Abstract
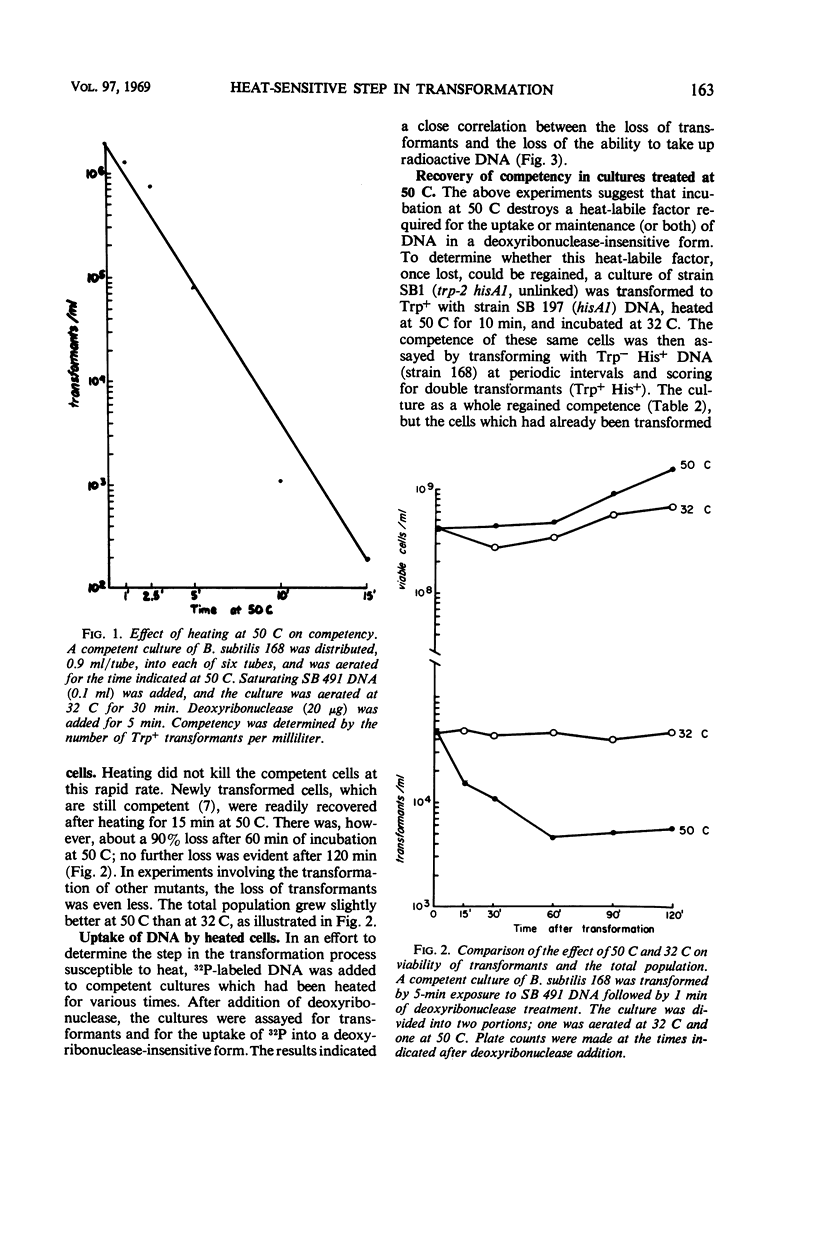
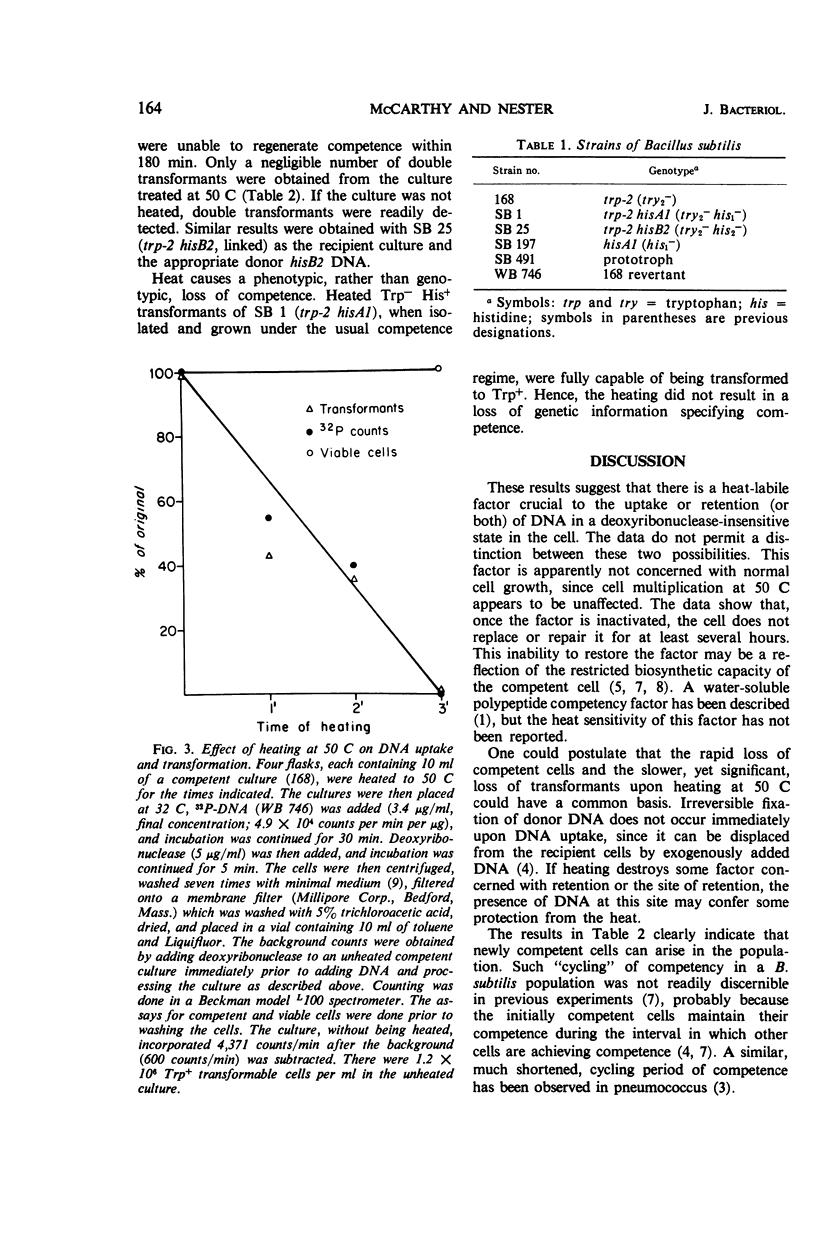
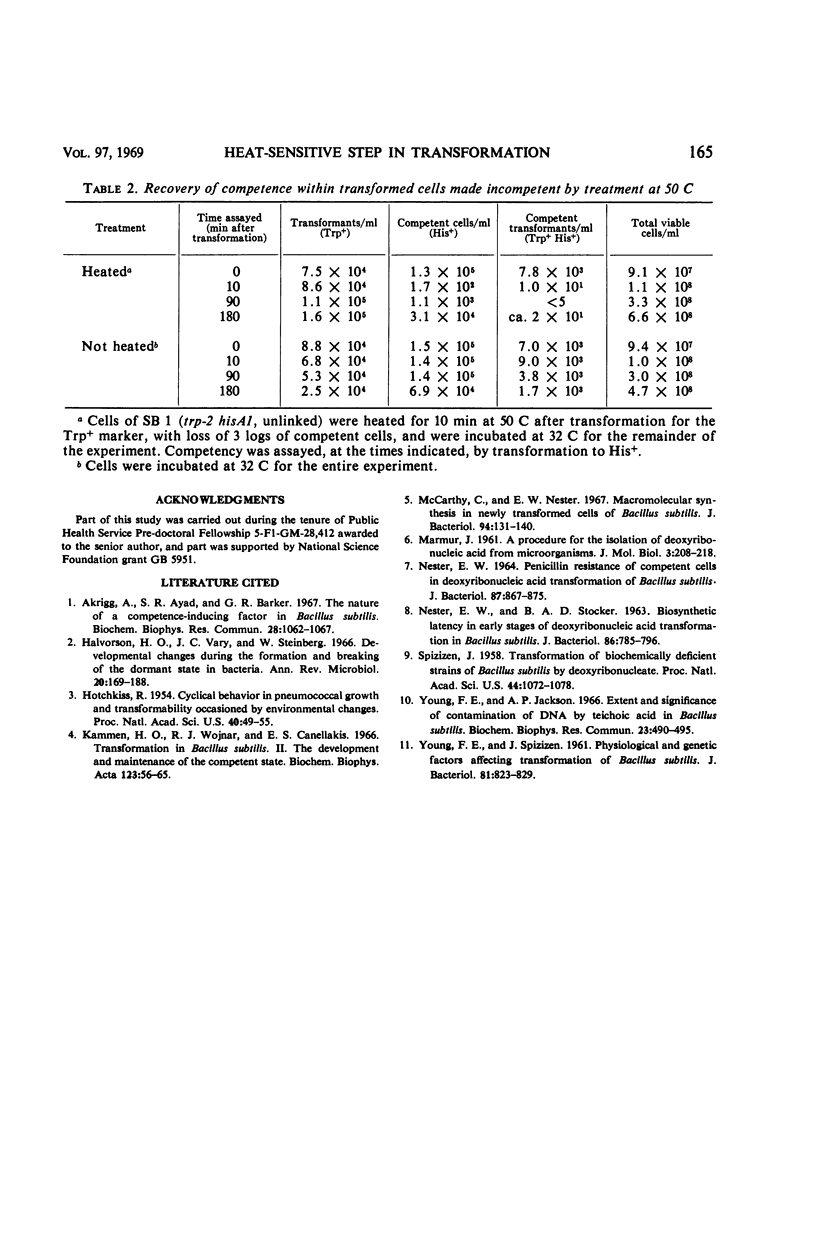
Over 90% of the competent cells in a population of Bacillus subtilis lost their competence after being heated to 50 C for 5 min. There was only a slight loss in the number of transformants if the culture was heated for 5 min after the termination of transformation, but 90% of the transformants were lost after 1 hr at 50 C. The population as a whole grew at a slightly faster rate at 50 C than at 32 C. We postulate that a heat-labile factor is required for the uptake or retention (or both) of deoxyribonucleic acid (DNA) in the cell, since uptake of 32P-DNA into a deoxyribonuclease-resistant form was inversely proportional to the time of exposure to heat. Cells that had lost competence after being heated did not regain their competence for at least several hours, although other cells in the population became competent. These data suggest that the heat-labile factor required for competence is synthesized only once during the period that a cell remains competent.