Abstract
About 50% of patients with autonomic failure suffer from supine hypertension, even those with very low plasma norepinephrine and renin. Because nitric oxide is arguably the most potent metabolic modulator of blood pressure, we hypothesized that impaired nitric oxide function contributes to supine hypertension in autonomic failure. However, we found that autonomic failure patients (n=14) were more sensitive to the pressor effects of the nitric oxide synthase inhibitor L-NMMA, suggesting increased nitric oxide function rather than deficiency; a lower dose of L-NMMA was needed to produce a similar increase in blood pressure in AF patients, as in healthy controls in whom autonomic failure was induced with the ganglionic blocker trimethaphan (171±37 vs. 512±81 mg, respectively, p=0.001). Furthermore, potentiation of the actions of endogenous nitric oxide with the phosphodiesterase inhibitor sildenafil 25 mg PO decreased nighttime supine systolic blood pressure from 182±11 to 138±4 mmHg in eight autonomic failure patients with supine hypertension (p=0.012 compared to placebo). Finally, autonomic failure patients tolerated a greater degree of upright tilt during infusion of L-NMMA (56±6° vs. 41±4° with placebo, n=7, p=0.014), an improvement in orthostatic tolerance similar to that obtained with equipressor doses of phenylephrine. In conclusion, autonomic failure patients do not have nitric oxide deficiency contributing to supine hypertension. Instead, they have increased nitric oxide function contributing to their orthostatic hypotension. Potentiation of nitric oxide could be used in the treatment of supine hypertension, and its inhibition offers a novel approach to improve orthostatic hypotension.
Keywords: Nitric Oxide, Orthostatic hypotension, Supine Hypertension, Pure Autonomic Failure, Shy Drager Syndrome, Blood Pressure, L-NMMA
Introduction
Autonomic failure is characterized by severe orthostatic hypotension that can occur from an autonomic neuropathy secondary to systemic illnesses, such as diabetes mellitus or amyloidosis, or as a primary neurodegenerative disorder. The primary forms include pure autonomic failure (PAF), which presents only with autonomic nervous system manifestations, and multiple system atrophy (MSA, Shy-Drager syndrome), which is associated with a movement disorder or truncal ataxia in addition to autonomic failure. Both MSA and PAF patients are characterized clinically by disabling orthostatic hypotension, as would be expected from their severe autonomic failure. In addition, approximately 50% of patients with autonomic impairment due to either PAF or MSA suffer from supine hypertension which can be severe, with systolic blood pressure (SBP) in many cases exceeding 200 mm Hg 1. In the case of MSA, we have previously shown that their hypertension may be explained by residual sympathetic tone, possibly acting on hypersensitive adrenoreceptors and unrestrained by the lack of baroreflex modulation 2. In contrast, the cause of hypertension in PAF remains unknown. It is important to note that hypertension in these patients is due to an increase in vascular resistance 3, despite having very low plasma norepinephrine and renin activity1. Therefore, the driving force for this increased vascular tone is not known, but is likely to be magnified by the lack of baroreflex buffering capacity resulting from their autonomic failure.
Because autonomic neural mechanisms do not explain the hypertension of PAF, it is likely that hormonal or metabolic factors are involved. We have recently shown that nitric oxide (NO) is arguably the most important metabolic regulator in normal subjects, tonically restraining blood pressure approximately 30 mm Hg4. Nitric oxide deficiency has been proposed to play a role in essential hypertension5 and other cardiovascular disorders6. We, therefore, hypothesized that the hypertension of PAF is due to impaired nitric oxide. Our results, however, suggest that autonomic failure is characterized by excess nitric oxide function, rather than a deficiency and that this excess may contribute to the orthostatic hypotension in these patients.
Material and Methods
Subjects
We studied 20 patients with autonomic failure. Fourteen were diagnosed with pure autonomic failure (age 67±2.5 years, 10 males) and six were diagnosed with multiple system atrophy (age 60± 3.6, 4 males). Patients were diagnosed following the criteria of the American Autonomic Society to differentiate between MSA and PAF7. Patients were excluded if they had secondary forms of autonomic failure (e.g. diabetes mellitus or amyloidosis) or renal failure. Subjects could be included in more than one protocol. Fourteen subjects participated in study 1 (to determine blood pressure response to systemic NO synthase inhibition), eight patients participated in study 2 (to evaluate the effects of NO potentiation on supine hypertension of autonomic failure), and seven subjects participated in study 3 (to determine if systemic NO synthase inhibition improves orthostatic intolerance comparable to alpha adrenergic activation). All studies were approved by the Institutional Review Board at Vanderbilt University.
