Abstract
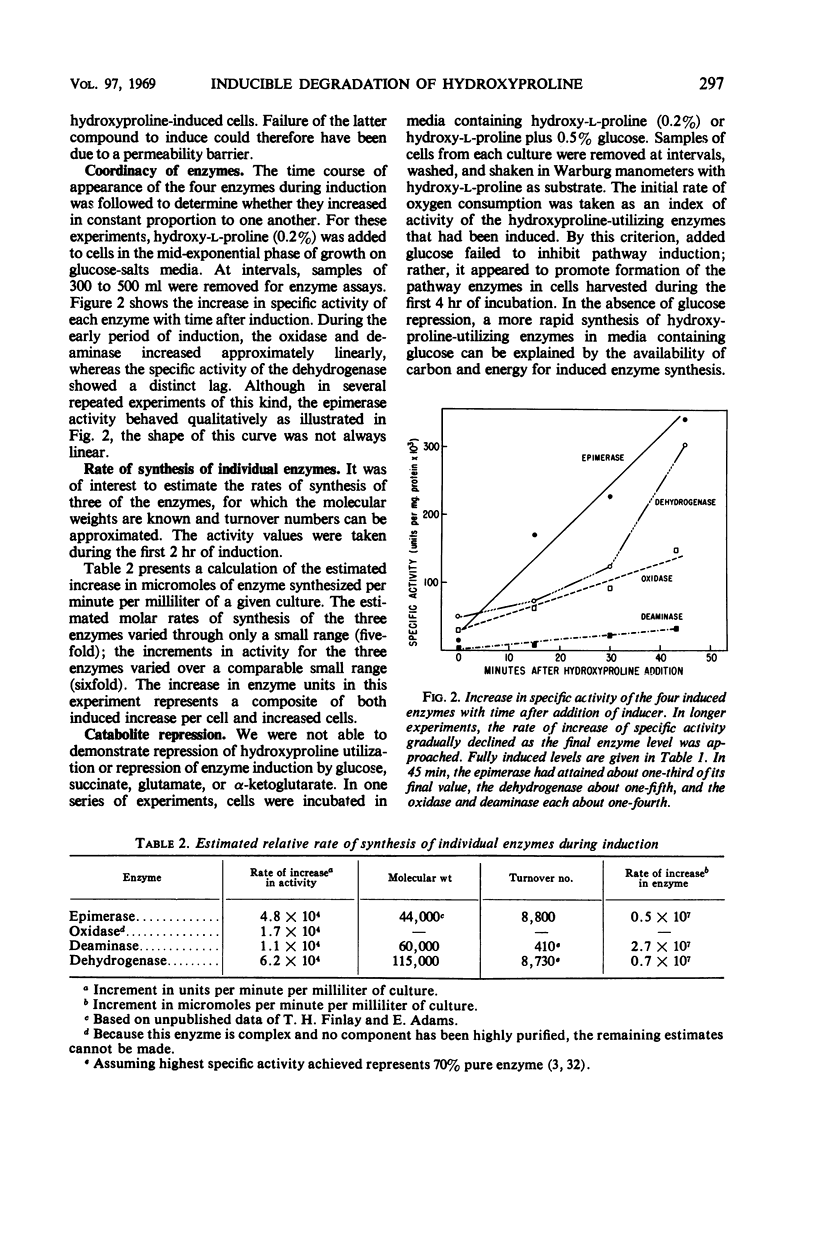
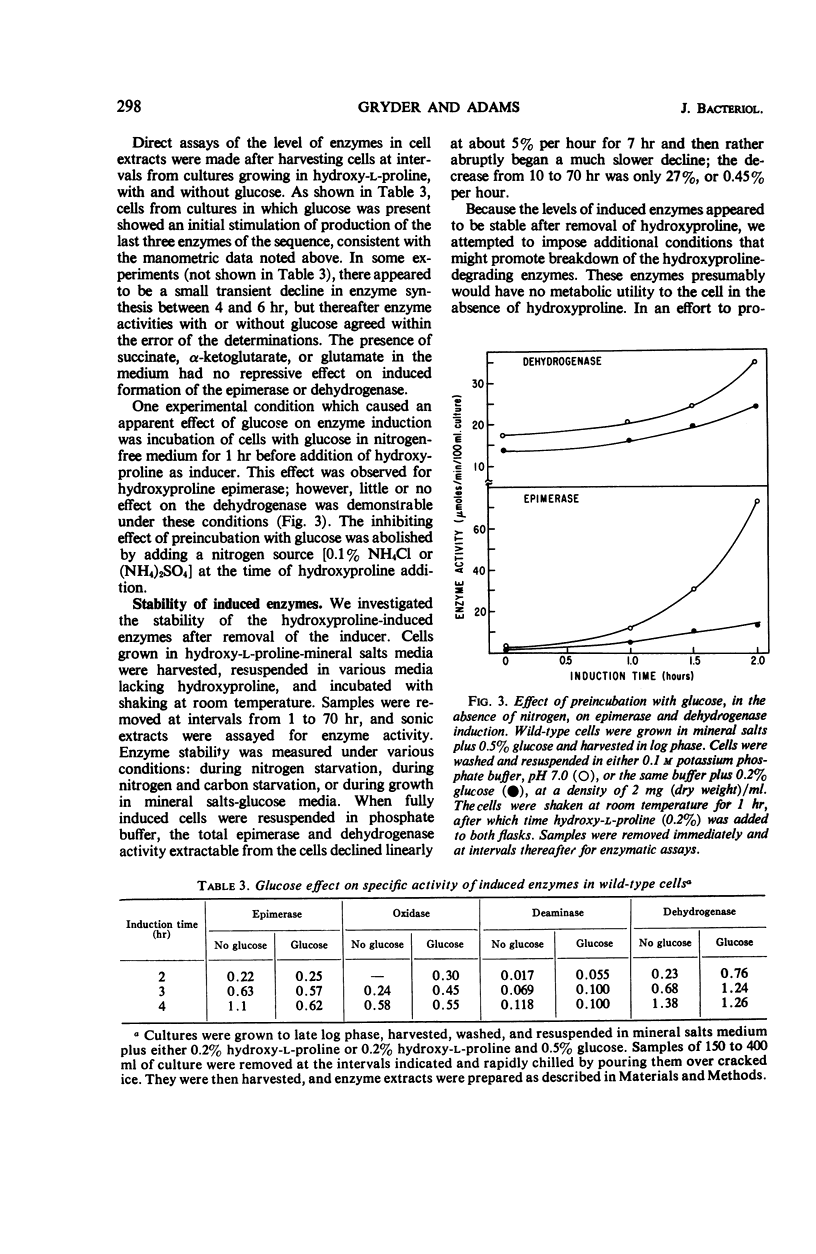
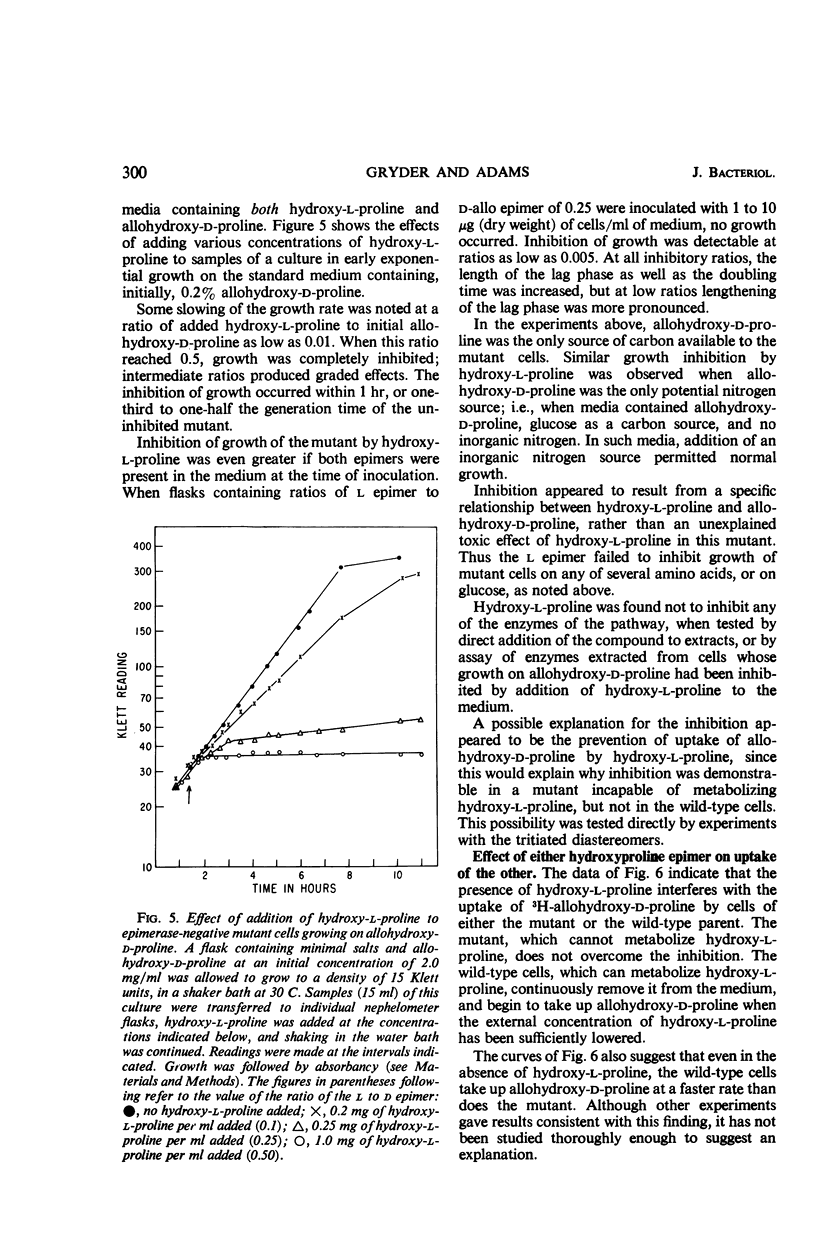
Studies in Pseudomonas putida of the inducible degradation of hydroxyproline to α-ketoglutarate have indicated that either of the two epimers, hydroxy-l-proline or allohydroxy-d-proline, acts as an inducer of all the pathway enzymes. In a mutant lacking the first enzyme of the sequence, hydroxyproline-2-epimerase, which interconverts these two hydroxyproline epimers, either epimer is still equally active as an inducer of the remaining three enzymes, suggesting that each epimer has intrinsic inducer activity. The second and third enzymes of the sequence were induced coordinately. The induction process appeared to be insensitive to catabolite repression under a number of experimental conditions. The induced enzymes were stable even under conditions of nitrogen starvation and other conditions designed to increase protein turnover. In addition to inducing the degradative enzymes, the two hydroxyproline epimers were also found to induce an uptake system that concentrates hydroxyproline intracellularly. Either amino acid induced the uptake system for its epimer as well as for itself.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADAMS E. Hydroxyproline metabolism. I. Conversion to alpha-ketoglutarate by extracts of Pseudomonas. J Biol Chem. 1959 Aug;234(8):2073–2084. [PubMed] [Google Scholar]
- ADAMS E., NORTON I. L. PURIFICATION AND PROPERTIES OF INDUCIBLE HYDROXYPROLINE 2-EPIMERASE FROM PSEUDOMONAS. J Biol Chem. 1964 May;239:1525–1535. [PubMed] [Google Scholar]
