Abstract
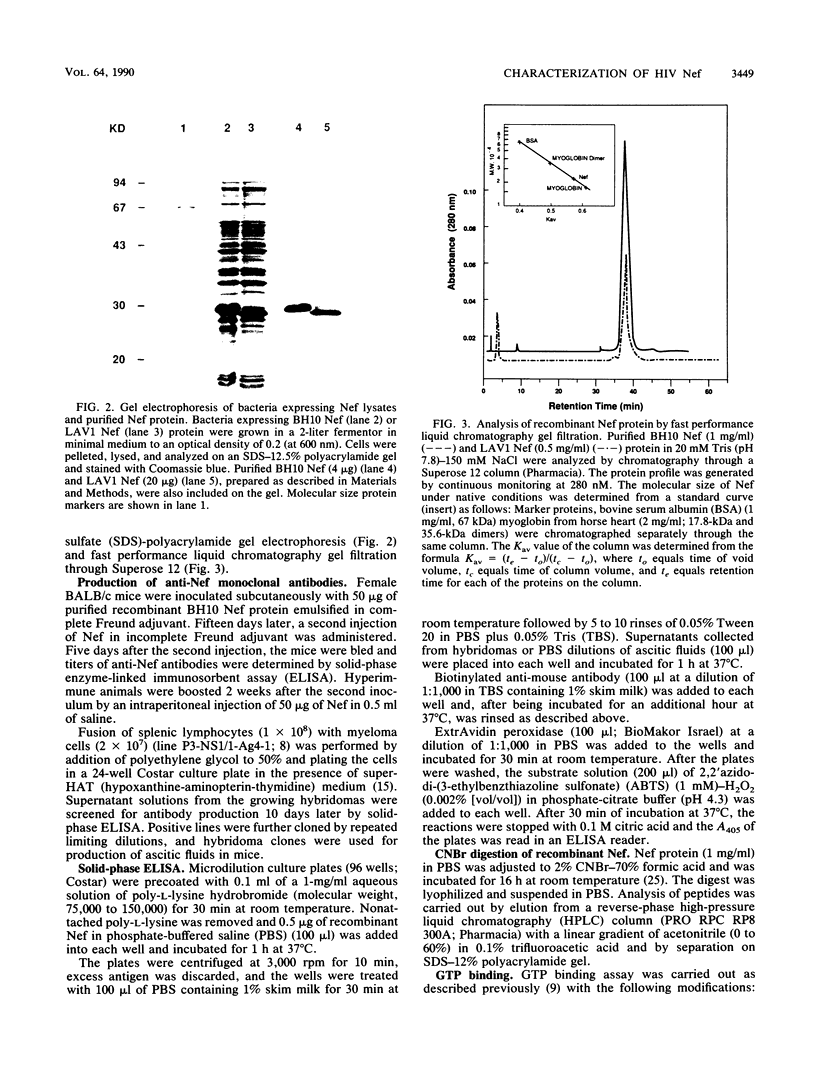
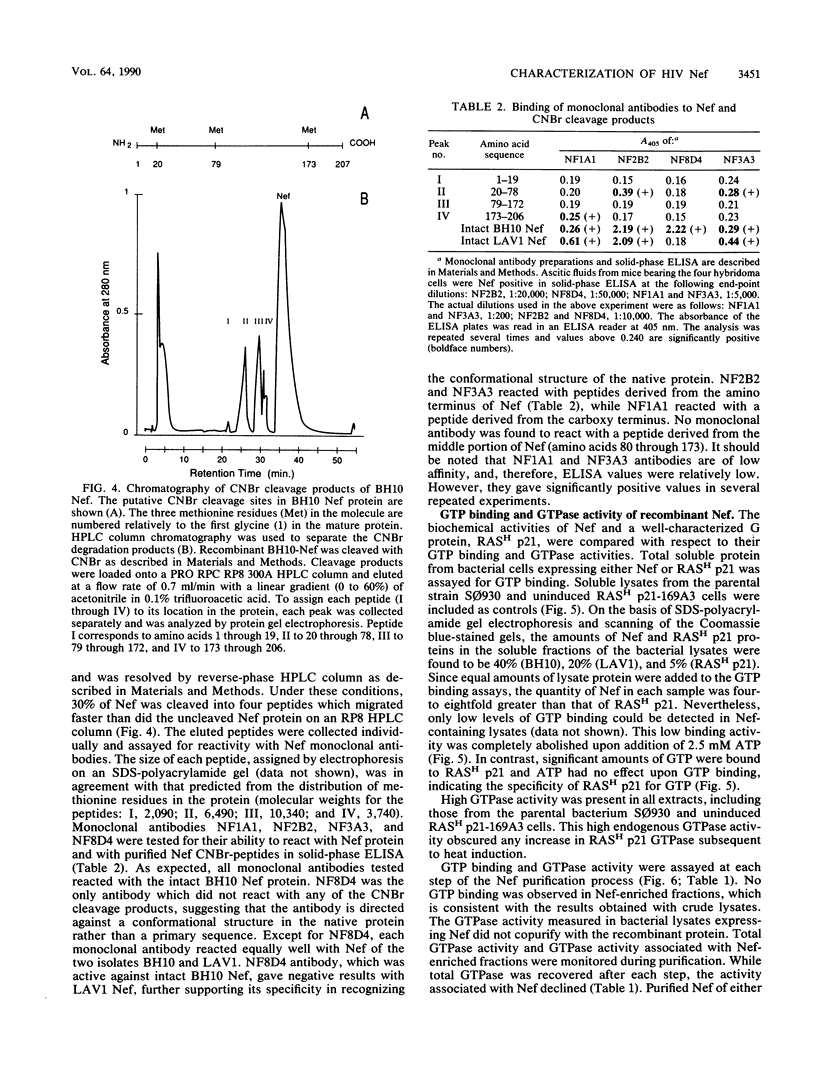
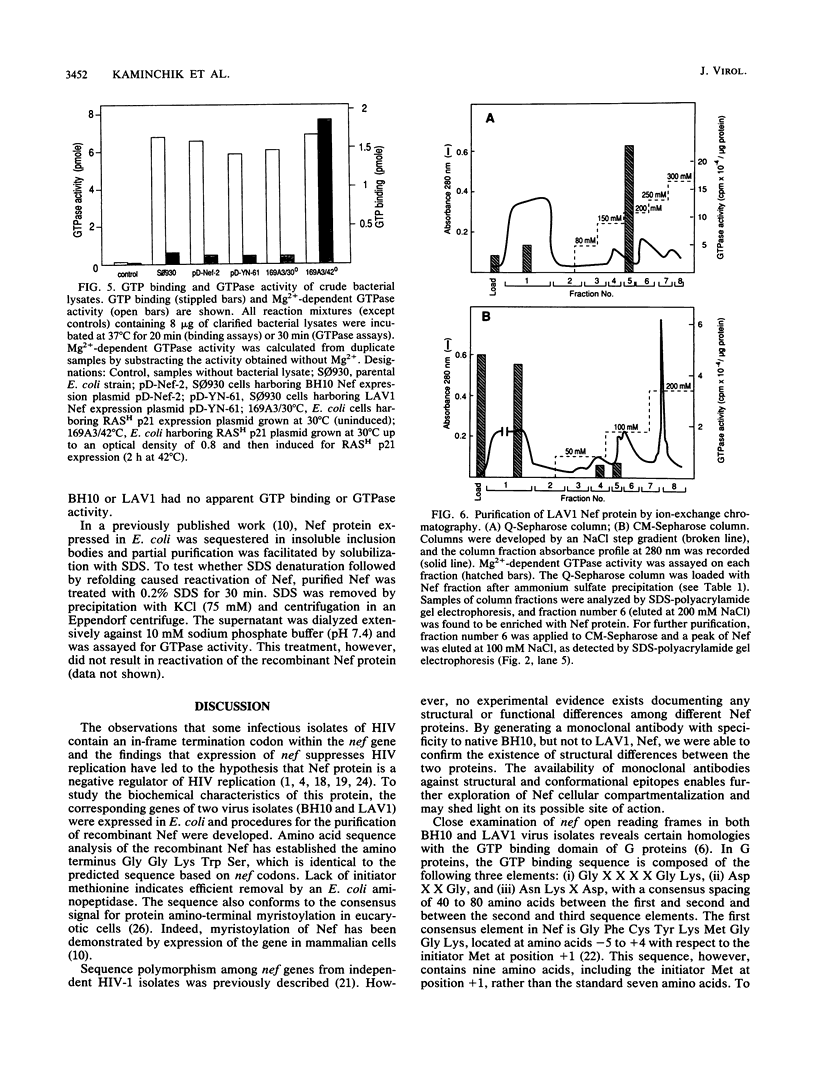
nef genes from human immunodeficiency virus type 1 isolates BH10 and LAV1 (lymphadenopathy-associated virus type 1) were expressed in Escherichia coli under the deo operon promoter. The two proteins found in the soluble compartment of the bacterial lysate were purified by ion-exchange column chromatography to apparent homogeneity. Determination of the amino-terminal sequence revealed glycine as the first amino acid in the Nef protein, indicating removal of the initiator methionine during expression in E. coli. Under native conditions, the recombinant Nef protein is a monomer of 23 kilodaltons. In denaturing polyacrylamide gels, however, BH10 and LAV1 Nef proteins migrate as 28 and 26 kilodaltons, respectively. GTP binding and GTPase activity were monitored during Nef protein purification. These activities did not copurify with the recombinant Nef protein from either the BH10 or the LAV1 isolate. Purified recombinant BH10 Nef protein was used as an immunogen to elicit mouse monoclonal antibodies. A series of monoclonal antibodies were obtained which reacted with sequences at either the amino or carboxy terminus of Nef. In addition, a conformational epitope reacting with native BH10, but not LAV1, Nef was isolated.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmad N., Venkatesan S. Nef protein of HIV-1 is a transcriptional repressor of HIV-1 LTR. Science. 1988 Sep 16;241(4872):1481–1485. doi: 10.1126/science.3262235. [DOI] [PubMed] [Google Scholar]
- Allan J. S., Coligan J. E., Lee T. H., McLane M. F., Kanki P. J., Groopman J. E., Essex M. A new HTLV-III/LAV encoded antigen detected by antibodies from AIDS patients. Science. 1985 Nov 15;230(4727):810–813. doi: 10.1126/science.2997921. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Cheng-Mayer C., Iannello P., Shaw K., Luciw P. A., Levy J. A. Differential effects of nef on HIV replication: implications for viral pathogenesis in the host. Science. 1989 Dec 22;246(4937):1629–1632. doi: 10.1126/science.2531920. [DOI] [PubMed] [Google Scholar]
