Abstract
Growth hormone (GH) controls the physiology and pathophysiology of the liver, and its signals are conducted by two members of the family of signal transducers and activators of transcription, STAT5A and STAT5B. Mice in which the Stat5a/b locus has been inactivated specifically in hepatocytes display GH resistance, the sex-specific expression of genes associated with liver metabolism and the cytochrome P-450 system is lost, and they develop hepatosteatosis. Several groups have shown by global gene expression profiling that a cadre of STAT5A/B target genes identify genetic cascades induced by GH and other cytokines. Evidence is accumulating that in the absence of STAT5A/B GH aberrantly activates STAT1 and STAT3 and their downstream target genes and thereby offers a partial explanation of some of the physiological alterations observed in Stat5a/b-null mice and human patients. We hypothesize that phenotypic changes observed in the absence of STAT5A/B are due to two distinct molecular consequences: first, the failure of STAT5A/B target genes to be activated by GH and second, the rerouting of GH signaling to other members of the STAT family. Rerouting of GH signaling to STAT1 and STAT3 might partially compensate for the loss of STAT5A/B, but it certainly activates biological programs distinct from STAT5A/B. Here we discuss the extent to which studies on global gene expression profiling have fostered a better understanding of the biology behind cytokine-STAT5A/B networks in hepatocytes. We also explore whether this wealth of information on gene activity can be used to further understand the roles of cytokines in liver disease.
Keywords: signal transducers and activators of transcription, knockout, metabolism
the ability to mutate individual genes in the mouse has permitted detailed studies on their roles in development, physiology, and disease. In particular, gene knockout mice have provided in-depth insight into the molecular structure and dynamics of signal transduction networks. However, in many cases the physiological consequences observed in mutant mice, or lack thereof, were unexpected and irreconcilable with the proposed functions of the protein under investigation. Genomewide gene expression profiling from gene knockout mice should provide an inroad into better understanding of the molecular consequences of disrupting complex information networks. The application of global approaches to the analysis of complex biological systems including organ development, physiology, and disease has gained considerable strength. Characterization of molecular networks that drive these biological processes has been facilitated by the emergence of affordable high-throughput technologies, including DNA microarrays that can monitor the activity of an entire genome. Such approaches have the promise to facilitate an understanding of molecular networks and how they respond to a fluctuating environment, including hormonal changes and tumorigenesis.
The physiology and pathophysiology of the liver are largely determined by cytokines and steroid hormones, which directly and indirectly activate downstream genetic pathways. Growth hormone (GH) and its downstream mediators JAK2 and STAT5 control liver metabolism (88). STAT5 was originally isolated from ovine mammary tissue as a prolactin-induced transcription factor named mammary gland factor (MGF) (86) and subsequently renamed STAT5A. STAT5B was identified independently by three groups and shown to be encoded by a distinct gene (3, 56, 60). Over the past decade it has become clear that STAT5A/B are activated by a wide variety of cytokines, such as growth hormones, interleukin (IL)-2, IL-3, IL-5, erythropoietin (EPO), thrombopoietin (TPO), and granulocyte-macrophage colony-stimulating factor (GM-CSF). A detailed understanding of the physiological roles of STAT5A and STAT5B has been provided through studies of mice from which the respective genes have been deleted [knockout (KO) mice]. As indicated by these studies, STAT5A/B control a variety of functions in diverse cell systems ranging from hematopoietic stem cells to hepatocytes and lactation.
Four distinct mouse models have been generated to explore the role of STAT5A/B in development, physiology, and disease: mice lacking either a functional Stat5a (57) or Stat5b (82) gene, mice carrying mutant Stat5a/b genes that encode STAT5A/B lacking the NH2-terminal tetramerization domain (Stat5ΔN mice) (81), and mice in which the entire Stat5a/b locus can be deleted in specific cell types with Cre-mediated recombination (Stat5−/− mice) (16). Defects observed in mice carrying deletions of either the Stat5a or Stat5b gene largely reflect the distribution pattern of the respective protein. STAT5A-deficient mice mature normally, but mammary development during pregnancy is impaired and females fail to lactate after parturition (57). In STAT5B-deficient male mice, body growth is decreased to levels observed in wild-type females (81, 82). STAT5ΔN mice have a reduced body size due to disrupted GH signaling, and females are infertile because of defects in the corpora lutea (81). In contrast to STAT5ΔN mice, Stat5a/b−/− mice die perinatally (16), and it was only possible to investigate the role of STAT5A/B in liver metabolism through the hepatocyte-specific deletion of the Stat5a and Stat5b genes.
Whole body gene deletions in mice often result in complex defects, and it is difficult to ascribe a defect to a specific cell type. In particular, the disruption of negative feedback loops in the hypothalamus and pituitary gland can lead to widespread systemic consequences. To overcome some of these obstacles and to permit a dissection of the contribution of STAT5A/B in individual cell types or developmental stages, the Cre/loxP recombination system has been used to generate mice in which the entire 110-kb Stat5a/b locus is flanked by loxP sites (16). With these mice, researchers were able to identify the functions of STAT5A/B in specific cell types, including mammary epithelium (16), pancreatic beta cells (49), T cells (31, 46, 85, 96, 97), B cells (17), muscle (41), and hepatocytes (15, 24). To delete the Stat5a/b locus in hepatocytes in the mouse, two distinct Cre transgenes were used (15, 24). In addition, mice carrying mutated growth hormone receptors (GHRs) unable to dock STAT5A/B (68, 69) have been studied. Extensive gene expression profiling has been performed on several mouse models in which the STAT5A/B signaling pathways were disrupted (12, 13, 15, 24, 32, 89), and this review focuses on the information that has been provided by these studies to further understand the role and intracellular network comprising STAT5A/B.
Cytokine-STAT5A/B Signaling in the Liver
Although the conventional gene knockout approach demonstrated a crucial role for STAT5B in postnatal body growth and liver metabolic pathways (82), a more complete set of STAT5A and STAT5B functions was only recognized upon the deletion of the respective genes in hepatocytes with Cre-loxP-mediated recombination. Deletion of Stat5B resulted in reduced growth of males but not females, and male-specific gene expression was reduced to female levels (82). In contrast, in females the expression of some gene sets was elevated. Moreover, these mice developed obesity. GH levels were increased, and IGF-I levels were decreased. Notably, STAT1 levels were increased in liver tissue of these mice, suggesting the acquisition of an altered STAT network. While STAT5B is a key component regulating sexual dimorphic expression of GH-induced genes in male mice, STAT5A regulates the expression of a subset of sex-specific genes in the female liver (13).
Deletion of the Stat5a/b locus with an Alb-Cre transgene, which is active postnatally, did not result in reduced body growth (15). However, these mice developed hepatosteatosis, glucose intolerance, insulin resistance, late-onset obesity, and impaired liver regeneration on partial hepatectomy (PHx). Deletion of the Stat5a/b locus with a Cre transgene under control of the albumin gene promoter and the α-fetoprotein enhancer provided overall similar results but also revealed distinct features (24). Mainly, these mice displayed impaired postnatal growth, indicating a different expression pattern of the Cre gene and loss of STAT5A/B at early stages. Importantly, this study demonstrated a physical and functional interaction between STAT5A/B and the glucocorticoid receptor. This interaction was shown to be critical for the regulation of specific subsets of metabolic genes (24).
STAT5A/B Target Genes
STAT5A/B target genes in liver tissue have been identified in several systems. Waxman and colleagues have performed microarray analyses on liver tissue from mice lacking STAT5A (13) or STAT5B (12) globally and from liver-specific Stat5a/b KO mice (32). Engblom and colleagues (24) have identified target genes from mice in which the Stat5a/b locus was deleted with the AFP-Cre transgene. Our laboratory (15) has profiled gene expression in liver tissue on deletion of the Stat5a/b locus with the Alb-Cre transgene. Rowland and colleagues (69) analyzed gene expression profiles in liver tissue from mice that carry truncated GHRs that impair STAT5A/B signaling, and Vidal et al. (84) analyzed gene expression in mice on GH stimulation. On the basis of these experiments, a large set of genes was identified whose expression was altered on the deletion of STAT5A/B. The use of different microarray platforms and possibly different mouse strains makes it challenging to fully compare different data sets. Regardless of these caveats, a coherent theme emerged.
