Abstract
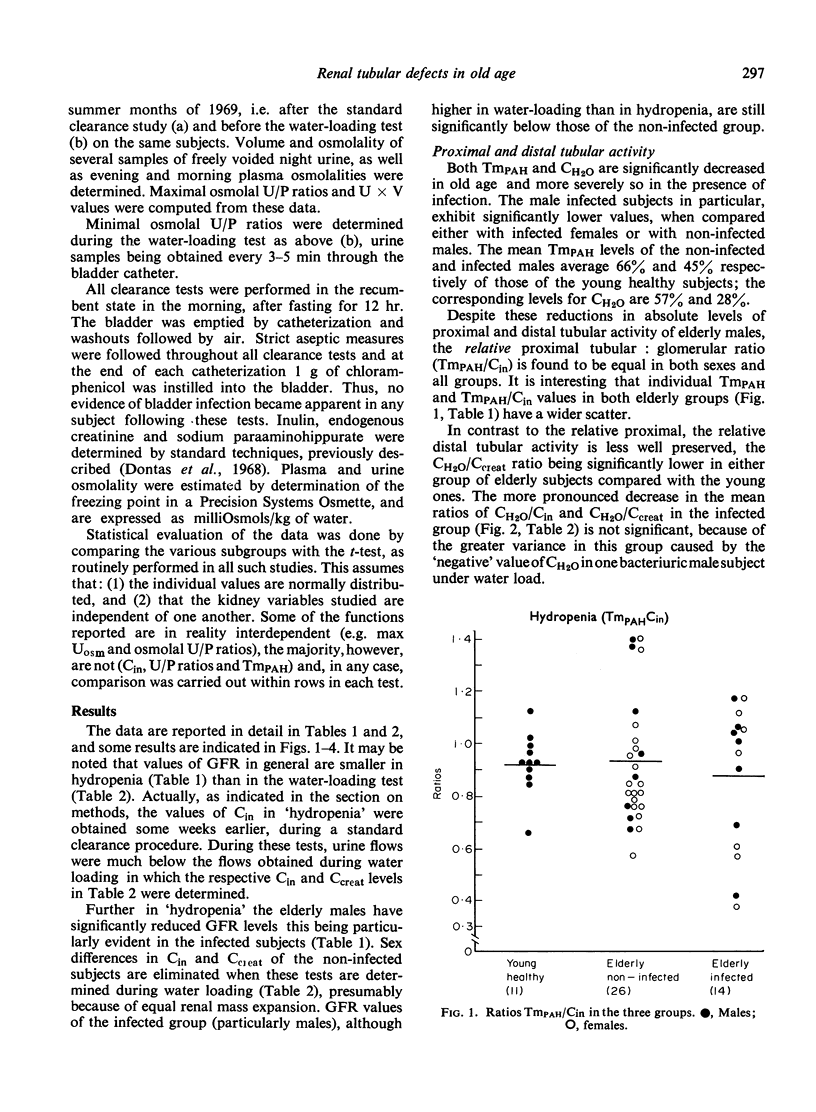
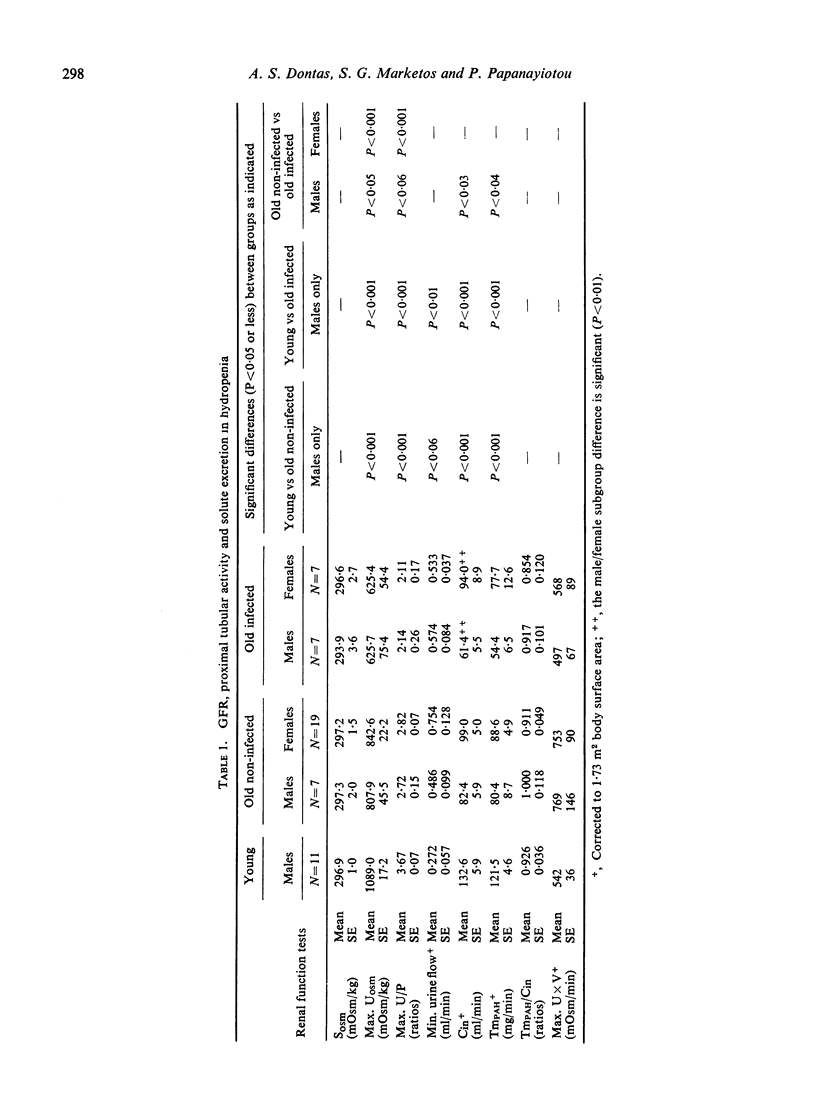
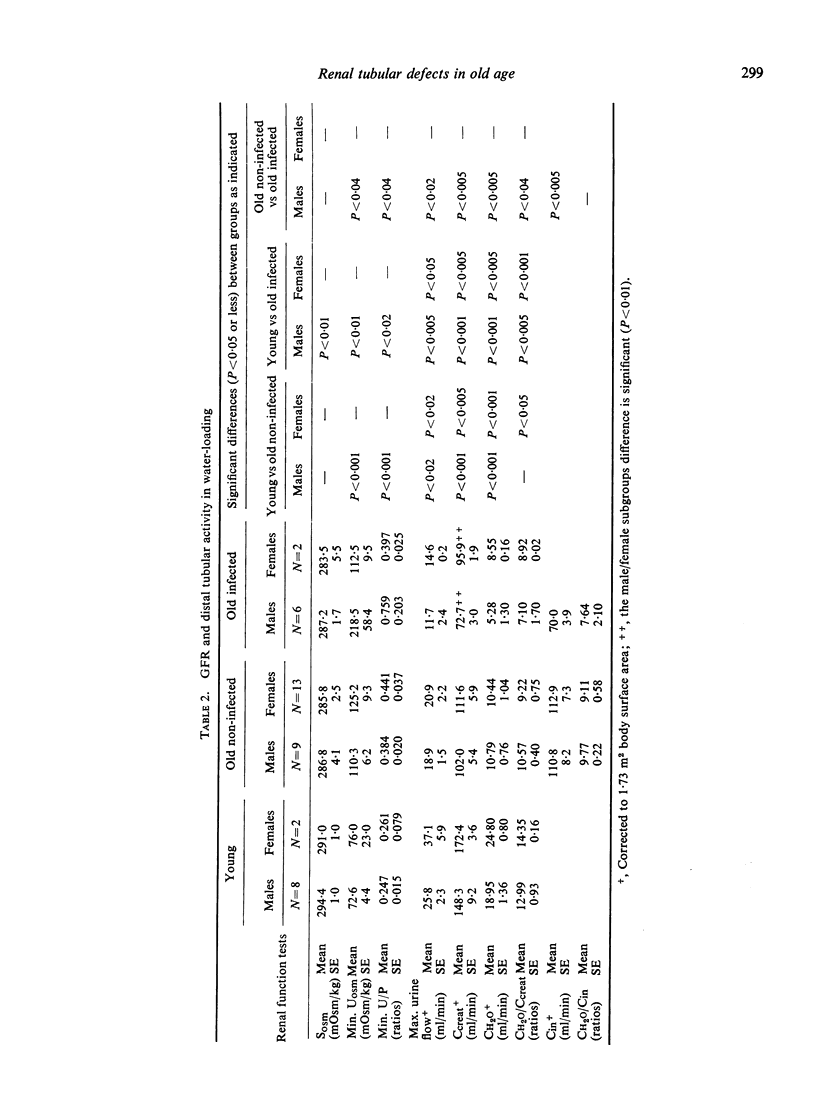
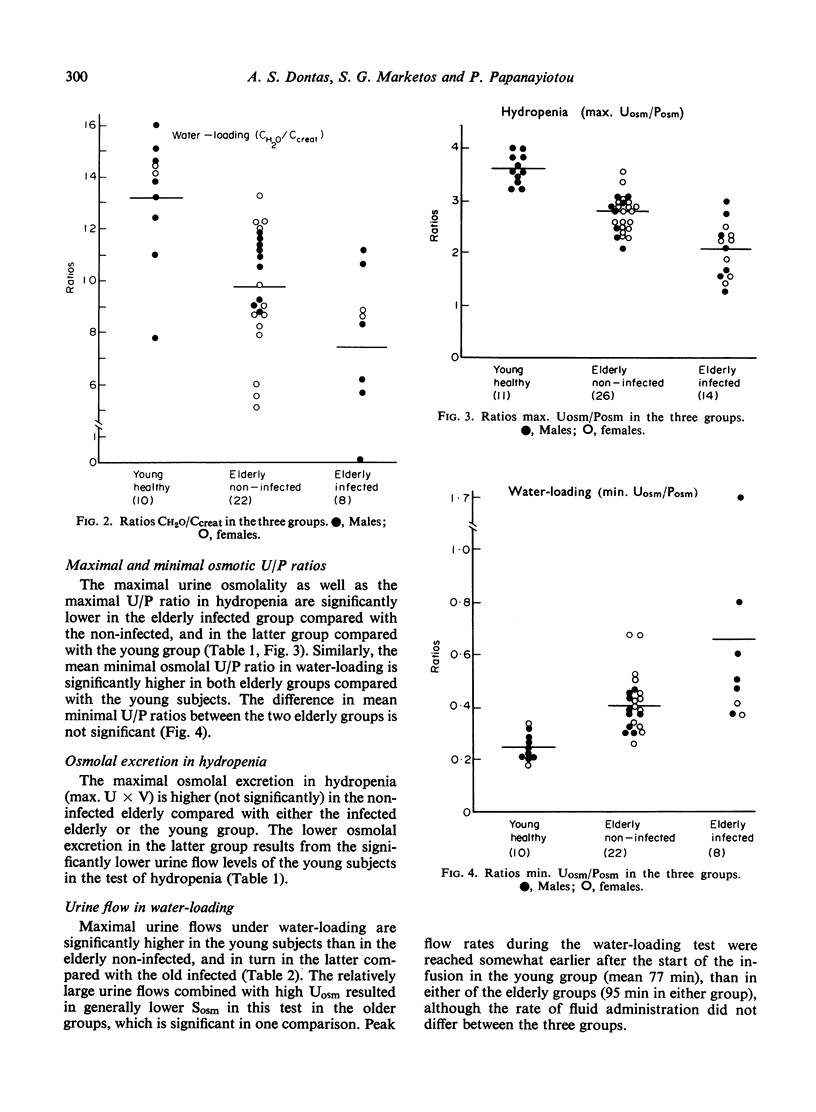
The mechanisms of renal tubular dysfunction in old age have been examined in twenty-eight clinically healthy elderly subjects without infection, and in fourteen subjects of similar age with laboratory evidence of intrarenal infection. The data were compared with those from thirteen clinically healthy young subjects. Studied were: proximal tubular (Tm(PAH)) and distal tubular (CH2O) activity, minimal and maximal osmolal U/P ratios, maximal osmolal excretion in hydropenia, and GFR levels under standard hydration and under water-loading. The reduction of GFR in old age is evident particularly in men under conditions of standard hydration: it is accentuated in the presence of renal infection. Proximal tubular activity is also significantly lower in elderly men, especially if they have chronic bacteriuria. The reduction is closely related to GFR levels, with identical Tm(PAH):C(in) ratios in all groups. This supports the intact nephron hypothesis for this part of the nephron. Distal tubular activity is depressed in old age in both sexes proportionately more than proximal tubular activity or the GFR. The lower CH2O: GFR ratios imply a selective distal tubular damage. Maximal osmolal U/P ratios in hydropenia are significantly higher in the young (mean 367) than in either the elderly non-infected (mean 279) or the