- DePierre J. W., Karnovsky M. L. Ecto-enzymes of the guinea pig polymorphonuclear leukocyte. I. Evidence for an ecto-adenosine monophosphatase, adenosine triphosphatase, and -p-nitrophenyl phosphates. J Biol Chem. 1974 Nov 25;249(22):7111–7120. [PubMed] [Google Scholar]
- Dever T. E., Glynias M. J., Merrick W. C. GTP-binding domain: three consensus sequence elements with distinct spacing. Proc Natl Acad Sci U S A. 1987 Apr;84(7):1814–1818. doi: 10.1073/pnas.84.7.1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folks T., Benn S., Rabson A., Theodore T., Hoggan M. D., Martin M., Lightfoote M., Sell K. Characterization of a continuous T-cell line susceptible to the cytopathic effects of the acquired immunodeficiency syndrome (AIDS)-associated retrovirus. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4539–4543. doi: 10.1073/pnas.82.13.4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galfre G., Howe S. C., Milstein C., Butcher G. W., Howard J. C. Antibodies to major histocompatibility antigens produced by hybrid cell lines. Nature. 1977 Apr 7;266(5602):550–552. doi: 10.1038/266550a0. [DOI] [PubMed] [Google Scholar]
- Gibbs J. B., Sigal I. S., Poe M., Scolnick E. M. Intrinsic GTPase activity distinguishes normal and oncogenic ras p21 molecules. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5704–5708. doi: 10.1073/pnas.81.18.5704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy B., Kieny M. P., Riviere Y., Le Peuch C., Dott K., Girard M., Montagnier L., Lecocq J. P. HIV F/3' orf encodes a phosphorylated GTP-binding protein resembling an oncogene product. Nature. 1987 Nov 19;330(6145):266–269. doi: 10.1038/330266a0. [DOI] [PubMed] [Google Scholar]
- Hammes S. R., Dixon E. P., Malim M. H., Cullen B. R., Greene W. C. Nef protein of human immunodeficiency virus type 1: evidence against its role as a transcriptional inhibitor. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9549–9553. doi: 10.1073/pnas.86.23.9549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori S., Ulsh L. S., Halliday K., Shih T. Y. Biochemical properties of a highly purified v-rasH p21 protein overproduced in Escherichia coli and inhibition of its activities by a monoclonal antibody. Mol Cell Biol. 1985 Jun;5(6):1449–1455. doi: 10.1128/mcb.5.6.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennett R. H., Denis K. A., Tung A. S., Klinman N. R. Hybrid plasmacytoma production: fusions with adult spleen cells, monoclonal spleen fragments, neonatal spleen cells and human spleen cells. Curr Top Microbiol Immunol. 1978;81:77–91. doi: 10.1007/978-3-642-67448-8_13. [DOI] [PubMed] [Google Scholar]
- Kim S., Ikeuchi K., Byrn R., Groopman J., Baltimore D. Lack of a negative influence on viral growth by the nef gene of human immunodeficiency virus type 1. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9544–9548. doi: 10.1073/pnas.86.23.9544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacal J. C., Santos E., Notario V., Barbacid M., Yamazaki S., Kung H., Seamans C., McAndrew S., Crowl R. Expression of normal and transforming H-ras genes in Escherichia coli and purification of their encoded p21 proteins. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5305–5309. doi: 10.1073/pnas.81.17.5305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciw P. A., Cheng-Mayer C., Levy J. A. Mutational analysis of the human immunodeficiency virus: the orf-B region down-regulates virus replication. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1434–1438. doi: 10.1073/pnas.84.5.1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederman T. M., Thielan B. J., Ratner L. Human immunodeficiency virus type 1 negative factor is a transcriptional silencer. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1128–1132. doi: 10.1073/pnas.86.4.1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratner L., Haseltine W., Patarca R., Livak K. J., Starcich B., Josephs S. F., Doran E. R., Rafalski J. A., Whitehorn E. A., Baumeister K. Complete nucleotide sequence of the AIDS virus, HTLV-III. Nature. 1985 Jan 24;313(6000):277–284. doi: 10.1038/313277a0. [DOI] [PubMed] [Google Scholar]