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akrigg A., Ayad S. R., Barker G. R. The nature of a competence-inducing factor in Bacillus subtilis. Biochem Biophys Res Commun. 1967 Sep 27;28(6):1062–1067. doi: 10.1016/0006-291x(67)90090-3. [DOI] [PubMed] [Google Scholar]
- Halvorson H. O., Vary J. C., Steinberg W. Developmental changes during the formation and breaking of the dormant state in bacteria. Annu Rev Microbiol. 1966;20:169–188. doi: 10.1146/annurev.mi.20.100166.001125. [DOI] [PubMed] [Google Scholar]
- Hotchkiss R. D. CYCLICAL BEHAVIOR IN PNEUMOCOCCAL GROWTH AND TRANSFORMABILITY OCCASIONED BY ENVIRONMENTAL CHANGES. Proc Natl Acad Sci U S A. 1954 Feb;40(2):49–55. doi: 10.1073/pnas.40.2.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kammen H. O., Wojnar R. J., Canellakis E. S. Transformation in Bacillus subtilis. II. The development and maintenance of the competent state. Biochim Biophys Acta. 1966 Jul 20;123(1):56–65. [PubMed] [Google Scholar]
- McCarthy C., Nester E. W. Macromolecular synthesis in newly transformed cells of Bacillus subtilis. J Bacteriol. 1967 Jul;94(1):131–140. doi: 10.1128/jb.94.1.131-140.