elderly infected subjects (mean 212). Conversely, minimal U/P ratios in water-loading are lower in the young (mean 0.247) than in either elderly group (means 0.418 and 0.668). Osmolal excretion in hydropenia is not different between the groups, but urine flows in water-loading clearly separate them. The data indicate that simple functions of the distal-collecting tubule (e.g. the CH2O), are less affected in old age than are functions involving several medullary structures (as is the maximal U(osm) or U/P ratio). They suggest that the main impairment of the distal tubular cell involves the failure to achieve a proper osmotic gradient between tubular fluid and blood, rather than an inability to excrete or re-absorb an adequate amount of solute. Finally, it appears that renal infection aggravates the larger glomerular and proximal tubular deficits observed in non-infected men: it depresses distal tubular function equally in both sexes.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADAMS J. R., BARROWS C. H., Jr Effect of age on PAH accumulation by kidney slices of female rats. J Gerontol. 1963 Jan;18:37–40. doi: 10.1093/geronj/18.1.37. [DOI] [PubMed] [Google Scholar]
- BOYARSKY S., SMITH H. W. Renal concentrating operation at low urine flows. J Urol. 1957 Nov;78(5):511–524. doi: 10.1016/S0022-5347(17)66472-3. [DOI] [PubMed] [Google Scholar]
- Bricker N. S. On the meaning of the intact nephron hypothesis. Am J Med. 1969 Jan;46(1):1–11. doi: 10.1016/0002-9343(69)90053-9. [DOI] [PubMed] [Google Scholar]
