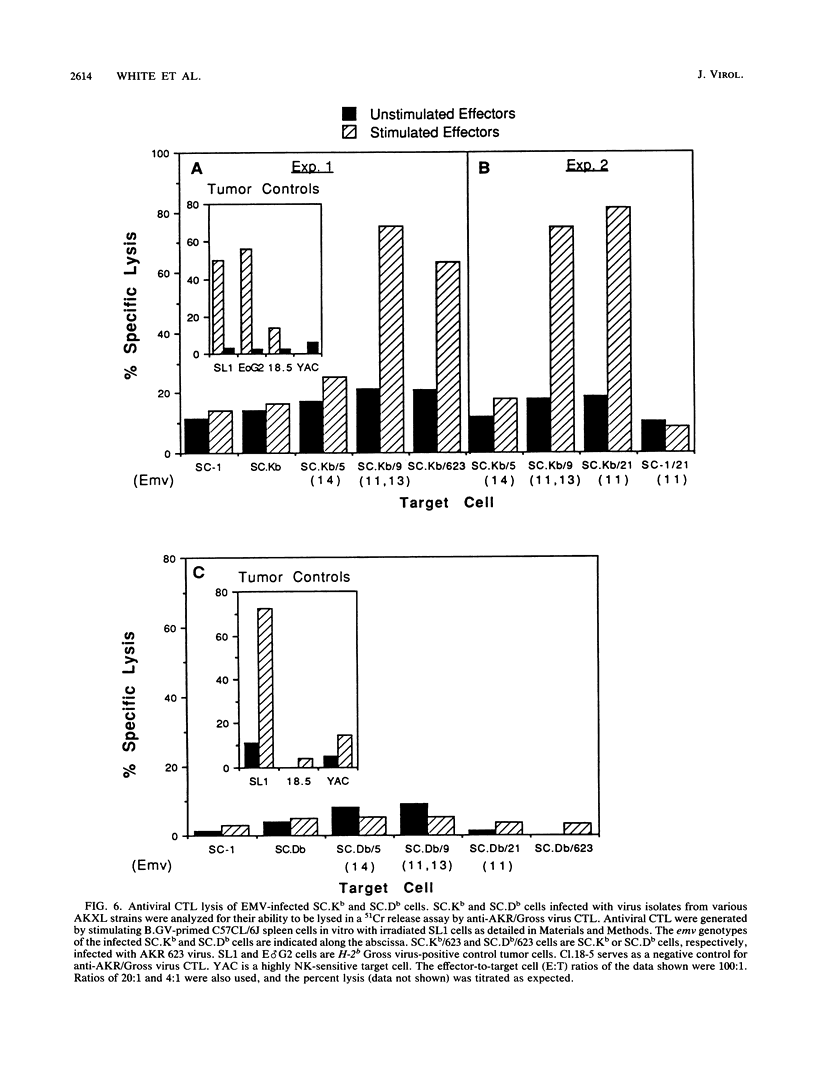
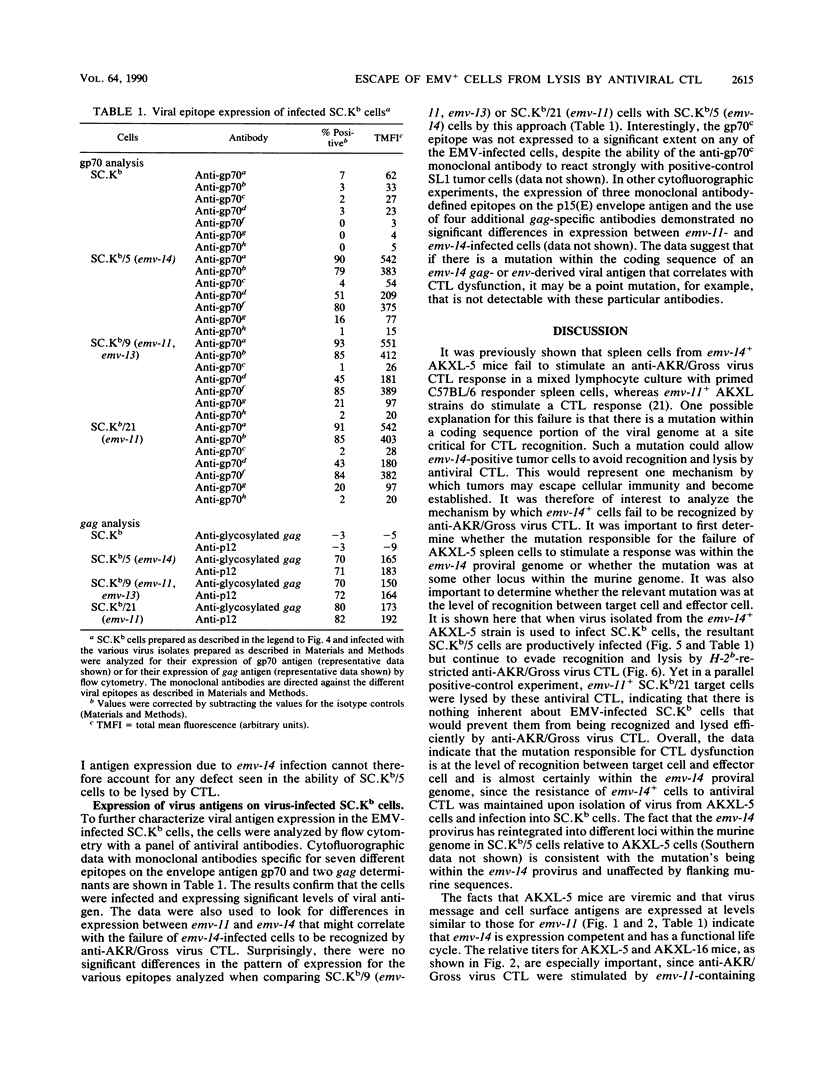
It was previously shown that spleen cells from endogenous ecotropic murine leukemia virus emv-14+ AKXL-5 mice fail to stimulate an anti-AKR/Gross virus cytolytic T-lymphocyte (CTL) response in a mixed lymphocyte culture with primed C57BL/6 responder spleen cells, whereas spleen cells from AKXL strains carrying the very similar emv-11 provirus do stimulate a response (Green and Graziano, Immunogenetics 23:106-110, 1986). We wished to determine whether the lack of response with AKXL-5 spleen cells was at the level of recognition between effector cell and target cell and whether the relevant mutation was within the emv-14 provirus. It is shown here that EMV-negative SC-1 fibroblast cells transfected with the major histocompatibility complex class I Kb gene and infected with virus isolated from the AKXL-5 strain (SC.Kb/5 cells) were not lysed by H-2b-restricted anti-AKR/Gross virus CTL. SC.Kb cells infected with virus isolated from emv-11+ strains, however, were efficiently lysed by anti-AKR/Gross virus CTL, indicating that there is nothing intrinsic to EMV-infected SC.Kb cells that would prevent them from being recognized and lysed efficiently by anti-AKR/Gross virus CTL. Analysis of virus expression for the infected SC.Kb cells by XC plaque assay and by flow cytometry indicated that emv-14 virus expression for SC.Kb/5 cells was not significantly different from that for emv-11-containing SC.Kb/9 or SC.Kb/21 cells. These data show that the mutation responsible for the lack of CTL recognition and lysis is at the level of recognition between target cell and effector cell. Furthermore, these data strongly suggest that the mutation is within the emv-14 genome. Flow cytometry experiments with monoclonal antibodies against a number of viral determinants indicated that there was no gross mutation detectable in the viral determinants analyzed. The data suggest that the relevant mutation may be a point mutation or a small insertion or deletion within a coding sequence that is critical for CTL recognition.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A., Dunn C. Y. High-frequency C-type virus induction by inhibitors of protein synthesis. Science. 1974 Feb 1;183(4123):422–424. doi: 10.1126/science.183.4123.422. [DOI] [PubMed] [Google Scholar]
- Abastado J. P., Plata F., Morello D., Daniel-Vedele F., Kourilsky P. H-2-restricted cytolysis of L cells doubly transformed with a cloned H-2Kb gene and cloned retroviral DNA. J Immunol. 1985 Nov;135(5):3512–3519. [PubMed] [Google Scholar]
- Allen H., Wraith D., Pala P., Askonas B., Flavell R. A. Domain interactions of H-2 class I antigens alter cytotoxic T-cell recognition sites. Nature. 1984 May 17;309(5965):279–281. doi: 10.1038/309279a0. [DOI] [PubMed] [Google Scholar]
