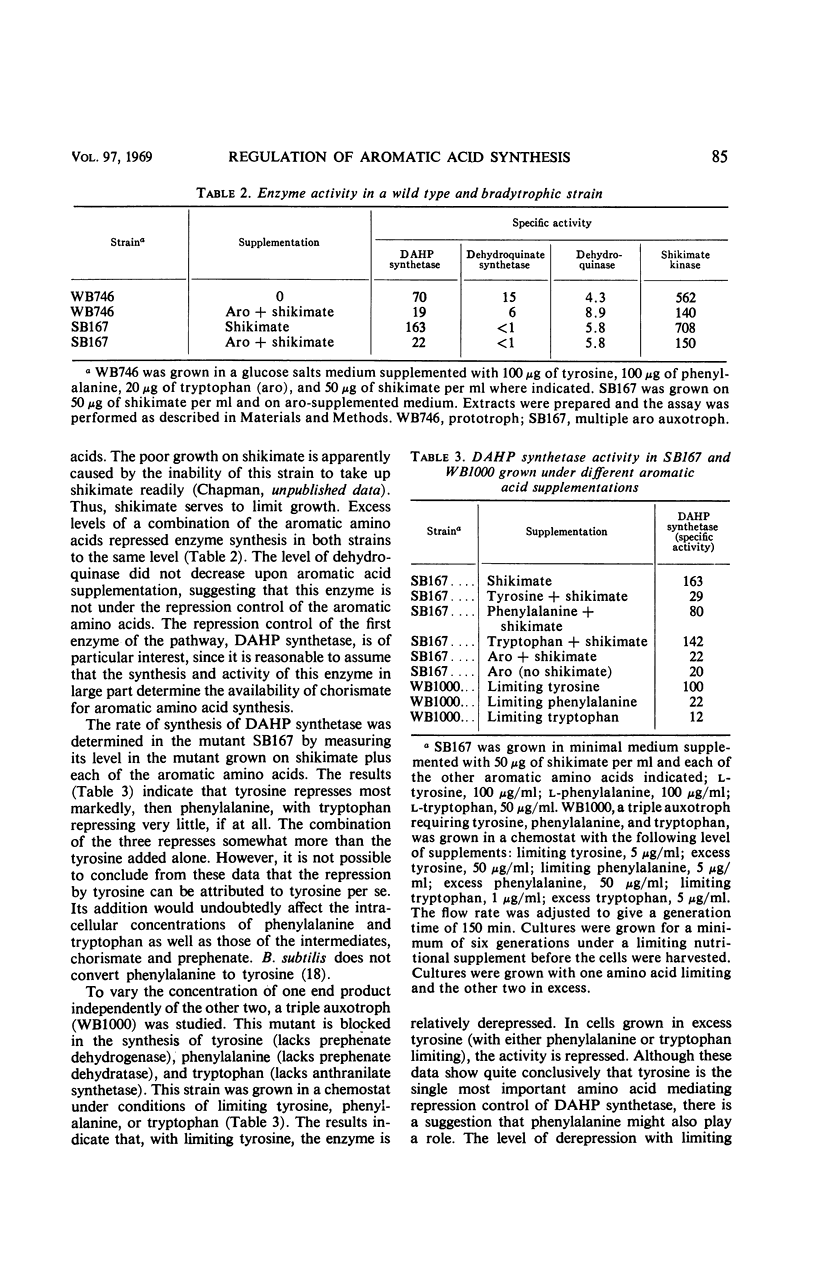
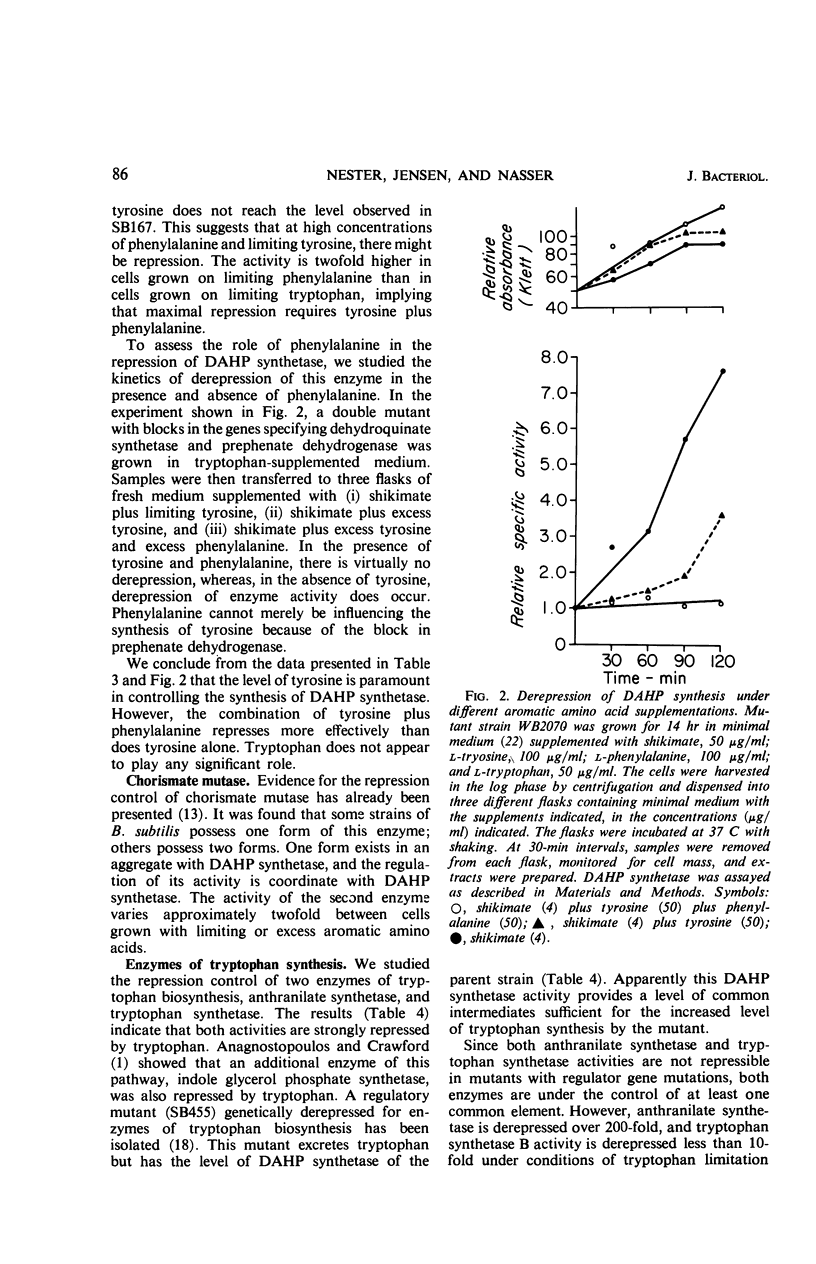
Abstract
The control of the synthesis of certain key enzymes of aromatic amino acid biosynthesis was studied. Tyrosine represses the first enzyme of the 3-deoxy-d-arabino heptulosonic acid 7-phosphate pathway, DAHP synthetase, as well as shikimate kinase and chorismate mutase about fivefold in cultures grown under conditions limiting the synthesis of the aromatic amino acids. A mixture of tyrosine and phenylalanine represses twofold further. Tryptophan does not appear to be involved in the control of these enzymes. The specific activity of at least one early enzyme, dehydroquinase, remains essentially constant under a variety of nutritional supplementations. Two enzymes in the terminal branches are repressed by the amino acids they help to synthesize: prephenate dehydrogenase can be repressed fourfold by tyrosine, and anthranilate synthetase can be repressed over 200-fold by tryptophan. There is no evidence that phenylalanine represses prephenate dehydratase. Regulatory mutants have been isolated in which various enzymes of the pathway are no longer repressible. One class is derepressed for several of the prechorismate enzymes, as well as chorismate mutase and prephenate dehydrogenase. In another mutant, several enzymes of tryptophan biosynthesis are no longer repressible. Thus, the rate of synthesis of enzymes at every stage of the pathway is under control of various aromatic amino acids. Tyrosine and phenylalanine control the synthesis of enzymes involved in the synthesis of the three aromatic amino acids. Each terminal branch is under the control of its end product.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANAGNOSTOPOULOS C., CRAWFORD I. P. Transformation studies on the linkage of markers in the tryptophan pathway in Bacillus subtilis. Proc Natl Acad Sci U S A. 1961 Mar 15;47:378–390. doi: 10.1073/pnas.47.3.