- Brod J. Study of renal function in the differential diagnosis of kidney disease. Br Med J. 1971 Jul 17;3(5767):135–143. doi: 10.1136/bmj.3.5767.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckalew V. M., Jr, Puschett J. B., Kintzel J. E., Goldberg M. Mechanism of exaggerated natriuresis in hypertensive man: impaired sodium transport in the loop of Henle. J Clin Invest. 1969 Jun;48(6):1007–1016. doi: 10.1172/JCI106057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon P. J. Effects of five per cent dextros-water infusions in normal and hypertensive man. Evidence for increased proximal and distal tubular sodium rejection by hypertensive patients and its relation to renal hemodynamics. Circulation. 1968 May;37(5):832–846. doi: 10.1161/01.cir.37.5.832. [DOI] [PubMed] [Google Scholar]
- Coleman A. J., Arias M., Carter N. W., Rector F. C., Seldin D. W. The mechanism of salt wastage in chronic renal disease. J Clin Invest. 1966 Jul;45(7):1116–1125. doi: 10.1172/JCI105418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIES D. F., SHOCK N. W. Age changes in glomerular filtration rate, effective renal plasma flow, and tubular excretory capacity in adult males. J Clin Invest. 1950 May;29(5):496–507. doi: 10.1172/JCI102286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dontas A. S., Papanayiotou P., Marketos S. G., Papanicolaou N. T. The effect of bacteriuria on renal functional patterns in old age. Clin Sci. 1968 Feb;34(1):73–81. [PubMed] [Google Scholar]