- Bennink J. R., Yewdell J. W., Smith G. L., Moss B. Anti-influenza virus cytotoxic T lymphocytes recognize the three viral polymerases and a nonstructural protein: responsiveness to individual viral antigens is major histocompatibility complex controlled. J Virol. 1987 Apr;61(4):1098–1102. doi: 10.1128/jvi.61.4.1098-1102.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borysiewicz L. K., Hickling J. K., Graham S., Sinclair J., Cranage M. P., Smith G. L., Sissons J. G. Human cytomegalovirus-specific cytotoxic T cells. Relative frequency of stage-specific CTL recognizing the 72-kD immediate early protein and glycoprotein B expressed by recombinant vaccinia viruses. J Exp Med. 1988 Sep 1;168(3):919–931. doi: 10.1084/jem.168.3.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchberg A. M., Taylor B. A., Jenkins N. A., Copeland N. G. Chromosomal localization of Emv-16 and Emv-17, two closely linked ecotropic proviruses of RF/J mice. J Virol. 1986 Dec;60(3):1175–1178. doi: 10.1128/jvi.60.3.1175-1178.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charpentier B., Michelson S., Martin B. Definition of human cytomegalovirus-specific target antigens recognized by cytotoxic T cells generated in vitro by using an autologous lymphocyte system. J Immunol. 1986 Jul 1;137(1):330–336. [PubMed] [Google Scholar]
- Chattopadhyay S. K., Lander M. R., Rands E., Lowy D. R. Structure of endogenous murine leukemia virus DNA in mouse genomes. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5774–5778. doi: 10.1073/pnas.77.10.5774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland N. G., Bedigian H. G., Thomas C. Y., Jenkins N. A. DNAs of two molecularly cloned endogenous ecotropic proviruses are poorly infectious in DNA transfection assays. J Virol. 1984 Feb;49(2):437–444. doi: 10.1128/jvi.49.2.437-444.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Flyer D. C., Burakoff S. J., Faller D. V. Cytotoxic T lymphocyte recognition of transfected cells expressing a cloned retroviral gene. 1983 Oct 27-Nov 2Nature. 305(5937):815–818. doi: 10.1038/305815a0. [DOI] [PubMed] [Google Scholar]
- Flyer D. C., Burakoff S. J., Faller D. V. Retrovirus-induced changes in major histocompatibility complex antigen expression influence susceptibility to lysis by cytotoxic T lymphocytes. J Immunol. 1985 Oct;135(4):2287–2292. [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Green W. R., Brown M. A. The specificity of H-2-restricted cytotoxic T lymphocytes directed to AKR/Gross leukemia virus-induced tumors. II. Altered gp70 display and production of noninfectious virus particles by an insusceptible variant tumor. Eur J Immunol. 1983 Nov;13(11):871–877. doi: 10.1002/eji.1830131103. [DOI] [PubMed] [Google Scholar]
- Green W. R. Cell surface expression of cytotoxic T lymphocyte-defined, AKR/Gross leukemia virus-associated tumor antigens by normal AKR.H-2b splenic B cells. J Immunol. 1983 Dec;131(6):3078–3084. [PubMed] [Google Scholar]
- Green W. R. Genetic control of the induction of cytolytic T lymphocyte responses to AKR/Gross viral leukemias. I. H-2-encoded dominant gene control. J Immunol. 1984 May;132(5):2658–2664. [PubMed] [Google Scholar]
- Green W. R. Genetic control of the induction of cytolytic T lymphocyte responses to AKR/Gross viral leukemias. II. Negative control by the Fv-1 locus in AKR mice of responder H-2b haplotype. J Immunol. 1984 May;132(5):2665–2671. [PubMed] [Google Scholar]