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauerle R. H., Margolin P. The functional organization of the tryptophan gene cluster in Salmonella typhimurium. Proc Natl Acad Sci U S A. 1966 Jul;56(1):111–118. doi: 10.1073/pnas.56.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coats J. H., Nester E. W. Regulation reversal mutation: characterization of end product-activated mutants of Bacillus subtilis. J Biol Chem. 1967 Nov 10;242(21):4948–4955. [PubMed] [Google Scholar]
- Cohen G. N. Regulation of enzyme activity in microorganisms. Annu Rev Microbiol. 1965;19:105–126. doi: 10.1146/annurev.mi.19.100165.000541. [DOI] [PubMed] [Google Scholar]
- DAVIS B. D. Aromatic biosynthesis. IV. Preferential conversion, in incompletely blocked mutants, of a common precursor of several metabolites. J Bacteriol. 1952 Nov;64(5):729–748. doi: 10.1128/jb.64.5.729-748.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. D. Autocatalytic growth of a mutant due to accumulation of unstable phenylalanine precursor. Science. 1953 Aug 28;118(3061):251–252. doi: 10.1126/science.118.3061.251. [DOI] [PubMed] [Google Scholar]
- Gollub E., Zalkin H., Sprinson D. B. Correlation of genes and enzymes, and studies on regulation of the aromatic pathway in Salmonella. J Biol Chem. 1967 Nov 25;242(22):5323–5328. [PubMed] [Google Scholar]
- Ito J., Crawford I. P. Regulation of the enzymes of the tryptophan pathway in Escherichia coli. Genetics. 1965 Dec;52(6):1303–1316. doi: 10.1093/genetics/52.6.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JENSEN R. A., NESTER E. W. THE REGULATORY SIGNIFICANCE OF INTERMEDIARY METABOLITES: CONTROL OF AROMATIC ACID BIOSYNTHESIS BY FEEDBACK INHIBITION IN BACILLUS SUBTILIS. J Mol Biol. 1965 Jun;12:468–481. doi: 10.1016/s0022-2836(65)80270-4. [DOI] [PubMed] [Google Scholar]
