Abstract
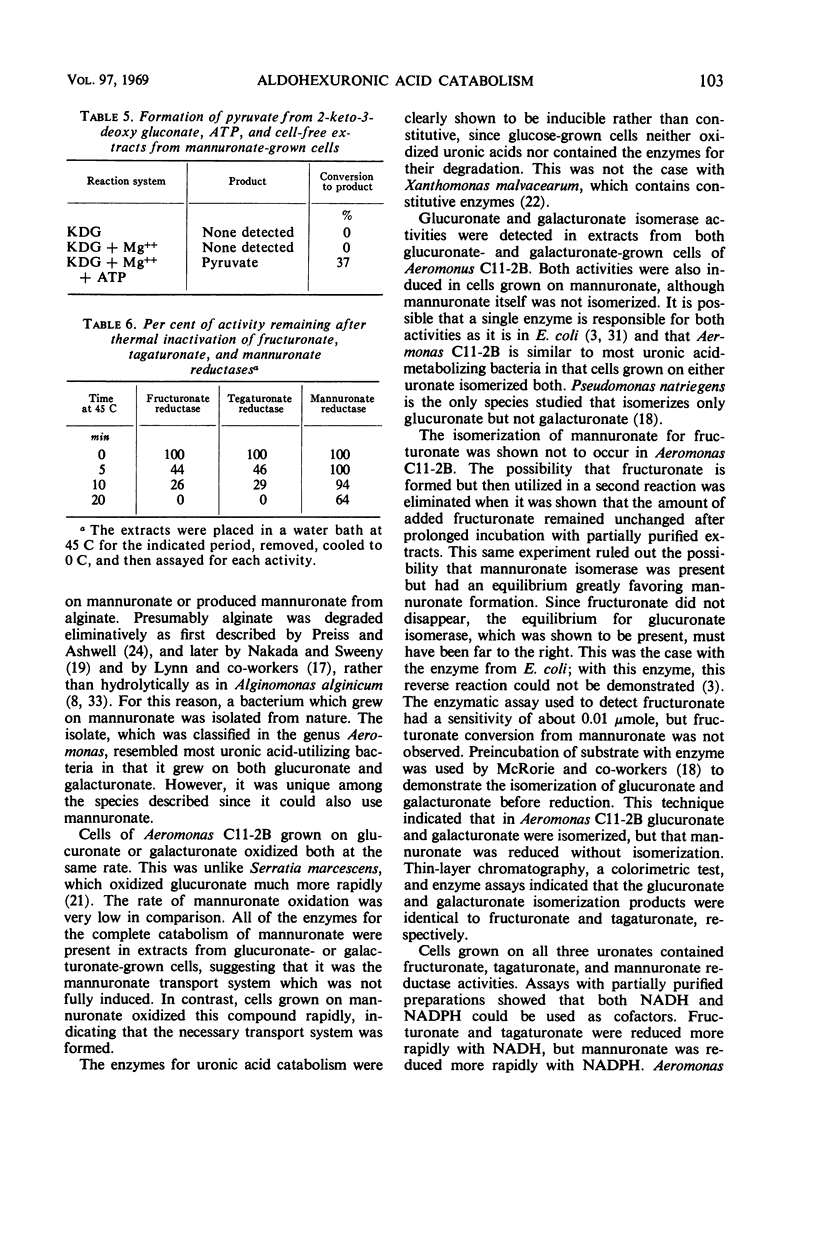
Bacteria which utilize mannuronic acid as an energy source were isolated from nature. One of the organisms, identified as a member of the genus Aeromonas, used glucuronate, galacturonate, and mannuronate as the sole source of carbon and energy. Glucuronate- and galacturonate-grown resting cells oxidized both glucuronate and galacturonate rapidly, but mannuronate slowly. Mannuronate-grown cells oxidized all three rapidly, with the rate of mannuronate utilization somewhat lower. Cell-free extracts from glucuronate-, galacturonate-, and mannuronate-grown Aeromonas C11-2B contained glucuronate and galacturonate isomerases, fructuronate, tagaturonate, and mannuronate reductases, and mannonate and altronate dehydratases, with the exception of glucuronate-grown cells which lacked altronate dehydratase. Thus, the pathway for glucuronate and galacturonate catabolism for Aeromonas was identical to Escherichia coli. Glucuronate and galacturonate were isomerized to d-fructuronate and d-tagaturonate which were then reduced by reduced nicotinamide adenine dinucleotide to d-mannonate and d-altronate, respectively. The hexonic acids were dehydrated to 2-keto-3-deoxy gluconate which was phosphorylated by adenosine triphosphate to 2-keto-3-deoxy-6-phospho gluconate. The latter was then cleaved to pyruvate and glyceraldehyde-3-phosphate. Mannuronate was reduced directly to d-mannonate by a reduced nicotinamide adenine dinucleotide phosphate-linked oxidoreductase. d-Mannonate was then further broken down as in the glucuronate pathway. The mannuronate reducing enzyme, for which the name d-mannonate:nicotinamide adenine dinucleotide (phosphate) oxidoreductase (d-mannuronate-forming) was proposed, was shown to be distinct from altronate and mannoate oxidoreductases. This is the first report of a bacterial oxidoreductase which reduces an aldohexuronic acid to a hexonic acid. The enzyme should prove to be a useful analytical tool for determining mannuronate in the presence of other uronic acids.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASHWELL A., WAHBA A. J., HICKMAN J. A new pathway of uronic acid metabolism. Biochim Biophys Acta. 1958 Oct;30(1):186–187. doi: 10.1016/0006-3002(58)90257-9. [DOI] [PubMed] [Google Scholar]
- ASHWELL G., WAHBA A. J., HICKMAN J. Uronic acid metabolism in bacteria. I. Purification and properties of uronic acid isomerase in Escherichia coli. J Biol Chem. 1960 Jun;235:1559–1565. [PubMed] [Google Scholar]
- CYNKIN M. A., ASHWELL G. Uronic acid metabolism in bacteria. IV. Purification and properties of 2-keto-3-deoxy-D-gluconokinase in Escherichia coli. J Biol Chem. 1960 Jun;235:1576–1579. [PubMed] [Google Scholar]
- DISCHE Z., BORENFREUND E. A new spectrophotometric method for the detection and determination of keto sugars and trioses. J Biol Chem. 1951 Oct;192(2):583–587. [PubMed] [Google Scholar]
- ELLER J., PAYNE W. J. Studies on bacterial utilization of uronic acids. 4. Alginolytic and mannuronic acid oxidizing isolates. J Bacteriol. 1960 Aug;80:193–199. doi: 10.1128/jb.80.2.193-199.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELLIOTT W. H. A new threonine metabolite. Biochim Biophys Acta. 1958 Aug;29(2):446–447. doi: 10.1016/0006-3002(58)90215-4. [DOI] [PubMed] [Google Scholar]