- Green W. R., Graziano R. F. Cytolytic T lymphocyte-defined retroviral antigens on normal cells: encoding by the Akv-1 proviral locus. Immunogenetics. 1986;23(2):106–110. doi: 10.1007/BF00377969. [DOI] [PubMed] [Google Scholar]
- Green W. R. H-2-restricted cytolytic T lymphocytes specific for a subclass of AKR endogenous leukemia virus-induced tumors: correlation of tumor cell susceptibility with expression of the gross cell surface antigen. J Immunol. 1980 Dec;125(6):2584–2590. [PubMed] [Google Scholar]
- Green W. R., Nowinski R. C., Henney C. S. Specificity of cytolytic T cells directed against AKR/Gross virus-induced syngeneic leukemias: antibodies directed against H-2K, but not against viral proteins, inhibit lysis. J Immunol. 1980 Aug;125(2):647–655. [PubMed] [Google Scholar]
- Green W. R., Nowinski R. C., Henney C. S. The generation and specificity of cytotoxic T cells raised against syngeneic tumor cells bearing AKR/Gross murine leukemia virus antigens. J Exp Med. 1979 Jul 1;150(1):51–66. doi: 10.1084/jem.150.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green W. R. The specificity of H-2-restricted cytotoxic T lymphocytes directed to AKR/Gross leukemia virus-induced tumors. I. Isolation of a selectively resistant variant tumor subclone. Eur J Immunol. 1983 Nov;13(11):863–870. doi: 10.1002/eji.1830131102. [DOI] [PubMed] [Google Scholar]
- Hany M., Oehen S., Schulz M., Hengartner H., Mackett M., Bishop D. H., Overton H., Zinkernagel R. M. Anti-viral protection and prevention of lymphocytic choriomeningitis or of the local footpad swelling reaction in mice by immunization with vaccinia-recombinant virus expressing LCMV-WE nucleoprotein or glycoprotein. Eur J Immunol. 1989 Mar;19(3):417–424. doi: 10.1002/eji.1830190302. [DOI] [PubMed] [Google Scholar]
- Hartley J. W., Rowe W. P. Clonal cells lines from a feral mouse embryo which lack host-range restrictions for murine leukemia viruses. Virology. 1975 May;65(1):128–134. doi: 10.1016/0042-6822(75)90013-6. [DOI] [PubMed] [Google Scholar]
- Hartley J. W., Wolford N. K., Old L. J., Rowe W. P. A new class of murine leukemia virus associated with development of spontaneous lymphomas. Proc Natl Acad Sci U S A. 1977 Feb;74(2):789–792. doi: 10.1073/pnas.74.2.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins N. A., Copeland N. G., Taylor B. A., Lee B. K. Dilute (d) coat colour mutation of DBA/2J mice is associated with the site of integration of an ecotropic MuLV genome. Nature. 1981 Oct 1;293(5831):370–374. doi: 10.1038/293370a0. [DOI] [PubMed] [Google Scholar]