- Adams E., Rosso G. Alpha-ketoglutaric semialdehyde dehydrogenase of Pseudomonas. Properties of the purified enzyme induced by hydroxyproline and of the glucarate-induced and constitutive enzymes. J Biol Chem. 1967 Apr 25;242(8):1802–1814. [PubMed] [Google Scholar]
- Creighton T. E., Yanofsky C. Indole-3-glycerol phosphate synthetase of Escherichia coli, an enzyme of the tryptophan operon. J Biol Chem. 1966 Oct 25;241(20):4616–4624. [PubMed] [Google Scholar]
- Cánovas J. L., Ornston L. N., Stanier R. Y. Evolutionary significance of metabolic control systems. The beta-ketoadipate pathway provides a case history in bacteria. Science. 1967 Jun 30;156(3783):1695–1699. doi: 10.1126/science.156.3783.1695. [DOI] [PubMed] [Google Scholar]
- GOLDSTONE A., ADAMS E. Metabolism of gamma-hydroxyglutamic acid. I. Conversion to alpha-hydroxy-gamma-ketoglutarate by purified glutamic-aspartic transaminase to rat liver. J Biol Chem. 1962 Nov;237:3476–3485. [PubMed] [Google Scholar]
- Hegeman G. D. Synthesis of the enzymes of the mandelate pathway by Pseudomonas putida. I. Synthesis of enzymes by the wild type. J Bacteriol. 1966 Mar;91(3):1140–1154. doi: 10.1128/jb.91.3.1140-1154.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegeman G. D. Synthesis of the enzymes of the mandelate pathway by Pseudomonas putida. II. Isolation and properties of blocked mutants. J Bacteriol. 1966 Mar;91(3):1155–1160. doi: 10.1128/jb.91.3.1155-1160.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IRREVERRE F., PIEZ K. A., WOLFF H. L. The separation and determination of cyclic imino acids. J Biol Chem. 1956 Dec;223(2):687–697. [PubMed] [Google Scholar]