In mice, pulsatile secretion of GH in males is distinct from that in females and sexual dimorphic expression patterns of genes in liver tissue have been observed (89). In liver, STAT5B is 20-fold more abundant than STAT5A, suggesting that the vast majority of GH signaling is conveyed through STAT5B. Studies on global Stat5a- and Stat5b-null mice confirmed STAT5B as the transcription factor conveying sexual dimorphism, but a distinct role for STAT5A has been established as well. STAT5B-deficient male mice are characterized by reduced body growth and a loss of sex-specific expression of genes encoding cytochrome P-450 (Cyp) enzymes (82) as well as other genes. In liver tissue of Stat5b-null mice 90% of male-predominant genes were suppressed and 60% of female-predominant genes were induced (12). Microarray studies published in this journal (13) identified several gene sets displaying sexual dimorphic expression. Notably, the expression of 15% of female-predominant genes was dependent on the presence of STAT5A. These studies highlight sex-specific roles of the two STAT5 isoforms, with STAT5A preferentially regulating gene expression in the female liver while STAT5B plays the predominant role in the male. It can be speculated that STAT5 binding sites (GAS sites) in regulatory sequences of 15% of female-predominant genes are recognized exclusively by STAT5A. Whole genome analyses, such as ChIP-seq using massive parallel sequencing, will be needed to elucidate the specificity of GAS sites for the two STAT5 isoforms. On the basis of their comparison of sexual dimorphic genes dependent on either STAT5 isoforms the authors defined STAT5A as a “feminizing” factor in female mouse liver and STAT5B as a “masculinizing” factor in male liver (13).
A comparison of gene expression data from mice in which the entire Stat5a/b locus was deleted from liver tissue with two independent Cre transgenes (15, 24) yielded several genes with an altered expression pattern that could, at least in part, explain the altered metabolism and pathology in these mice (Table 1). As expected, IGF-I mRNA levels were reduced in the absence of STAT5A/B. GH activates hepatic Igf-1 gene expression through STAT5B (5, 27), and systemic IGF-I promotes growth of target tissues, such as muscle and bone. Surprisingly, however, mice with a Alb-Cre-mediated liver-specific STAT5A/B deletion grew normally without effect on body size (15). In these mice the targeted Igf-1 deletion resulted in an ∼75% decrease in circulating IGF-I and elevated levels of plasma GH (74, 95). These results indicate that hepatic IGF-I is not essential for normal postnatal growth. Furthermore, skeletal muscle-specific STAT5A/B-null mice displayed a >60% reduction in muscle IGF-I mRNA content but only a 15% reduction of circulating IGF-I and showed significantly reduced postnatal growth and skeletal size (41). These data support the concept that autocrine/paracrine-derived IGF-I is essential for normal postnatal growth. In contrast to the liver-specific Stat5fl/fl;Alb-Cre mice generated in our laboratory, mice in which the Stat5a/b locus has been deleted with the α-fetoprotein Cre transgene displayed a reduced body growth similar to that seen in complete STAT5B-null mice (82). The reason for this discrepancy is not known. At 4 wk of age IGF-I levels in both strains were reduced. It is possible that the temporal expression pattern of the two Cre transgenes or slight differences in their cell specificity are the underlying causes, because the Afp gene is expressed in fetal tissues, such as liver, kidney, and yolk sac (28). Stat5fl/fl;Alb-Cre mice develop obesity and display glucose intolerance, which could to some extent compensate for the reduced body weight caused by growth retardation. Alternatively, strain differences might have an effect as well. For example, while Stat5-null mice in a C57BL/6 background die perinatally, fetal death at embryonic day 14.5 is observed in a BALB/c background (G. W. Robinson and L. Hennighausen, unpublished observations). To fully understand the differences observed between these two studies, it would be necessary to perform them in the same strain background and monitor both the temporal and cell-specific deletion of the Stat5a/b locus imposed by the two Cre strains.
Table 1.
Genes induced in hepatocytes lacking STAT5
| Gene | STAT5 fl/fl: Alb-Cre | STAT5 fl/fl: Alb/AFP-Cre (KO/Con)* | ||
|---|---|---|---|---|
| STAT5 target genes involved in liver metabolism and body growth | ||||
| IGF-1 | ↓ | ↓ | ||
| Glutathione S-transferase (mu 6) | ↓ | ↓ | ||
| Serine protease inhibitor (clade A, member 3K) | ↓ | ↓ | ||
| Prolactin receptor | ↓ | ↓ | ||
| IGF binding protein 3 | ↓ | ↓ | ||
| STAT5 target genes involved in negative regulation of JAK-STAT pathway | ||||
| SOCS2 | ↓ | N/A | ||
| SOCS3 | ↓ | N/A | ||
| CIS | ↓ | N/A | ||
| IFN-STAT1 target genes | ||||
| IFN-α-inducible protein | ↑ | ↑ | ||
| IFN-γ-inducible protein | ↑ | ↑ | ||
| IFN-γ-induced GTPase | ↑ | ↑ | ||
| IFN regulatory factor-1 | ↑ | N/A | ||
| Guanylate-binding protein | ↑ | N/A | ||
| Complement protein C3 | ↑ | N/A | ||
| Stat1 | ↑ | ↑ | ||
| STAT3 target genes | ||||
| C-reactive protein | → | → | ||
| Haptoglobin | ↑ | N/A | ||
| Complement C3 | ↑ | N/A | ||
| Fibrinogen α | ↑ | N/A | ||
| Fibrinogen β | ↑ | N/A | ||
| Fibrinogen γ | ↑ | N/A | ||
| C/EBP β | ↑ | N/A | ||
| p21 | ↑ | N/A | ||
| Cyclin D1 | ↑ | ↑ | ||
| Bcl-2 | ↑ | N/A | ||
| Mcl-1 | → | N/A | ||
On the basis of current evidence it is clear that STAT5A/B are essential for Igf-1 gene expression in hepatocytes, but it is still an open question as to what extent the hepatic STAT5A/B-IGF-I axis is responsible for body growth. Severely reduced expression of other bona fide STAT5A/B target genes, such as those encoding glutathione S-transferase and IGF binding protein 3, were found in the different mouse models (Table 1).
Negative Regulation of STATs Through Members of the SOCS Family
As negative regulators of the JAK/STAT pathway, suppressors of cytokine signaling (SOCS) and cytokine-inducible SH2-containing proteins (CIS) are intimately connected to STAT5A/B. The strength and duration of STAT signals are regulated by SOCS and CIS proteins, which attenuate or terminate cytokine/growth factor signals (30). Excessive or constitutive activation of STAT pathways can induce oncogenic transformation, tumor cell invasion, and metastasis (18, 29). Defects in negative-feedback regulation of STAT pathways have been identified in diverse tumors (62, 77, 80). As a negative feedback, SOCS/CIS proteins inhibit cytokine-stimulated STAT5A/B signaling through several mechanisms (65). Several reports have provided evidence that cytokines such as GH, IL-6, and interferon (IFN) induce Socs/Cis gene expression, and that these inhibitory molecules suppress STAT signaling by means of negative feedback (reviewed in Refs. 61, 98). Adams and colleagues (2) first reported that GH activates the genes encoding SOCS1, SOCS2, SOCS3, and CIS through STAT5A/B, which is abrogated in liver-specific STAT5A/B-null mice (Table 1). Gene profiling experiments have shown that the levels of SOCS2 (15), SOCS3, and CIS mRNA are greatly reduced in liver tissue from liver-specific STAT5A/B-null mice (Table 1).