- Jenkins N. A., Copeland N. G., Taylor B. A., Lee B. K. Organization, distribution, and stability of endogenous ecotropic murine leukemia virus DNA sequences in chromosomes of Mus musculus. J Virol. 1982 Jul;43(1):26–36. doi: 10.1128/jvi.43.1.26-36.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joly E., Salvato M., Whitton J. L., Oldstone M. B. Polymorphism of cytotoxic T-lymphocyte clones that recognize a defined nine-amino-acid immunodominant domain of lymphocytic choriomeningitis virus glycoprotein. J Virol. 1989 May;63(5):1845–1851. doi: 10.1128/jvi.63.5.1845-1851.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan A. S., Laigret F., Rodi C. P. Expression of mink cell focus-forming murine leukemia virus-related transcripts in AKR mice. J Virol. 1987 Mar;61(3):876–882. doi: 10.1128/jvi.61.3.876-882.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koszinowski U. H., Keil G. M., Schwarz H., Schickedanz J., Reddehase M. J. A nonstructural polypeptide encoded by immediate-early transcription unit 1 of murine cytomegalovirus is recognized by cytolytic T lymphocytes. J Exp Med. 1987 Jul 1;166(1):289–294. doi: 10.1084/jem.166.1.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilly F., Duran-Reynals M. L., Rowe W. P. Correlation of early murine leukemia virus titer and H-2 type with spontaneous leukemia in mice of the BALB/c times AKR cross: a genetic analysis. J Exp Med. 1975 Apr 1;141(4):882–889. [PMC free article] [PubMed] [Google Scholar]
- Lilly F. The role of genetics in Gross virus leukemogenesis. Bibl Haematol. 1970;(36):213–220. doi: 10.1159/000391710. [DOI] [PubMed] [Google Scholar]
- Lowy D. R., Rands E., Chattopadhyay S. K., Garon C. F., Hager G. L. Molecular cloning of infectious integrated murine leukemia virus DNA from infected mouse cells. Proc Natl Acad Sci U S A. 1980 Jan;77(1):614–618. doi: 10.1073/pnas.77.1.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manjunath R., Graziano R. F., Green W. R. The specificity of H-2-restricted cytotoxic T lymphocytes directed to AKR/Gross leukemia virus-induced tumors. III. Coordinate alterations in viral gp70 antigen expression and restoration of CTL-susceptibility to insusceptible variant tumors. J Immunol. 1986 Mar 15;136(6):2271–2279. [PubMed] [Google Scholar]
- Martin S., Courtney R. J., Fowler G., Rouse B. T. Herpes simplex virus type 1-specific cytotoxic T lymphocytes recognize virus nonstructural proteins. J Virol. 1988 Jul;62(7):2265–2273. doi: 10.1128/jvi.62.7.2265-2273.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx J. L. What T cells see and how they see it. Science. 1988 Nov 11;242(4880):863–865. doi: 10.1126/science.2460921. [DOI] [PubMed] [Google Scholar]
- Nowinski R. C., Brown M., Doyle T., Prentice R. L. Genetic and viral factors influencing the development of spontaneous leukemia in AKR mice. Virology. 1979 Jul 15;96(1):186–204. doi: 10.1016/0042-6822(79)90184-3. [DOI] [PubMed] [Google Scholar]
- O'Connell K. A., Gooding L. R. Cloned cytotoxic T lymphocytes recognize cells expressing discrete fragments of the SV40 tumor antigen. J Immunol. 1984 Feb;132(2):953–958. [PubMed] [Google Scholar]
- Oldstone M. B., Whitton J. L., Lewicki H., Tishon A. Fine dissection of a nine amino acid glycoprotein epitope, a major determinant recognized by lymphocytic choriomeningitis virus-specific class I-restricted H-2Db cytotoxic T lymphocytes. J Exp Med. 1988 Aug 1;168(2):559–570. doi: 10.1084/jem.168.2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozato K., Sachs D. H. Monoclonal antibodies to mouse MHC antigens. III. Hybridoma antibodies reacting to antigens of the H-2b haplotype reveal genetic control of isotype expression. J Immunol. 1981 Jan;126(1):317–321. [PubMed] [Google Scholar]