- EPSTEIN F. H., KLEEMAN C. R., PURSEL S., HENDRIKX A. The effect of feeding protein and urea on the renal concentrating process. J Clin Invest. 1957 May;36(5):635–641. doi: 10.1172/JCI103463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eknoyan G., Suki W. N., Rector F. C., Jr, Seldin D. W. Functional characteristics of the diluting segment of the dog nephron and the effect of extracellular volume expansion on its reabsorptive capacity. J Clin Invest. 1967 Jul;46(7):1178–1188. doi: 10.1172/JCI105611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayslett J. P., Kashgarian M., Epstein F. H. Changes in proximal and distal tubular reabsorption produced by rapid expansion of extracellular fluid. J Clin Invest. 1967 Jul;46(7):1254–1263. doi: 10.1172/JCI105618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVITT M. F., LEVY M. S., POLIMEROS D. The effect of a fall in filtration rate on solute and water excretion in hydropenic man. J Clin Invest. 1959 Mar;38(3):463–473. doi: 10.1172/JCI103822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LINDEMAN R. D., VAN BUREN H. C., RAISZ L. G. Osmolar renal concentrating ability in healthy young men and hospitalized patients without renal disease. N Engl J Med. 1960 Jun 30;262:1306–1309. doi: 10.1056/NEJM196006302622603. [DOI] [PubMed] [Google Scholar]
- Lindeman R. D., Lee T. D., Jr, Yiengst M. J., Shock N. W. Influence of age, renal disease, hypertension, diuretics, and calcium on the antidiuretic responses to suboptimal infusions of vasopressin. J Lab Clin Med. 1966 Aug;68(2):206–223. [PubMed] [Google Scholar]