An important concept emerges from the observation that loss of STAT5 results in the disruption of negative feedback loops that rely on SOCS2, SOCS3, and CIS; namely, abrogation of SOCS-mediated inhibition could permit an enhanced activation of other STATs (Fig. 1). Denson's work demonstrates that IL-6 inhibits hepatic GH signaling through an increase of CIS and SOCS3, and thus causes acquired GH resistance in patients with inflammatory diseases or sepsis (10, 21).
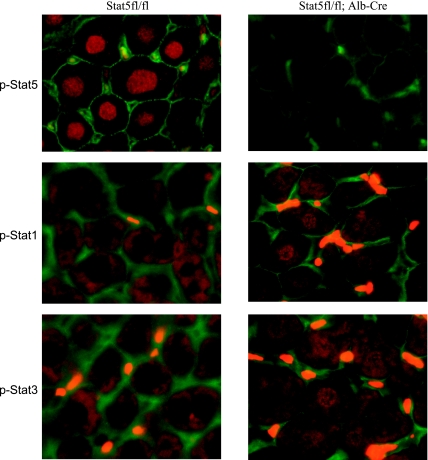
Fig. 1.
Growth hormone (GH)-induced activation of signal transducers and activators of transcription (STATs) in hepatocytes. GH was injected subcutaneously into control and liver-specific STAT5A/B-null mice (Stat5fl/fl;Alb-Cre) mice, and liver tissue was isolated after 15 min. Immunohistochemical analyses were performed with antibodies from Cell Signaling that were specific to phospho (p)-STAT1, p-STAT3, and p-STAT5. Nuclear localization of STATs indicated that they had been activated by GH. Nuclear STAT5 was detected in control mice on GH injection but not in STAT5-null liver tissue. Cytoplasmic STAT1 and STAT3 were observed in control mice, and nuclear STAT1 and STAT3 were detected on GH injection in liver tissue from Stat5fl/fl;Alb-Cre mice. The bright yellow staining between cells is attributed to autofluorescent red blood cells.
Balancing STAT Signaling
Evidence has been accumulating that loss of a given STAT member can result in the activation of “illegitimate” STATs (61, 83). Although this concept appears to be universal, cell-specific differences at the levels of individual STAT members would need to be taken into account. In mouse liver, GH activates STAT5B preferentially (Fig. 1) and little or no activation of STAT1 or STAT3 can be detected. In contrast, extensive hepatic nuclear STAT1 and STAT3 can be observed in Stat5fl/fl;Alb-Cre mice (Fig. 1). Because each STAT member targets its own set of genes, which possibly includes some common ones, the aberrant activation of STAT1 and STAT3 is likely not to result in compensation but rather in a gain of function (Fig. 2). Evidence for this comes from gene profiling of liver tissue in the presence and absence of STAT5. Notably, induction of STAT1 and STAT3 target genes was detected (17).
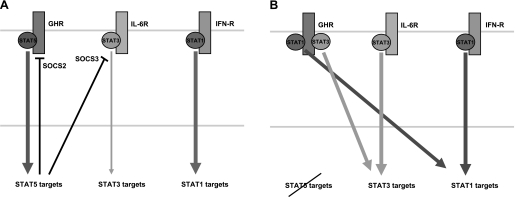
Fig. 2.
JAK-STAT signaling in hepatocytes in the presence and absence of STAT5A/B. A: cytokine-induced signaling in the presence of STAT5A/B. The growth hormone receptor (GHR) specifically recruits and preferentially activates STAT5A/B, which in turn induce the expression of specific sets of genes and elicit a STAT5 response. Suppressors of cytokine signaling (SOCS)2 and SOCS3, 2 negative regulators of the JAK/STAT pathway, are encoded by genes that are controlled by STAT5. Members of the interleukin (IL)-6 family of cytokines activate STAT3 and elicit a STAT3 response in the cell, while interferons (IFNs) activate STAT1. B: cytokine-induced signaling in the absence of STAT5A/B. In the absence of STAT5, both STAT1 and STAT3 can be recruited to the GHR, although with lower affinity than STAT5. Thus GH can aberrantly activate a STAT1 and a STAT3 program in hepatocytes as demonstrated by gene expression profiling. Moreover, impaired expression of negative regulators from the SOCS family will exacerbate STAT3 signaling.
STAT1 Target Genes
IFNs regulate cellular antiviral, antiproliferative, and immunologic responses. In liver, STAT1 is activated by both IFN-α/β and IFN-γ (19). Although STAT1 is also activated by other cytokines and growth factors, including IL-2, EGF, and GH, STAT1-deficient mice exhibit selective signaling defects only in their response to IFNs (23, 68). It is not clear whether the activation of STAT1 by cytokines other than IFNs is an evolutionary footprint merely pointing to the fact that the SH2 domains of all STAT members exhibit some promiscuity or whether there is an underlying biological significance. Such relevance could be masked by the fact that EGF mainly activates STAT3 and GH and IL-2 preferentially activate STAT5. STAT1 target genes shown in Table 1 are induced in Stat5fl/fl;Alb-Cre mice, further supporting the notion that STAT1 can efficiently dock to the GHR and transduce its signal in the absence of STAT5. Surprisingly, STAT1 mRNA levels were also elevated in the absence of STAT5, suggesting the presence of a positive feedback loop.
STAT1 is a key regulator of the gene encoding IFN regulatory factor-1 (IRF-1), which was originally identified as a nuclear factor binding to the upstream regulatory region of the human IFN-β gene (52, 59). In liver, STAT1 and IRF-1 are essential in liver injury induced by lipopolysaccharide LPS/d-galactosamine through inducible nitric oxide synthase (iNOS) and nitric oxide (NO) production, as well as elevated reactive oxygen species (ROS) production (39, 48). STAT1 activation not only contributes to liver injury but blocks liver repair through inhibition of hepatocyte proliferation in the PHx model (79). Stat5fl/fl;Alb-Cre mice have impaired proliferation of hepatocytes on PHx, suggesting upon first view a role of STAT5 in liver regeneration. However, this defect is ameliorated on the concomitant deletion of STAT1, suggesting that defective liver regeneration in the absence of STAT5 was the result of an aberrant activation of STAT1. STAT1 hyperactivation in the absence of STAT5 is probably mainly due to GH and IL-2 stimulation. Inhibition of SOCS/CIS expression, described above, could exacerbate STAT1 and STAT3 activation. Taken together, loss of STAT5A/B causes not only impaired GH signaling in liver but inhibition of SOCS expression followed by the activation of other STATs, which results in impaired proliferation of hepatocytes on PHx (Fig. 3).
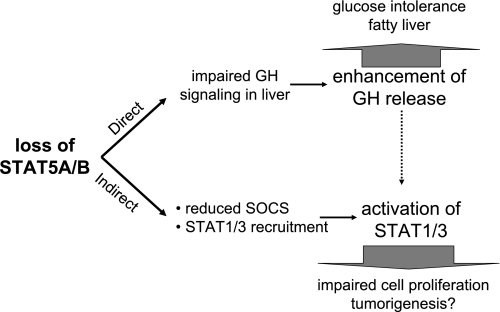
Fig. 3.
Loss of STAT5 modifies the physiology of liver cells through direct and indirect pathways. Several distinct mechanisms are responsible for the altered physiology of hepatocytes in the absence of STAT5A/B. First, reduced expression of specific classes of STAT5A/B target genes, such as those encoding different members of the cytochrome P-450 family, results in an altered metabolism of hepatocytes. Among the physiological changes are the emergence of glucose intolerance and hepatosteatosis. Second, reduced levels of SOCS proteins result in enhanced STAT3 signaling through receptors containing the gp130 subunit, such as IL-6. Third, in the absence of STAT5, STAT1 and STAT3 can dock to the GHR and thus elicit their respective biological responses on GH stimulation. This includes the activation of genes involved in cell proliferation and survival. Finally, the development of GH resistance results in elevated GH levels, which exacerbate aberrant STAT1/3 signaling.