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NESTER E. W. PENICILLIN RESISTANCE OF COMPETENT CELLS IN DEOXYRIBONUCLEIC ACID TRANSFORMATION OF BACILLUS SUBTILIS. J Bacteriol. 1964 Apr;87:867–875. doi: 10.1128/jb.87.4.867-875.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NESTER E. W., STOCKER B. A. BIOSYNTHETIC LATENCY IN EARLY STAGES OF DEOXYRIBONUCLEIC ACIDTRANSFORMATION IN BACILLUS SUBTILIS. J Bacteriol. 1963 Oct;86:785–796. doi: 10.1128/jb.86.4.785-796.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spizizen J. TRANSFORMATION OF BIOCHEMICALLY DEFICIENT STRAINS OF BACILLUS SUBTILIS BY DEOXYRIBONUCLEATE. Proc Natl Acad Sci U S A. 1958 Oct 15;44(10):1072–1078. doi: 10.1073/pnas.44.10.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOUNG F. E., SPIZIZEN J. Physiological and genetic factors affecting transformation of Bacillus subtilis. J Bacteriol. 1961 May;81:823–829. doi: 10.1128/jb.81.5.823-829.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young F. E., Jackson A. P. Extent and significance of contamination of DNA by teichoic acid in Bacillus subtilis. Biochem Biophys Res Commun. 1966 May 25;23(4):490–495. doi: 10.1016/0006-291x(66)90755-8. [DOI] [PubMed] [Google Scholar]