- Pals S. T., Zijstra M., Radaszkiewicz T., Quint W., Cuypers H. T., Schoenmakers H. J., Melief C. J., Berns A., Gleichmann E. Immunologic induction of malignant lymphoma: graft-vs-host reaction-induced B cell lymphomas contain integrations of predominantly ecotropic murine leukemia proviruses. J Immunol. 1986 Jan;136(1):331–339. [PubMed] [Google Scholar]
- Pan S., Knowles B. B. Monoclonal antibody to SV40 T-antigen blocks lysis of cloned cytotoxic T-cell line specific for SV40 TASA. Virology. 1983 Feb;125(1):1–7. doi: 10.1016/0042-6822(83)90058-2. [DOI] [PubMed] [Google Scholar]
- Pillemer E. A., Kooistra D. A., Witte O. N., Weissman I. L. Monoclonal antibody to the amino-terminal L sequence of murine leukemia virus glycosylated gag polyproteins demonstrates their unusual orientation in the cell membrane. J Virol. 1986 Feb;57(2):413–421. doi: 10.1128/jvi.57.2.413-421.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinter A., Honnen W. J., Tung J. S., O'Donnell P. V., Hämmerling U. Structural domains of endogenous murine leukemia virus gp70s containing specific antigenic determinants defined by monoclonal antibodies. Virology. 1982 Jan 30;116(2):499–516. doi: 10.1016/0042-6822(82)90143-x. [DOI] [PubMed] [Google Scholar]
- Plata F., Langlade-Demoyen P., Abastado J. P., Berbar T., Kourilsky P. Molecular definition of retrovirus-induced antigens recognized by tumour-specific H-2-restricted cytolytic T lymphocytes. J Immunogenet. 1986 Apr-Jun;13(2-3):263–268. doi: 10.1111/j.1744-313x.1986.tb01110.x. [DOI] [PubMed] [Google Scholar]
- Plata F., Langlade-Demoyen P., Abastado J. P., Berbar T., Kourilsky P. Retrovirus antigens recognized by cytolytic T lymphocytes activate tumor rejection in vivo. Cell. 1987 Jan 30;48(2):231–240. doi: 10.1016/0092-8674(87)90426-0. [DOI] [PubMed] [Google Scholar]
- Plata F., Lilly F. Viral specificity of H-2-restricted T killer cells directed against syngeneic tumors induced by Gross, Friend, or Rauscher leukemia virus. J Exp Med. 1979 Nov 1;150(5):1174–1186. doi: 10.1084/jem.150.5.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risser R., Horowitz J. M., McCubrey J. Endogenous mouse leukemia viruses. Annu Rev Genet. 1983;17:85–121. doi: 10.1146/annurev.ge.17.120183.000505. [DOI] [PubMed] [Google Scholar]
- Rowe W. P., Pugh W. E., Hartley J. W. Plaque assay techniques for murine leukemia viruses. Virology. 1970 Dec;42(4):1136–1139. doi: 10.1016/0042-6822(70)90362-4. [DOI] [PubMed] [Google Scholar]
- Schäfer A., Schmidt W. Resistance to cellular immune response in AKR leukemias. Eur J Immunol. 1986 Jul;16(7):753–759. doi: 10.1002/eji.1830160707. [DOI] [PubMed] [Google Scholar]
- Stallcup K. C., Springer T. A., Mescher M. F. Characterization of an anti-H-2 monoclonal antibody and its use in large-scale antigen purification. J Immunol. 1981 Sep;127(3):923–930. [PubMed] [Google Scholar]