STAT3 Target Genes
The presence of STAT3 is critical for acute-phase response (66, 91), protection against liver injury, promotion of liver regeneration (6, 92), glucose homeostasis, and hepatic lipid metabolism (34). STAT3 is mainly activated by IL-6 family members, such as IL-6, IL-11, leukemia inhibitory factor (LIF), oncostatin M (OSM), ciliary neurotrophic factor, and cardiotrophin-1, which utilize combinations of a common signal-transducing subunit, gp-130, and various ligand-binding subunits (40). Among these cytokines, IL-6 has been extensively investigated, and is thought to play the most important role in the liver. IL-6 stimulates hepatocytes to produce a variety of target genes, which include acute-phase proteins, such as C-reactive protein, complement C3, fibrinogen, and haptoglobin, and cell cycle-related and apoptosis-related genes, such as cyclin D1, C/EBPβ, p21WAF1/Cip, Bcl-2, and Bcl-xL. Expression of most of these genes was elevated in liver tissue from Stat5fl/fl;Alb-Cre mice, some of which are listed in Table 1, further supporting the notion that loss of STAT5A/B leads to an aberrant activation of STAT3 signaling (Figs. 1 and 2). While STAT3 functions as an important factor contributing to the hepatoprotective and hepatomitogenic effect (14, 54), it has also been linked to tumorigenesis (reviewed in Refs. 7, 29). Experimentally, constitutively activated STAT3 mutants can induce some aspects of cell transformation (9), and dominant-negative STAT3 blocks STAT-dependent transcription and transformation induced by activated tyrosine kinases. It has been proposed that constitutively activated STAT3 facilitates cellular transformation by inducing the expression of genes critical to the initiation and/or maintenance of transformation. STAT3 regulates the expression of genes that mediate proliferation (e.g., c-myc and cyclin D1), suppress apoptosis [e.g., B-cell leukemia/lymphoma (Bcl)-xL and Bcl-2], or promote angiogenesis (e.g., VEGF).
Some inhibitory molecules are also regulated by STATs. Expression levels of p21WAF1/CIP1 are related to malignant tissue formation (4, 22, 63) as STAT1 activation in response to IFN-γ results in induction of p21WAF1/CIP1 and growth arrest (8, 11, 33). Therefore, in contrast to STAT3 and STAT5, STAT1 is thought to be a tumor suppressor. This is supported by studies using Stat1-mutant mice, which develop more frequent and more rapidly growing tumors when exposed to chemical carcinogens (38). In part this can be explained by impaired immune responses, because these mice do not respond to IFN-γ and have defects in natural killer cell activity. Moreover, STAT1 is also required for growth inhibition through the induction of p21WAF1/CIP1. Since STAT3 also induces p21WAF1/CIP1 through binding to the p21WAF1/CIP1 gene promoter (73), one can hypothesize that two independent signaling pathways induce the same, but possibly cell-specific, program. In Stat5fl/fl;Alb-Cre mice, levels of p21 and cyclin D1 are elevated. We propose that this is the result of an aberrant activation of STAT1 and STAT3. At this point it is not known whether this illegitimate activation leads to the onset of tumors.
Oncogenesis is frequently associated with a deregulated expression of pro- and antiapoptotic genes. Two principal pathways in apoptosis activate intracellular cysteine proteases, so-called caspases (1). One is the mitochondrion-dependent intrinsic (stress) cell death pathway, and another is the extrinsic (death receptor) pathway. The two pathways are largely independent; however, in hepatocytes these two pathways intersect through processing the proapoptotic protein BID into its active truncated form (tBID) (36). The antiapoptotic Bcl-2 family proteins, Bcl-2, Bcl-xL, and myeloid cell leukemia sequence 1 (Mcl-1), play important roles mainly by inhibiting intrinsic cell death pathways (26). This pathway induced by cytokine deprivation, intracellular damage, and oncogene expression is initiated by Bcl-2 homology domain (BH)3-only proteins, such as Bad, Bik, Bid, Bim, Bmf, Hrk, Noxa, and Puma, which inactivate Bcl-2 family proteins and thereby unleash Bax and Bak. Expression of the Bcl-2 family is regulated to some extent by the STAT3 pathway, and a strong correlation exists between elevated levels of these family members and human cancer (9, 25, 35, 55, 67, 70). Proliferation of cancer cell lines can be controlled by inhibiting STAT3 activity with Stat3 small interfering RNA (siRNA), decoy, or selective inhibitors (53, 71, 78, 90, 93). Additional attention needs to be paid to the fact that STAT5 also contributes to the induction of the Bcl-2 family (75, 76). In the erythroid lineage, Epo-dependent activation of STAT5 is used by Epo-responsive progenitor cells to induce the expression of bcl-xL and consequently to inhibit apoptosis (72). De Groot et al. (20) also reported that bcl-xL is induced through BCR-Abl. STAT1 is known to induce apoptosis by activating proapoptotic regulatory genes, such as Bak, Bax, caspases 3 and 8, Fas, and Fas ligand (33, 47, 50, 64, 94). Furthermore, STAT1 plays a role in the expression of Bcl-2 and Bcl-xL (43). Thus cell apoptosis or survival is determined partly by STAT activation, including STAT1, STAT3, and STAT5, through expression of pro-/antiapoptotic regulatory genes. The balance of each STAT activation is also important for the determination of cell fate as in the case of cell growth.
Contribution of STAT5 in Liver to Human Physiology and Disease
Patients with homozygous inactivating mutations in the Stat5B gene provide some answers to the question of whether cytokine-STAT5 signaling in the liver contributes to the physiology and pathophysiology of the human body (42, 87). Poor weight gain and growth failure were noted in these patients, reminiscent of patients with classical GH insufficiency (44). Circulating GH levels were elevated, and serum concentrations of IGF-I and IGF-binding protein-3 (IGFBP-3) were markedly reduced, with poor response to daily GH injections. However, these patients lack functional STAT5B not only in liver but globally, and it is difficult to ascertain that the phenotype is due to its absence in liver. Fibroblasts of these patients displayed elevated levels of STAT1 and aberrant GH- and prolactin-induced activation of STAT1 and STAT3, reminiscent of what has been seen in liver-specific Stat5a/b-null mice. While these patients will provide insight into the global function of STAT5B in human physiology and disease, Stat5fl/fl;Alb-Cre mice as well as other cell-specific STAT5-KO mice will be instrumental in understanding the molecular underpinnings.
Is there a link between STAT5A/B activation and liver disease? Notably, STAT5 activation has been observed in clinical samples from patients with hepatocellular carcinoma (HCC) by some (51) but not by others (37). Even if activated STAT5 is detected in HCC, it is not clear whether the activation of STAT5 is a primary event in the development of cancer or the result of malignant transformation. Because HCC is accompanied by chronic hepatitis (CH) and liver cirrhosis (LC), it would be informative to analyze STAT5 activity in patients with CH or LC. Moreover, it will be necessary to have a more global view and compare the activation status of other STATs as well as how their balance might determine the physiology of the cell.
In contrast to a constitutive activation of STAT5 in some diseases, STAT5 activity in liver is inhibited in several syndromes, such as dwarfism and Laron syndrome (45). In dwarfism, GH secretion is suppressed as a consequence of different genetic defects, such as impaired pituitary function. In patients with GHR mutations STAT5 fails to be activated and expression of STAT5 target genes, such as IGF-I, is severely impaired. As pointed out above, recent studies on mutant mice suggest a more complicated mechanism that could involve the deregulation of feedback loops composed of IL-6 and SOCS3. Impaired STAT5 activation has also been observed in sepsis with an accompanying endotoxin release. In these patients hepatic expression of SOCS-1, SOCS-3, and CIS was transiently increased during sepsis and temporally associated with the development of hepatic GH resistance (10, 99). It is likely that increased SOCS/CIS expression is the result of elevated IL-6 levels that accompany severe inflammation.