- Jacoby G. A. The induction and repression of amino acid oxidation in Pseudomonas fluorescens. Biochem J. 1964 Jul;92(1):1–8. doi: 10.1042/bj0920001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaraman K., Radhakrishnan A. N. Bacterial metabolism of hydroxyproline. I. Metabolism of L-allohydroxyproline by a Pseudomonas. Indian J Biochem. 1965 Sep;2(3):145–153. [PubMed] [Google Scholar]
- Jayaraman K., Radhakrishnan A. N. Bacterial metabolism of hydroxyproline. II. Metabolism of L-hydroxyproline in Achromobacter. Indian J Biochem. 1965 Sep;2(3):153–158. [PubMed] [Google Scholar]
- KIHARA H., SNELL E. E. Peptides and bacterial growth. II. L-alanine peptides and growth of Lactobacillus casei. J Biol Chem. 1952 May;197(2):791–805. [PubMed] [Google Scholar]
- Kaback H. R., Stadtman E. R. Proline uptake by an isolated cytoplasmic membrane preparation of Escherichia coli. Proc Natl Acad Sci U S A. 1966 Apr;55(4):920–927. doi: 10.1073/pnas.55.4.920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOVELESS A., HOWARTH S. Mutation of bacteria at high levels of survival by ethyl methane sulphonate. Nature. 1959 Dec 5;184:1780–1782. doi: 10.1038/1841780a0. [DOI] [PubMed] [Google Scholar]
- Lessie T. G., Neidhardt F. C. Formation and operation of the histidine-degrading pathway in Pseudomonas aeruginosa. J Bacteriol. 1967 Jun;93(6):1800–1810. doi: 10.1128/jb.93.6.1800-1810.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANDELSTAM J., JACOBY G. A. INDUCTION AND MULTI-SENSITIVE END-PRODUCT REPRESSION IN THE ENZYMIC PATHWAY DEGRADING MANDELATE IN PSEUDOMONAS FLUORESCENS. Biochem J. 1965 Mar;94:569–577. doi: 10.1042/bj0940569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magasanik B., Lund P., Neidhardt F. C., Schwartz D. T. Induction and repression of the histidine-degrading enzymes in Aerobacter aerogenes. J Biol Chem. 1965 Nov;240(11):4320–4324. [PubMed] [Google Scholar]