- Jensen R. A., Nasser D. S., Nester E. W. Comparative control of a branch-point enzyme in microorganisms. J Bacteriol. 1967 Nov;94(5):1582–1593. doi: 10.1128/jb.94.5.1582-1593.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen R. A., Nester E. W. Regulatory enzymes of aromatic amino acid biosynthesis in Bacillus subtilis. I. Purification and properties of 3-deoxy-D-arabino-heptulosonate 7-phosphate synthetase. J Biol Chem. 1966 Jul 25;241(14):3365–3372. [PubMed] [Google Scholar]
- KATAGIRI M., SATO R. Accumulation of phenylalanine by a phenylalanineless mutant of Escherichia coli. Science. 1953 Aug 28;118(3061):250–251. doi: 10.1126/science.118.3061.250. [DOI] [PubMed] [Google Scholar]
- Lorence J. H., Nester E. W. Multiple molecular forms of chorismate mutase in Bacillus subtillis. Biochemistry. 1967 May;6(5):1541–1553. doi: 10.1021/bi00857a041. [DOI] [PubMed] [Google Scholar]
- MITSUHASHI S., DAVIS B. D. Aromatic biosynthesis. XII. Conversion of 5-dehydroquinic acid to 5-dehydroshikimic acid dy 5-dehydroquinase. Biochim Biophys Acta. 1954 Sep;15(1):54–61. doi: 10.1016/0006-3002(54)90093-1. [DOI] [PubMed] [Google Scholar]
- Nasser D., Nester E. W. Aromatic amino acid biosynthesis: gene-enzyme relationships in Bacillus subtilis. J Bacteriol. 1967 Nov;94(5):1706–1714. doi: 10.1128/jb.94.5.1706-1714.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nester E W, Schafer M, Lederberg J. Gene Linkage in DNA Transfer: A Cluster of Genes Concerned with Aromatic Biosynthesis in Bacillus Subtilis. Genetics. 1963 Apr;48(4):529–551. doi: 10.1093/genetics/48.4.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nester E. W., Jensen R. A. Control of aromatic acid biosynthesis in Bacillus subtilis: sequenial feedback inhibition. J Bacteriol. 1966 Apr;91(4):1594–1598. doi: 10.1128/jb.91.4.1594-1598.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nester E. W., Lorence J. H., Nasser D. S. An enzyme aggregate involved in the biosynthesis of aromatic amino acids in Bacillus subtilis. Its possible function in feedback regulation. Biochemistry. 1967 May;6(5):1553–1563. doi: 10.1021/bi00857a042. [DOI] [PubMed] [Google Scholar]