Conclusions
Mice that carry mutations in the GHR-JAK2-STAT5A/B signaling pathway have been used extensively to explore the function of GH in the physiology and pathophysiology of mammals. Gene expression profiling of cells lacking functional STAT5A/B has provided a glimpse into the molecular mechanisms underlying the phenotypes observed in mutant mice and human patients. This review has focused on the link between molecular and physiological consequences encountered on the deletion of two highly conserved individual transcription factors (STAT5A/B), the archeological founding members of the STAT family. The seven members of the STAT family are the integral components of a communication network that is used by the majority of cytokines. Loss of STAT5A/B leads not only to the deregulated expression of its target genes but also to the aberrant activation of other members of the STAT family, which leads to a qualitative shift in the JAK-STAT communication network (Fig. 2). While the aberrant activation of STAT1 and STAT3 by GH might compensate for some functions of STAT5A/B, it is clear that STAT1 and STAT3 activate their own set of target genes and thus promote a different biology (Fig. 3). Emerging technologies, such as ChIP-seq, which require massive parallel sequencing, will be essential in our understanding of how cytokines use the JAK-STAT network to achieve specificity.
GRANTS
This work was supported by the intramural program of the National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases.
Acknowledgments
We thank Gertraud W. Robinson for her extensive insight into this work and comments on the manuscript.
Address for reprint requests and other correspondence: L. Hennighausen, Laboratory of Genetics and Physiology, National Institutes of Health/NIDDK, 8 Center Dr., Rm. 101, Bethesda, MD 20892-0822 (e-mail: lotharh@mail.nih.gov); A. Hosui, Dept. of Gastroenterology and Hepatology, Osaka Univ. Graduate School of Medicine, 2-2 Yamadaoka, Suita, Osaka 565-0871, Japan (e-mail: hosui@medone.med.osaka-u.ac.jp).
REFERENCES
- 1.Adams JM Ways of dying: multiple pathways to apoptosis. Genes Dev 17: 2481–2495, 2003. [DOI] [PubMed] [Google Scholar]
- 2.Adams TE, Hansen JA, Starr R, Nicola NA, Hilton DJ, Billestrup N. Growth hormone preferentially induces the rapid, transient expression of SOCS-3, a novel inhibitor of cytokine receptor signaling. J Biol Chem 273: 1285–1287, 1998. [DOI] [PubMed] [Google Scholar]
- 3.Azam M, Erdjument-Bromage H, Kreider BL, Xia M, Quelle F, Basu R, Saris C, Tempst P, Ihle JN, Schindler C. Interleukin-3 signals through multiple isoforms of Stat5. EMBO J 14: 1402–1411, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barboule N, Baldin V, Jozan S, Vidal S, Valette A. Increased level of p21 in human ovarian tumors is associated with increased expression of cdk2, cyclin A and PCNA. Int J Cancer 76: 891–896, 1998. [DOI] [PubMed] [Google Scholar]
- 5.Bichell DP, Kikuchi K, Rotwein P. Growth hormone rapidly activates insulin-like growth factor I gene transcription in vivo. Mol Endocrinol 6: 1899–1908, 1992. [DOI] [PubMed] [Google Scholar]
- 6.Blindenbacher A, Wang X, Langer I, Savino R, Terracciano L, Heim MH. Interleukin 6 is important for survival after partial hepatectomy in mice. Hepatology 38: 674–682, 2003. [DOI] [PubMed] [Google Scholar]
- 7.Bowman T, Garcia R, Turkson J, Jove R. STATs in oncogenesis. Oncogene 19: 2474–2488, 2000. [DOI] [PubMed] [Google Scholar]
- 8.Bromberg JF, Horvath CM, Wen Z, Schreiber RD, Darnell JE Jr. Transcriptionally active Stat1 is required for the antiproliferative effects of both interferon alpha and interferon gamma. Proc Natl Acad Sci USA 93: 7673–7678, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bromberg JF, Wrzeszczynska MH, Devgan G, Zhao Y, Pestell RG, Albanese C, Darnell JE Jr. Stat3 as an oncogene. Cell 98: 295–303, 1999. [DOI] [PubMed] [Google Scholar]
- 10.Chen Y, Sun D, Krishnamurthy VM, Rabkin R. Endotoxin attenuates growth hormone-induced hepatic insulin-like growth factor I expression by inhibiting JAK2/STAT5 signal transduction and STAT5b DNA binding. Am J Physiol Endocrinol Metab 292: E1856–E1862, 2007. [DOI] [PubMed] [Google Scholar]
- 11.Chin YE, Kitagawa M, Su WC, You ZH, Iwamoto Y, Fu XY. Cell growth arrest and induction of cyclin-dependent kinase inhibitor p21 WAF1/CIP1 mediated by STAT1. Science 272: 719–722, 1996. [DOI] [PubMed] [Google Scholar]
- 12.Clodfelter KH, Holloway MG, Hodor P, Park SH, Ray WJ, Waxman DJ. Sex-dependent liver gene expression is extensive and largely dependent upon signal transducer and activator of transcription 5b (STAT5b): STAT5b-dependent activation of male genes and repression of female genes revealed by microarray analysis. Mol Endocrinol 20: 1333–1351, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Clodfelter KH, Miles GD, Wauthier V, Holloway MG, Zhang X, Hodor P, Ray WJ, Waxman DJ. Role of STAT5a in regulation of sex-specific gene expression in female but not male mouse liver revealed by microarray analysis. Physiol Genomics 31: 63–74, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cressman DE, Greenbaum LE, DeAngelis RA, Ciliberto G, Furth EE, Poli V, Taub R. Liver failure and defective hepatocyte regeneration in interleukin-6-deficient mice. Science 274: 1379–1383, 1996. [DOI] [PubMed] [Google Scholar]
- 15.Cui Y, Hosui A, Sun R, Shen K, Gavrilova O, Chen W, Cam MC, Gao B, Robinson GW, Hennighausen L. Loss of signal transducer and activator of transcription 5 leads to hepatosteatosis and impaired liver regeneration. Hepatology 46: 504–513, 2007. [DOI] [PubMed] [Google Scholar]
- 16.Cui Y, Riedlinger G, Miyoshi K, Tang W, Li C, Deng CX, Robinson GW, Hennighausen L. Inactivation of Stat5 in mouse mammary epithelium during pregnancy reveals distinct functions in cell proliferation, survival, and differentiation. Mol Cell Biol 24: 8037–8047, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dai X, Chen Y, Di L, Podd A, Li G, Bunting KD, Hennighausen L, Wen R, Wang D. Stat5 is essential for early B cell development but not for B cell maturation and function. J Immunol 179: 1068–1079, 2007. [DOI] [PubMed] [Google Scholar]
- 18.Darnell JE Validating Stat3 in cancer therapy. Nat Med 11: 595–596, 2005. [DOI] [PubMed] [Google Scholar]
- 19.Darnell JE, Kerr IM, Stark GR. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science 264: 1415–1421, 1994. [DOI] [PubMed] [Google Scholar]
- 20.de Groot RP, Raaijmakers JA, Lammers JW, Koenderman L. STAT5-dependent cyclinD1 and Bcl-xL expression in Bcr-Abl-transformed cells. Mol Cell Biol Res Commun 3: 299–305, 2000. [DOI] [PubMed] [Google Scholar]