General Protocol
Patients were admitted to the General Clinical Research Center at Vanderbilt University Medical Center. Medications with cardiovascular/autonomic effects were discontinued for ≥ 5 half-lives before admission. Patients were placed in a metabolic ward on a sodium balanced diet. The diet consisted of low monoamine, caffeine-free food containing 150 milliequivalents of sodium and 70 milliequivalents of potassium per day. Studies were conducted ≥ 2.5 hours after a meal. The screening consisted of a comprehensive medical history, physical examination, 12-lead ECG, and laboratory assessments. Standardized autonomic function tests were performed to assess the severity of autonomic impairment8. These included orthostatic stress test, Valsalva maneuver, the cold pressor test, handgrip and sinus arrhythmia, as previously described.9 Brachial BP and heart rate during all of these tests were obtained using an automated cuff-oscillometric sphygmomanometer (Dinamap, GE Medical Systems Information Technologies).
Laboratory measurements
Plasma norepinephrine levels were determined by high-performance liquid chromatography with electrochemical detection.10. In a subset of AF patients (n=12) and normal controls (n=11), baseline determination of NO products (NOx) was performed using the chemiluminescence assay. This was performed in plasma samples taken at the initial evaluation and kept frozen at −80 until time of analysis. The chemiluminescence assay was done after reduction to NO by using a vanadium HCl catalyst. The NO generated was reacted in an ozone chamber and detected by chemiluminescence in an NO analyzer (Sievers 280i NOA, Boulder, CO).11
Study 1: To determine blood pressure response to systemic nitric oxide synthase (NOS) inhibition in patients with autonomic failure
The studies were conducted in the morning with the patient in the recumbent position ≥ 8 hours after their last meal. Heart rate was determined with continuous ECG monitoring, blood pressure through the volume clamp method (Finapres 2300; Ohmeda), and also automated brachial cuff-oscillometric sphygmomanometer (Dinamap). An intravenous line for NG-monomethyl-L-arginine (L-NMMA) infusion was placed in a large antecubital vein in the left arm.
After a stable baseline was reached, patients were gradually tilted head up until a systolic blood pressure of about 110 mm Hg was reached. After stabilization at this new baseline, L-NMMA was infused at 4 different doses for 15 minutes each (33, 83, 167 and 250 μg/kg per minute) or until SBP reached 150 mm Hg.
For comparison, we used a group of ten healthy controls that were subjected to transient pharmacological autonomic withdrawal with trimethaphan as described before.4 Briefly, an intravenous line with three infusion ports connected to the catheter was placed in a large antecubital vein in the left arm, one port was for trimethaphan infusion, the second for infusion of phenylephrine, and the third for L-NMMA. After a stable baseline was reached, NN-cholinergic receptors were blocked by continuous infusion of trimethaphan (Cambridge Pharmaceuticals) at 4 μg/min. We have shown previously that this dose induces virtually complete autonomic blockade and a mild but significant lowering of blood pressure.12 Blood pressure was then restored to pre-trimethaphan levels (or increased up to a systolic blood pressure of 110 mm Hg if baseline levels were higher than 120 mm Hg) by infusing phenylephrine at individually titrated doses, starting with 0.05 μg/kg per minute. L-NMMA was then infused at 2 different doses for 15 minutes each (250 and 500 μg/kg per minute) or until SBP reached 150 mm Hg.
Study 2: To evaluate the effects of NO potentiation on supine hypertension of autonomic failure
We compared the effect of 25 mg of sildenafil to placebo on overnight supine blood pressure in eight patients with autonomic failure and supine hypertension. The order of the intervention was randomized. The medication was administered orally with 50 mL of tap water at 8:00 P.M. and ≥2.5 hours after the last meal. Patients were instructed to remain supine throughout the night, fluid intake was restrained, and blood pressure was measured at 2-hour intervals by an automated sphygmomanometer.
All patients were males with primary autonomic failure. All of the patients had night time supine hypertension defined as supine systolic blood pressure >150 mm Hg and/or supine diastolic blood pressure >90 mm Hg.
Study 3: To determine if systemic NO synthase inhibition improves orthostatic intolerance comparable to alpha adrenergic activation
Seven autonomic failure patients were studied on 2 separate study days randomly assigned at least 1 day apart using a crossover design. On both occasions orthostatic tolerance to passive head up tilt was tested before and after either NOS blockade or alpha adrenergic activation.
The studies were conducted in the morning with the subject in the recumbent position ≥ 8 hours after their last meal. Heart rate was determined with continuous ECG monitoring, blood pressure through the volume clamp method (Finapres 2300; Ohmeda), and also automated brachial cuff-oscillometric sphygmomanometry (Dinamap). One intravenous line was connected to a catheter placed in a large antecubital vein in the left arm for infusion of L-NMMA (NO day) or phenylephrine (Phenylephrine day).