- SCHWARTZ A. K., BONNER D. M. TRYPTOPHAN SYNTHETASE IN BACILLUS SUBTILIS: EFFECTS OF HIGH POTASSIUM ION CONCENTRATION ON A TWO COMPONENT ENZYME. Biochim Biophys Acta. 1964 Aug 26;89:337–347. doi: 10.1016/0926-6569(64)90223-8. [DOI] [PubMed] [Google Scholar]
- SCHWINCK I., ADAMS E. Aromatic biosynthesis. XVI. Aromatization of prephenic acid to p-hydroxyphenylpyruvic acid, a step in tyrosine biosynthesis in Escherichia coli. Biochim Biophys Acta. 1959 Nov;36:102–117. doi: 10.1016/0006-3002(59)90074-5. [DOI] [PubMed] [Google Scholar]
- SMITH L. C., RAVEL J. M., LAX S. R., SHIVE W. The control of 3-deoxy-D-arabino-heptulosonic acid 7-phosphate synthesis by phenylalanine and tyrosine. J Biol Chem. 1962 Nov;237:3566–3570. [PubMed] [Google Scholar]
- SRINIVASAN P. R., ROTHSCHILD J., SPRINSON D. B. THE ENZYMIC CONVERSION OF 3-DEOXY-D-ARABINO-HEPTULOSONIC ACID 7-PHOSPHATE TO 5-DEHYDROQUINATE. J Biol Chem. 1963 Oct;238:3176–3182. [PubMed] [Google Scholar]
- SRINIVASAN P. R., SPRINSON D. B. 2-Keto-3-deoxy-D-arabo-heptonic acid 7-phosphate synthetase. J Biol Chem. 1959 Apr;234(4):716–722. [PubMed] [Google Scholar]
- Spizizen J. TRANSFORMATION OF BIOCHEMICALLY DEFICIENT STRAINS OF BACILLUS SUBTILIS BY DEOXYRIBONUCLEATE. Proc Natl Acad Sci U S A. 1958 Oct 15;44(10):1072–1078. doi: 10.1073/pnas.44.10.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sueoka N. CORRELATION BETWEEN BASE COMPOSITION OF DEOXYRIBONUCLEIC ACID AND AMINO ACID COMPOSITION OF PROTEIN. Proc Natl Acad Sci U S A. 1961 Aug;47(8):1141–1149. doi: 10.1073/pnas.47.8.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]