- 21.Denson LA, Held MA, Menon RK, Frank SJ, Parlow AF, Arnold DL. Interleukin-6 inhibits hepatic growth hormone signaling via upregulation of Cis and Socs-3. Am J Physiol Gastrointest Liver Physiol 284: G646–G654, 2003. [DOI] [PubMed] [Google Scholar]
- 22.DiGiuseppe JA, Redston MS, Yeo CJ, Kern SE, Hruban RH. p53-independent expression of the cyclin-dependent kinase inhibitor p21 in pancreatic carcinoma. Am J Pathol 147: 884–888, 1995. [PMC free article] [PubMed] [Google Scholar]
- 23.Durbin JE, Hackenmiller R, Simon MC, Levy DE. Targeted disruption of the mouse Stat1 gene results in compromised innate immunity to viral disease. Cell 84: 443–450, 1996. [DOI] [PubMed] [Google Scholar]
- 24.Engblom D, Kornfeld JW, Schwake L, Tronche F, Reimann A, Beug H, Hennighausen L, Moriggl R, Schutz G. Direct glucocorticoid receptor-Stat5 interaction in hepatocytes controls body size and maturation-related gene expression. Genes Dev 21: 1157–1162, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fukada T, Hibi M, Yamanaka Y, Takahashi-Tezuka M, Fujitani Y, Yamaguchi T, Nakajima K, Hirano T. Two signals are necessary for cell proliferation induced by a cytokine receptor gp130: involvement of STAT3 in anti-apoptosis. Immunity 5: 449–460, 1996. [DOI] [PubMed] [Google Scholar]
- 26.Green DR, Kroemer G. The pathophysiology of mitochondrial cell death. Science 305: 626–629, 2004. [DOI] [PubMed] [Google Scholar]
- 27.Gronowski AM, Le Stunff C, Rotwein P. Acute nuclear actions of growth hormone (GH): cycloheximide inhibits inducible activator protein-1 activity, but does not block GH-regulated signal transducer and activator of transcription activation or gene expression. Endocrinology 137: 55–64, 1996. [DOI] [PubMed] [Google Scholar]
- 28.Hammer RE, Krumlauf R, Camper SA, Brinster RL, Tilghman SM. Diversity of alpha-fetoprotein gene expression in mice is generated by a combination of separate enhancer elements. Science 235: 53–58, 1987. [DOI] [PubMed] [Google Scholar]
- 29.Haura EB, Turkson J, Jove R. Mechanisms of disease: insights into the emerging role of signal transducers and activators of transcription in cancer. Nat Clin Pract Oncol 2: 315–324, 2005. [DOI] [PubMed] [Google Scholar]
- 30.Hilton DJ Negative regulators of cytokine signal transduction. Cell Mol Life Sci 55: 1568–1577, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoelbl A, Kovacic B, Kerenyi MA, Simma O, Warsch W, Cui Y, Beug H, Hennighausen L, Moriggl R, Sexl V. Clarifying the role of Stat5 in lymphoid development and Abelson-induced transformation. Blood 107: 4898–4906, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Holloway MG, Cui Y, Laz EV, Hosui A, Hennighausen L, Waxman DJ. Loss of sexually dimorphic liver gene expression upon hepatocyte-specific deletion of Stat5a-Stat5b locus. Endocrinology 148: 1977–1986, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang YQ, Li JJ, Karpatkin S. Thrombin inhibits tumor cell growth in association with up-regulation of p21(waf/cip1) and caspases via a p53-independent, STAT-1-dependent pathway. J Biol Chem 275: 6462–6468, 2000. [DOI] [PubMed] [Google Scholar]
- 34.Inoue H, Ogawa W, Ozaki M, Haga S, Matsumoto M, Furukawa K, Hashimoto N, Kido Y, Mori T, Sakaue H, Teshigawara K, Jin S, Iguchi H, Hiramatsu R, LeRoith D, Takeda K, Akira S, Kasuga M. Role of STAT-3 in regulation of hepatic gluconeogenic genes and carbohydrate metabolism in vivo. Nat Med 10: 168–174, 2004. [DOI] [PubMed] [Google Scholar]
- 35.Isomoto H, Kobayashi S, Werneburg NW, Bronk SF, Guicciardi ME, Frank DA, Gores GJ. Interleukin 6 upregulates myeloid cell leukemia-1 expression through a STAT3 pathway in cholangiocarcinoma cells. Hepatology 42: 1329–1338, 2005. [DOI] [PubMed] [Google Scholar]
- 36.Jurkiewicz M, Averill-Bates DA, Marion M, Denizeau F. Involvement of mitochondrial and death receptor pathways in tributyltin-induced apoptosis in rat hepatocytes. Biochim Biophys Acta 1693: 15–27, 2004. [DOI] [PubMed] [Google Scholar]
- 37.Kannangai R, Sahin F, Torbenson MS. EGFR is phosphorylated at Tyr845 in hepatocellular carcinoma. Mod Pathol 19: 1456–1461, 2006. [DOI] [PubMed] [Google Scholar]
- 38.Kaplan DH, Shankaran V, Dighe AS, Stockert E, Aguet M, Old LJ, Schreiber RD. Demonstration of an interferon gamma-dependent tumor surveillance system in immunocompetent mice. Proc Natl Acad Sci USA 95: 7556–7561, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim WH, Hong F, Radaeva S, Jaruga B, Fan S, Gao B. STAT1 plays an essential role in LPS/d-galactosamine-induced liver apoptosis and injury. Am J Physiol Gastrointest Liver Physiol 285: G761–G768, 2003. [DOI] [PubMed] [Google Scholar]
- 40.Kishimoto T, Akira S, Narazaki M, Taga T. Interleukin-6 family of cytokines and gp130. Blood 86: 1243–1254, 1995. [PubMed] [Google Scholar]
- 41.Klover P, Hennighausen L. Postnatal body growth is dependent on the transcription factors signal transducers and activators of transcription 5a/b in muscle: a role for autocrine/paracrine insulin-like growth factor I. Endocrinology 148: 1489–1497, 2007. [DOI] [PubMed] [Google Scholar]
- 42.Kofoed EM, Hwa V, Little B, Woods KA, Buckway CK, Tsubaki J, Pratt KL, Bezrodnik L, Jasper H, Tepper A, Heinrich JJ, Rosenfeld RG. Growth hormone insensitivity associated with a STAT5b mutation. N Engl J Med 349: 1139–1147, 2003. [DOI] [PubMed] [Google Scholar]
- 43.Koshiji M, Adachi Y, Sogo S, Taketani S, Oyaizu N, Than S, Inaba M, Phawa S, Hioki K, Ikehara S. Apoptosis of colorectal adenocarcinoma (COLO 201) by tumour necrosis factor-alpha (TNF-alpha) and/or interferon-gamma (IFN-gamma), resulting from down-modulation of Bcl-2 expression. Clin Exp Immunol 111: 211–218, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Laron Z Natural history of the classical form of primary growth hormone (GH) resistance (Laron syndrome). J Pediatr Endocrinol Metab 12, Suppl 1: 231–249, 1999. [PubMed] [Google Scholar]
- 45.Laron Z, Sarel R, Pertzelan A. Puberty in Laron type dwarfism. Eur J Pediatr 134: 79–83, 1980. [DOI] [PubMed] [Google Scholar]
- 46.Laurence A, Tato CM, Davidson TS, Kanno Y, Chen Z, Yao Z, Blank RB, Meylan F, Siegel R, Hennighausen L, Shevach EM, O'Shea JJ. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity 26: 371–381, 2007. [DOI] [PubMed] [Google Scholar]
- 47.Lee CK, Smith E, Gimeno R, Gertner R, Levy DE. STAT1 affects lymphocyte survival and proliferation partially independent of its role downstream of IFN-gamma. J Immunol 164: 1286–1292, 2000. [DOI] [PubMed] [Google Scholar]