- MILLER J. H., SHOCK N. W. Age differences in the renal tubular response to antidiuretic hormone. J Gerontol. 1953 Oct;8(4):446–450. doi: 10.1093/geronj/8.4.446. [DOI] [PubMed] [Google Scholar]
- Marketos S. G., Papanayiotou P. C., Dontas A. S. Bacteriuria and non-obstructive renovascular disease in old age. J Gerontol. 1969 Jan;24(1):33–36. doi: 10.1093/geronj/24.1.33. [DOI] [PubMed] [Google Scholar]
- Morrin P. A., Joynt S. K., Handa S. P., Frame J. The effect of uremic environment on renal concentrating and diluting mechanisms in a severely damaged nephron population. Invest Urol. 1970 Nov;8(3):273–283. [PubMed] [Google Scholar]
- SOMMERS S. C., GONICK H. C., KALMANSON G. M., GUZE L. B. PATHOGENESIS OF CHRONIC PYELONEPHRITIS. II. EFFECT OF REPETITIVE INFECTION. Am J Pathol. 1964 Nov;45:729–739. [PMC free article] [PubMed] [Google Scholar]
- STEINMETZ P. R., EISINGER R. P., GOMBOS E. A., CHASIS H., BALDWIN D. S. EXCRETION OF FREE WATER AND SOLUTE DURING MAXIMAL WATER DIURESIS IN NORMAL AND HYPERTENSIVE SUBJECTS. J Lab Clin Med. 1964 Aug;64:238–256. [PubMed] [Google Scholar]
- Stein R. M., Abramson R. G., Kahn T., Levitt M. F. Effects of hypotonic saline loading in hydrated dog: evidence for a saline-induced limit on distal tubular sodium transport. J Clin Invest. 1967 Jul;46(7):1205–1214. doi: 10.1172/JCI105614. [DOI] [PMC free article] [PubMed] [Google Scholar]