- NEUMAN R. E., LOGAN M. A. The determination of hydroxyproline. J Biol Chem. 1950 May;184(1):299–306. [PubMed] [Google Scholar]
- NORTON J. E., BULMER G. S., SOKATCH J. R. THE OXIDATION OF D-ALANINE BY CELL MEMBRANES OF PSEUDOMONAS AERUGINOSA. Biochim Biophys Acta. 1963 Oct 8;78:136–147. doi: 10.1016/0006-3002(63)91619-6. [DOI] [PubMed] [Google Scholar]
- Ornston L. N. The conversion of catechol and protocatechuate to beta-ketoadipate by Pseudomonas putida. IV. Regulation. J Biol Chem. 1966 Aug 25;241(16):3800–3810. [PubMed] [Google Scholar]
- PALLERONI N. J., STANIER R. Y. REGULATORY MECHANISMS GOVERNING SYNTHESIS OF THE ENZYMES FOR TRYPTOPHAN OXIDATION BY PSEUDOMONAS FLUORESCENS. J Gen Microbiol. 1964 May;35:319–334. doi: 10.1099/00221287-35-2-319. [DOI] [PubMed] [Google Scholar]
- PASIEKA A. E., MORGAN J. F. Specific determination of hydroxy-L-proline in biological materials. Proc Soc Exp Biol Med. 1956 May;92(1):96–99. doi: 10.3181/00379727-92-22395. [DOI] [PubMed] [Google Scholar]
- Pine M. J. Metabolic control of intracellular proteolysis in growing and resting cells of Escherichia coli. J Bacteriol. 1966 Oct;92(4):847–850. doi: 10.1128/jb.92.4.847-850.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCRIVER C. R., EFRON M. L., SCHAFER I. A. RENAL TUBULAR TRANSPORT OF PROLINE, HYDROXYPROLINE, AND GLYCINE IN HEALTH AND IN FAMILIAL HYPERPROLINEMIA. J Clin Invest. 1964 Mar;43:374–385. doi: 10.1172/JCI104922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STADTMAN E. R., NOVELLI G. D., LIPMANN F. Coenzyme A function in and acetyl transfer by the phosphotransacetylase system. J Biol Chem. 1951 Jul;191(1):365–376. [PubMed] [Google Scholar]