- 48.Lee HJ, Oh YK, Rhee M, Lim JY, Hwang JY, Park YS, Kwon Y, Choi KH, Jo I, Park SI, Gao B, Kim WH. The role of STAT1/IRF-1 on synergistic ROS production and loss of mitochondrial transmembrane potential during hepatic cell death induced by LPS/d-GalN. J Mol Biol 369: 967–984, 2007. [DOI] [PubMed] [Google Scholar]
- 49.Lee JY, Gavrilova O, Davani B, Na R, Robinson GW, Hennighausen L. The transcription factors Stat5a/b are not required for islet development but modulate pancreatic beta-cell physiology upon aging. Biochim Biophys Acta 1773: 1455–1461, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee SJ, Zhou T, Choi C, Wang Z, Benveniste EN. Differential regulation and function of Fas expression on glial cells. J Immunol 164: 1277–1285, 2000. [DOI] [PubMed] [Google Scholar]
- 51.Lee TK, Man K, Poon RT, Lo CM, Yuen AP, Ng IO, Ng KT, Leonard W, Fan ST. Signal transducers and activators of transcription 5b activation enhances hepatocellular carcinoma aggressiveness through induction of epithelial-mesenchymal transition. Cancer Res 66: 9948–9956, 2006. [DOI] [PubMed] [Google Scholar]
- 52.Lehtonen A, Matikainen S, Julkunen I. Interferons up-regulate STAT1, STAT2, and IRF family transcription factor gene expression in human peripheral blood mononuclear cells and macrophages. J Immunol 159: 794–803, 1997. [PubMed] [Google Scholar]
- 53.Leong PL, Andrews GA, Johnson DE, Dyer KF, Xi S, Mai JC, Robbins PD, Gadiparthi S, Burke NA, Watkins SF, Grandis JR. Targeted inhibition of Stat3 with a decoy oligonucleotide abrogates head and neck cancer cell growth. Proc Natl Acad Sci USA 100: 4138–4143, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li W, Liang X, Kellendonk C, Poli V, Taub R. STAT3 contributes to the mitogenic response of hepatocytes during liver regeneration. J Biol Chem 277: 28411–28417, 2002. [DOI] [PubMed] [Google Scholar]
- 55.Lin Q, Lai R, Chirieac LR, Li C, Thomazy VA, Grammatikakis I, Rassidakis GZ, Zhang W, Fujio Y, Kunisada K, Hamilton SR, Amin HM. Constitutive activation of JAK3/STAT3 in colon carcinoma tumors and cell lines: inhibition of JAK3/STAT3 signaling induces apoptosis and cell cycle arrest of colon carcinoma cells. Am J Pathol 167: 969–980, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu X, Robinson GW, Gouilleux F, Groner B, Hennighausen L. Cloning and expression of Stat5 and an additional homologue (Stat5b) involved in prolactin signal transduction in mouse mammary tissue. Proc Natl Acad Sci USA 92: 8831–8835, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu X, Robinson GW, Wagner KU, Garrett L, Wynshaw-Boris A, Hennighausen L. Stat5a is mandatory for adult mammary gland development and lactogenesis. Genes Dev 11: 179–186, 1997. [DOI] [PubMed] [Google Scholar]
- 58.Meraz MA, White JM, Sheehan KC, Bach EA, Rodig SJ, Dighe AS, Kaplan DH, Riley JK, Greenlund AC, Campbell D, Carver-Moore K, DuBois RN, Clark R, Aguet M, Schreiber RD. Targeted disruption of the Stat1 gene in mice reveals unexpected physiologic specificity in the JAK-STAT signaling pathway. Cell 84: 431–442, 1996. [DOI] [PubMed] [Google Scholar]
- 59.Miyamoto M, Fujita T, Kimura Y, Maruyama M, Harada H, Sudo Y, Miyata T, Taniguchi T. Regulated expression of a gene encoding a nuclear factor, IRF-1, that specifically binds to IFN-beta gene regulatory elements. Cell 54: 903–913, 1988. [DOI] [PubMed] [Google Scholar]
- 60.Mui AL, Wakao H, O'Farrell AM, Harada N, Miyajima A. Interleukin-3, granulocyte-macrophage colony stimulating factor and interleukin-5 transduce signals through two STAT5 homologs. EMBO J 14: 1166–1175, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Murray PJ The JAK-STAT signaling pathway: input and output integration. J Immunol 178: 2623–2629, 2007. [DOI] [PubMed] [Google Scholar]
- 62.Niwa Y, Kanda H, Shikauchi Y, Saiura A, Matsubara K, Kitagawa T, Yamamoto J, Kubo T, Yoshikawa H. Methylation silencing of SOCS-3 promotes cell growth and migration by enhancing JAK/STAT and FAK signalings in human hepatocellular carcinoma. Oncogene 24: 6406–6417, 2005. [DOI] [PubMed] [Google Scholar]
- 63.Noda H, Maehara Y, Irie K, Kakeji Y, Yonemura T, Sugimachi K. Growth pattern and expressions of cell cycle regulator proteins p53 and p21WAF1/CIP1 in early gastric carcinoma. Cancer 92: 1828–1835, 2001. [DOI] [PubMed] [Google Scholar]
- 64.Ossina NK, Cannas A, Powers VC, Fitzpatrick PA, Knight JD, Gilbert JR, Shekhtman EM, Tomei LD, Umansky SR, Kiefer MC. Interferon-gamma modulates a p53-independent apoptotic pathway and apoptosis-related gene expression. J Biol Chem 272: 16351–16357, 1997. [DOI] [PubMed] [Google Scholar]
- 65.Ram PA, Waxman DJ. SOCS/CIS protein inhibition of growth hormone-stimulated STAT5 signaling by multiple mechanisms. J Biol Chem 274: 35553–35561, 1999. [DOI] [PubMed] [Google Scholar]
- 66.Ramadori G, Christ B. Cytokines and the hepatic acute-phase response. Semin Liver Dis 19: 141–155, 1999. [DOI] [PubMed] [Google Scholar]
- 67.Reed JC Mechanisms of apoptosis avoidance in cancer. Curr Opin Oncol 11: 68–75, 1999. [DOI] [PubMed] [Google Scholar]
- 68.Rowland JE, Kerr LM, White M, Noakes PG, Waters MJ. Heterozygote effects in mice with partial truncations in the growth hormone receptor cytoplasmic domain: assessment of growth parameters and phenotype. Endocrinology 146: 5278–5286, 2005. [DOI] [PubMed] [Google Scholar]
- 69.Rowland JE, Lichanska AM, Kerr LM, White M, d'Aniello EM, Maher SL, Brown R, Teasdale RD, Noakes PG, Waters MJ. In vivo analysis of growth hormone receptor signaling domains and their associated transcripts. Mol Cell Biol 25: 66–77, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sepulveda P, Encabo A, Carbonell-Uberos F, Minana MD. BCL-2 expression is mainly regulated by JAK/STAT3 pathway in human CD34+ hematopoietic cells. Cell Death Differ 14: 378–380, 2007. [DOI] [PubMed] [Google Scholar]
- 71.Siddiquee K, Zhang S, Guida WC, Blaskovich MA, Greedy B, Lawrence HR, Yip ML, Jove R, McLaughlin MM, Lawrence NJ, Sebti SM, Turkson J. Selective chemical probe inhibitor of Stat3, identified through structure-based virtual screening, induces antitumor activity. Proc Natl Acad Sci USA 104: 7391–7396, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Silva M, Benito A, Sanz C, Prosper F, Ekhterae D, Nunez G, Fernandez-Luna JL. Erythropoietin can induce the expression of bcl-xL through Stat5 in erythropoietin-dependent progenitor cell lines. J Biol Chem 274: 22165–22169, 1999. [DOI] [PubMed] [Google Scholar]
- 73.Sinibaldi D, Wharton W, Turkson J, Bowman T, Pledger WJ, Jove R. Induction of p21WAF1/CIP1 and cyclin D1 expression by the Src oncoprotein in mouse fibroblasts: role of activated STAT3 signaling. Oncogene 19: 5419–5427, 2000. [DOI] [PubMed] [Google Scholar]