- Steffen D. L., Taylor B. A., Weinberg R. A. Continuing germ line integration of AKV proviruses during the breeding of AKR mice and derivative recombinant inbred strains. J Virol. 1982 Apr;42(1):165–175. doi: 10.1128/jvi.42.1.165-175.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone M. R., Nowinski R. C. Topological mapping of murine leukemia virus proteins by competition-binding assays with monoclonal antibodies. Virology. 1980 Jan 30;100(2):370–381. doi: 10.1016/0042-6822(80)90528-0. [DOI] [PubMed] [Google Scholar]
- Takahashi H., Cohen J., Hosmalin A., Cease K. B., Houghten R., Cornette J. L., DeLisi C., Moss B., Germain R. N., Berzofsky J. A. An immunodominant epitope of the human immunodeficiency virus envelope glycoprotein gp160 recognized by class I major histocompatibility complex molecule-restricted murine cytotoxic T lymphocytes. Proc Natl Acad Sci U S A. 1988 May;85(9):3105–3109. doi: 10.1073/pnas.85.9.3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H., Merli S., Putney S. D., Houghten R., Moss B., Germain R. N., Berzofsky J. A. A single amino acid interchange yields reciprocal CTL specificities for HIV-1 gp160. Science. 1989 Oct 6;246(4926):118–121. doi: 10.1126/science.2789433. [DOI] [PubMed] [Google Scholar]
- Tevethia S. S., Tevethia M. J. Cytotoxic T-lymphocyte recognition of simian virus 40 antigens. Cancer Surv. 1985;4(1):101–114. [PubMed] [Google Scholar]
- Townsend A. R., Gotch F. M., Davey J. Cytotoxic T cells recognize fragments of the influenza nucleoprotein. Cell. 1985 Sep;42(2):457–467. doi: 10.1016/0092-8674(85)90103-5. [DOI] [PubMed] [Google Scholar]
- Townsend A. R., McMichael A. J., Carter N. P., Huddleston J. A., Brownlee G. G. Cytotoxic T cell recognition of the influenza nucleoprotein and hemagglutinin expressed in transfected mouse L cells. Cell. 1984 Nov;39(1):13–25. doi: 10.1016/0092-8674(84)90187-9. [DOI] [PubMed] [Google Scholar]
- Townsend A. R., Skehel J. J. The influenza A virus nucleoprotein gene controls the induction of both subtype specific and cross-reactive cytotoxic T cells. J Exp Med. 1984 Aug 1;160(2):552–563. doi: 10.1084/jem.160.2.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker B. D., Chakrabarti S., Moss B., Paradis T. J., Flynn T., Durno A. G., Blumberg R. S., Kaplan J. C., Hirsch M. S., Schooley R. T. HIV-specific cytotoxic T lymphocytes in seropositive individuals. Nature. 1987 Jul 23;328(6128):345–348. doi: 10.1038/328345a0. [DOI] [PubMed] [Google Scholar]
- Walker B. D., Flexner C., Paradis T. J., Fuller T. C., Hirsch M. S., Schooley R. T., Moss B. HIV-1 reverse transcriptase is a target for cytotoxic T lymphocytes in infected individuals. Science. 1988 Apr 1;240(4848):64–66. doi: 10.1126/science.2451288. [DOI] [PubMed] [Google Scholar]
- Waneck G. L., Sherman D. H., Calvin S., Allen H., Flavell R. A. Tissue-specific expression of cell-surface Qa-2 antigen from a transfected Q7b gene of C57BL/10 mice. J Exp Med. 1987 May 1;165(5):1358–1370. doi: 10.1084/jem.165.5.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]