- Ratner L., Starcich B., Josephs S. F., Hahn B. H., Reddy E. P., Livak K. J., Petteway S. R., Jr, Pearson M. L., Haseltine W. A., Arya S. K. Polymorphism of the 3' open reading frame of the virus associated with the acquired immune deficiency syndrome, human T-lymphotropic virus type III. Nucleic Acids Res. 1985 Nov 25;13(22):8219–8229. doi: 10.1093/nar/13.22.8219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel K. P., Seth A., Konopka A., Lautenberger J. A., Papas T. S. The 3'-orf protein of human immunodeficiency virus shows structural homology with the phosphorylation domain of human interleukin-2 receptor and the ATP-binding site of the protein kinase family. FEBS Lett. 1987 Jun 22;218(1):81–86. doi: 10.1016/0014-5793(87)81023-2. [DOI] [PubMed] [Google Scholar]
- Sodroski J., Goh W. C., Rosen C., Tartar A., Portetelle D., Burny A., Haseltine W. Replicative and cytopathic potential of HTLV-III/LAV with sor gene deletions. Science. 1986 Mar 28;231(4745):1549–1553. doi: 10.1126/science.3006244. [DOI] [PubMed] [Google Scholar]
- Terwilliger E., Sodroski J. G., Rosen C. A., Haseltine W. A. Effects of mutations within the 3' orf open reading frame region of human T-cell lymphotropic virus type III (HTLV-III/LAV) on replication and cytopathogenicity. J Virol. 1986 Nov;60(2):754–760. doi: 10.1128/jvi.60.2.754-760.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisdale M., Ertl P., Larder B. A., Purifoy D. J., Darby G., Powell K. L. Characterization of human immunodeficiency virus type 1 reverse transcriptase by using monoclonal antibodies: role of the C terminus in antibody reactivity and enzyme function. J Virol. 1988 Oct;62(10):3662–3667. doi: 10.1128/jvi.62.10.3662-3667.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towler D. A., Adams S. P., Eubanks S. R., Towery D. S., Jackson-Machelski E., Glaser L., Gordon J. I. Myristoyl CoA:protein N-myristoyltransferase activities from rat liver and yeast possess overlapping yet distinct peptide substrate specificities. J Biol Chem. 1988 Feb 5;263(4):1784–1790. [PubMed] [Google Scholar]