- 74.Sjogren K, Liu JL, Blad K, Skrtic S, Vidal O, Wallenius V, LeRoith D, Tornell J, Isaksson OG, Jansson JO, Ohlsson C. Liver-derived insulin-like growth factor I (IGF-I) is the principal source of IGF-I in blood but is not required for postnatal body growth in mice. Proc Natl Acad Sci USA 96: 7088–7092, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Socolovsky M, Fallon AE, Wang S, Brugnara C, Lodish HF. Fetal anemia and apoptosis of red cell progenitors in Stat5a−/−5b−/− mice: a direct role for Stat5 in Bcl-XL induction. Cell 98: 181–191, 1999. [DOI] [PubMed] [Google Scholar]
- 76.Socolovsky M, Nam H, Fleming MD, Haase VH, Brugnara C, Lodish HF. Ineffective erythropoiesis in Stat5a−/−5b−/− mice due to decreased survival of early erythroblasts. Blood 98: 3261–3273, 2001. [DOI] [PubMed] [Google Scholar]
- 77.Sultan AS, Xie J, LeBaron MJ, Ealley EL, Nevalainen MT, Rui H. Stat5 promotes homotypic adhesion and inhibits invasive characteristics of human breast cancer cells. Oncogene 24: 746–760, 2005. [DOI] [PubMed] [Google Scholar]
- 78.Sun J, Blaskovich MA, Jove R, Livingston SK, Coppola D, Sebti SM. Cucurbitacin Q: a selective STAT3 activation inhibitor with potent antitumor activity. Oncogene 24: 3236–3245, 2005. [DOI] [PubMed] [Google Scholar]
- 79.Sun R, Park O, Horiguchi N, Kulkarni S, Jeong WI, Sun HY, Radaeva S, Gao B. STAT1 contributes to dsRNA inhibition of liver regeneration after partial hepatectomy in mice. Hepatology 44: 955–966, 2006. [DOI] [PubMed] [Google Scholar]
- 80.Sutherland KD, Lindeman GJ, Choong DY, Wittlin S, Brentzell L, Phillips W, Campbell IG, Visvader JE. Differential hypermethylation of SOCS genes in ovarian and breast carcinomas. Oncogene 23: 7726–7733, 2004. [DOI] [PubMed] [Google Scholar]
- 81.Teglund S, McKay C, Schuetz E, van Deursen JM, Stravopodis D, Wang D, Brown M, Bodner S, Grosveld G, Ihle JN. Stat5a and Stat5b proteins have essential and nonessential, or redundant, roles in cytokine responses. Cell 93: 841–850, 1998. [DOI] [PubMed] [Google Scholar]
- 82.Udy GB, Towers RP, Snell RG, Wilkins RJ, Park SH, Ram PA, Waxman DJ, Davey HW. Requirement of STAT5b for sexual dimorphism of body growth rates and liver gene expression. Proc Natl Acad Sci USA 94: 7239–7244, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.van Boxel-Dezaire AH, Stark GR. Cell type-specific signaling in response to interferon-gamma. Curr Top Microbiol Immunol 316: 119–154, 2007. [DOI] [PubMed] [Google Scholar]
- 84.Vidal OM, Merino R, Rico-Bautista E, Fernandez-Perez L, Chia DJ, Woelfle J, Ono M, Lenhard B, Norstedt G, Rotwein P, Flores-Morales A. In vivo transcript profiling and phylogenetic analysis identifies suppressor of cytokine signaling 2 as a direct signal transducer and activator of transcription 5b target in liver. Mol Endocrinol 21: 293–311, 2007. [DOI] [PubMed] [Google Scholar]
- 85.Villarino AV, Tato CM, Stumhofer JS, Yao Z, Cui YK, Hennighausen L, O'Shea JJ, Hunter CA. Helper T cell IL-2 production is limited by negative feedback and STAT-dependent cytokine signals. J Exp Med 204: 65–71, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wakao H, Gouilleux F, Groner B. Mammary gland factor (MGF) is a novel member of the cytokine regulated transcription factor gene family and confers the prolactin response. EMBO J 13: 2182–2191, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Walenkamp MJ, Vidarsdottir S, Pereira AM, Karperien M, van Doorn J, van Duyvenvoorde HA, Breuning MH, Roelfsema F, Kruithof MF, van Dissel J, Janssen R, Wit JM, Romijn JA. Growth hormone secretion and immunological function of a male patient with a homozygous STAT5b mutation. Eur J Endocrinol 156: 155–165, 2007. [DOI] [PubMed] [Google Scholar]
- 88.Waters MJ, Hoang HN, Fairlie DP, Pelekanos RA, Brown RJ. New insights into growth hormone action. J Mol Endocrinol 36: 1–7, 2006. [DOI] [PubMed] [Google Scholar]
- 89.Waxman DJ, O'Connor C. Growth hormone regulation of sex-dependent liver gene expression. Mol Endocrinol 20: 2613–2629, 2006. [DOI] [PubMed] [Google Scholar]
- 90.Weerasinghe P, Garcia GE, Zhu Q, Yuan P, Feng L, Mao L, Jing N. Inhibition of Stat3 activation and tumor growth suppression of non-small cell lung cancer by G-quartet oligonucleotides. Int J Oncol 31: 129–136, 2007. [PubMed] [Google Scholar]
- 91.Wegenka UM, Lutticken C, Buschmann J, Yuan J, Lottspeich F, Muller-Esterl W, Schindler C, Roeb E, Heinrich PC, Horn F. The interleukin-6-activated acute-phase response factor is antigenically and functionally related to members of the signal transducer and activator of transcription (STAT) family. Mol Cell Biol 14: 3186–3196, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wuestefeld T, Klein C, Streetz KL, Betz U, Lauber J, Buer J, Manns MP, Muller W, Trautwein C. Interleukin-6/glycoprotein 130-dependent pathways are protective during liver regeneration. J Biol Chem 278: 11281–11288, 2003. [DOI] [PubMed] [Google Scholar]
- 93.Xi S, Gooding WE, Grandis JR. In vivo antitumor efficacy of STAT3 blockade using a transcription factor decoy approach: implications for cancer therapy. Oncogene 24: 970–979, 2005. [DOI] [PubMed] [Google Scholar]
- 94.Xu X, Fu XY, Plate J, Chong AS. IFN-gamma induces cell growth inhibition by Fas-mediated apoptosis: requirement of STAT1 protein for up-regulation of Fas and FasL expression. Cancer Res 58: 2832–2837, 1998. [PubMed] [Google Scholar]
- 95.Yakar S, Liu JL, Stannard B, Butler A, Accili D, Sauer B, LeRoith D. Normal growth and development in the absence of hepatic insulin-like growth factor I. Proc Natl Acad Sci USA 96: 7324–7329, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yao Z, Cui Y, Watford WT, Bream JH, Yamaoka K, Hissong BD, Li D, Durum SK, Jiang Q, Bhandoola A, Hennighausen L, O'Shea JJ. Stat5a/b are essential for normal lymphoid development and differentiation. Proc Natl Acad Sci USA 103: 1000–1005, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yao Z, Kanno Y, Kerenyi M, Stephens G, Durant L, Watford WT, Laurence A, Robinson GW, Shevach EM, Moriggl R, Hennighausen L, Wu C, O'Shea JJ. Nonredundant roles for Stat5a/b in directly regulating Foxp3. Blood 109: 4368–4375, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yoshimura A, Naka T, Kubo M. SOCS proteins, cytokine signalling and immune regulation. Nat Rev Immunol 7: 454–465, 2007. [DOI] [PubMed] [Google Scholar]
- 99.Yumet G, Shumate ML, Bryant DP, Lang CH, Cooney RN. Hepatic growth hormone resistance during sepsis is associated with increased suppressors of cytokine signaling expression and impaired growth hormone signaling. Crit Care Med 34: 1420–1427, 2006. [DOI] [PubMed] [Google Scholar]