- STADTMAN T. C., ELLIOTT P. Studies on the enzymic reduction of amino acids. II. Purification and properties of D-proline reductase and a proline racemase from Clostridium sticklandii. J Biol Chem. 1957 Oct;228(2):983–997. [PubMed] [Google Scholar]
- STRAUSS B. S. Response of Escherichia coli auxotrophs to heat after treatment with mutagenic alkyl methanesulfonates. J Bacteriol. 1962 Feb;83:241–249. doi: 10.1128/jb.83.2.241-249.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger S., Scotto P., Magasanik B. Exogenous and endogenous induction of the histidine-degrading enzymes in Aerobacter aerogenes. J Biol Chem. 1965 Nov;240(11):4331–4337. [PubMed] [Google Scholar]
- Schlessinger D., Ben-Hamida F. Turnover of protein in Escherichia coli starving for nitrogen. Biochim Biophys Acta. 1966 Apr 18;119(1):171–182. doi: 10.1016/0005-2787(66)90048-7. [DOI] [PubMed] [Google Scholar]
- Singh R. M., Adams E. Enzymatic deamination of delta-1-pyrroline-4-hydroxy-2-carboxylate to 2,5-dioxovalerate (alpha-ketoglutaric semialdehyde). J Biol Chem. 1965 Nov;240(11):4344–4351. [PubMed] [Google Scholar]
- Singh R. M., Adams E. Isolation and identification of 2,5-dioxovalerate, an intermediate in the bacterial oxidation of hydroxyproline. J Biol Chem. 1965 Nov;240(11):4352–4356. [PubMed] [Google Scholar]
- Stanier R. Y., Palleroni N. J., Doudoroff M. The aerobic pseudomonads: a taxonomic study. J Gen Microbiol. 1966 May;43(2):159–271. doi: 10.1099/00221287-43-2-159. [DOI] [PubMed] [Google Scholar]
- YONEYA T., ADAMS E. Hydroxyproline metabolism. V. Inducible allohydroxy-D-proline oxidase of Pseudomonas. J Biol Chem. 1961 Dec;236:3272–3279. [PubMed] [Google Scholar]