- HICKMAN J., ASHWELL G. Uronic acid metabolism in bacteria. II. Purification and properties of D-altronic acid and D-mannonic acid dehydrogenases in Escherichia coli. J Biol Chem. 1960 Jun;235:1566–1570. [PubMed] [Google Scholar]
- HUGH R., LEIFSON E. The taxonomic significance of fermentative versus oxidative metabolism of carbohydrates by various gram negative bacteria. J Bacteriol. 1953 Jul;66(1):24–26. doi: 10.1128/jb.66.1.24-26.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KILGORE W. W., STARR M. P. Metabolism of hexuronates and 5-ketohexonates by Erwinia carotovora. Biochim Biophys Acta. 1958 Dec;30(3):652–653. doi: 10.1016/0006-3002(58)90123-9. [DOI] [PubMed] [Google Scholar]
- KILGORE W. W., STARR M. P. Uronate oxidation by phytopathogenic pseudomonads. Nature. 1959 May 16;183(4672):1412–1413. doi: 10.1038/1831412a0. [DOI] [PubMed] [Google Scholar]
- LEHNINGER A. L., UL HASSAN M. Enzymatic formation of ascorbic acid in rat liver extracts. J Biol Chem. 1956 Nov;223(1):123–138. [PubMed] [Google Scholar]
- McRORIE R. A., WILLIAMS A. K., PAYNE W. J. Alduronic acid metabolism by bacteria. J Bacteriol. 1959 Feb;77(2):212–216. doi: 10.1128/jb.77.2.212-216.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakada H. I., Sweeny P. C. Alginic acid degradation by eliminases from abalone hepatopancreas. J Biol Chem. 1967 Mar 10;242(5):845–851. [PubMed] [Google Scholar]
- PALLERONI N. J., DOUDOROFF M. Metabolism of carbohydrates by Pseudomonas saccharophila. III. Oxidation of D-arabinose. J Bacteriol. 1957 Aug;74(2):180–185. doi: 10.1128/jb.74.2.180-185.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAYNE W. J., CARLSON A. B. Studies on bacterial utilization of uronic acids. II. Growth response and oxidative activity of various species. J Bacteriol. 1957 Oct;74(4):502–506. doi: 10.1128/jb.74.4.502-506.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAYNE W. J. Studies on bacterial utilization of uronic acids. I. Serratia marcescens. J Bacteriol. 1956 Dec;72(6):834–838. doi: 10.1128/jb.72.6.834-838.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PREISS J., ASHWELL G. Alginic acid metabolism in bacteria. I. Enzymatic formation of unsaturated oligosac-charides and 4-deoxy-L-erythro-5-hexoseulose uronic acid. J Biol Chem. 1962 Feb;237:309–316. [PubMed] [Google Scholar]
- SMILEY J. D., ASHWELL G. Uronic acid metabolism in bacteria. III. Purification and properties of D-altronic acid and D-mannonic acid dehydrases in Escherichia coli. J Biol Chem. 1960 Jun;235:1571–1575. [PubMed] [Google Scholar]
- Sugg W. L., Lynch L. J., Webb W. R. Effects of counterpulsation on body fluid compartments, plasma hemoglobin, and hematocrit. J Thorac Cardiovasc Surg. 1968 Jul;56(1):33–38. [PubMed] [Google Scholar]
- TAKAGI Y., KANDA M., NAKATA Y. Studies on a D-galacturonic acid isomerase. Biochim Biophys Acta. 1959 Jan;31(1):264–265. doi: 10.1016/0006-3002(59)90471-8. [DOI] [PubMed] [Google Scholar]
- WARREN L. Thiobarbituric acid spray reaction for deoxy sugars and sialic acids. Nature. 1960 Apr 16;186:237–237. doi: 10.1038/186237a0. [DOI] [PubMed] [Google Scholar]