In both days an orthostatic tolerance test was performed at baseline before any drug was administrated. The orthostatic tolerance test consisted of a graded head up tilt at 5 degrees intervals for 4 minutes at each degree, until patients reached a systolic blood pressure of less than 70 mm Hg or developed symptoms of pre-syncope. Subjects were then returned to the supine position.
After a resting period, subjects were then gradually tilted head up until a systolic blood pressure of about 110 mm Hg was induced and, after stabilization, new baseline measurements were taken. L-NMMA was infused at increasing doses for 15 minutes each (33, 83, 167 and 250 μg/kg per minute) or until SBP reached 150 mm Hg. The orthostatic tolerance test performed at the beginning of the study was then repeated. A similar protocol was followed on the phenylephrine day, but instead of L-NMMA subjects received phenylephrine starting at 0.1 μg/kg/min and increasing it every 6 minutes until SBP reached 150 mm Hg.
Statistical Analysis
For study one, the primary outcome of interest was the cumulative dose of L-NMMA needed to increase SBP to 150 mm Hg. The study design used two parallel groups (autonomic failure patients and healthy controls). Differences between group means were tested by the Mann-Whitney U test. In study two, the outcome of interest was nighttime systolic blood pressure measured every two hours from 8 p.m. to 8 a.m. in a crossover design (the same subject was studied twice, once while receiving placebo and on a different occasion after receiving sildenafil). The area under the curve for systolic blood pressure overnight was used to summarize the data and the difference between treatments was analyzed with the Wilcoxon signed-rank test. In study three, the primary outcome of interest was the degree of tolerance to head up tilt during NOS inhibition with L-NMMA or during α-stimulation with phenylephrine at similar systolic blood pressure levels. Kaplan-Meir curves were compared using the log-rank test.
Data are reported as mean ± SEM. All tests were 2-tailed. A value of p<0.05 was considered significant. Statistical analyses were performed using SPSS for Windows version 15.0 (SPSS, Chicago, IL).
Results
General
Hemodynamic responses to posture are shown in table 1. Mean systolic and diastolic blood pressure were 162±11/90±6 mm Hg while supine and decreased to 79±6/52±4 mm Hg on standing, respectively. The compensatory increase in heart rate was inadequate considering the profound decrease in blood pressure, indicating failure in baroreflex modulation. Supine plasma norepinephrine was lower in pure autonomic failure (PAF) patients compared to multiple system atrophy (MSA) 93±17 and 174±23 pg/nl, respectively), but did not increase adequately on standing in either group (168±32 and 410±152 pg/mL), considering the severity of orthostatic hypotension. Systolic blood pressure decreased significantly during phase II of the Valsalva maneuver (from 160±11 to 104±14 mm Hg) and the expected phase IV blood pressure overshoot was absent. Both findings indicate impaired sympathetic function. Respiratory sinus arrhythmia was reduced (1.1±0.01 mm Hg; normal values, >1.2) consistent with parasympathetic dysfunction. Furthermore, the blood pressure response to the cold pressor test was blunted (9±3 mm Hg; normal, >20 mm Hg). Plasma NOx concentration were similar between AF patients and controls (30.9±5.2 μM for AF patients and 32.4±6.9 μM for controls, p= 0.928 by Mann-Whitney U test).
Table 1.
Baseline characteristics
| Patient | Diagnosis | Protocols | Gender | Blood Pressure
|
Heart Rate
|
Norepinephrine
|
|||
|---|---|---|---|---|---|---|---|---|---|
| Supine | Standing | Supine | Standing | Supine | Standing | ||||
| 1 | PAF | 1,3 | F | 147/88 | 65/39 | 72 | 84 | <25 | 131 |
| 2 | PAF | 1,3 | M | 207/108 | 116/69 | 70 | 68 | 103 | 152 |
| 3 | PAF | 1,3 | F | 176/107 | 83/50 | 74 | 87 | 68 | 139 |
| 4 | MSA | 1 | M | 172/97 | 93/55 | 72 | 87 | 105 | 254 |
| 5 | PAF | 1 | F | 150/84 | 101/78 | 61 | 60 | 28 | 45 |
| 6 | PAF | 1 | F | 104/60 | 78/52 | 81 | 82 | 40 | 131 |
| 7 | PAF | 1,2,3 | M | 168/76 | 92/54 | 68 | 80 | 112 | 167 |
| 8 | MSA | 1,3 | M | 165/86 | 60/48 | 80 | 89 | 139 | 185 |
| 9 | MSA | 1 | F | 159/105 | 74/51 | 93 | 102 | 193 | 338 |
| 10 | PAF | 1,3 | M | 107/72 | 61/43 | 62 | 74 | 55 | 116 |
| 11 | PAF | 1 | F | 171/88 | 72/43 | 76 | 85 | 250 | 494 |
| 12 | PAF | 1 | F | 162/80 | 66/44 | 70 | 83 | 69 | 52 |
| 13 | MSA | 1 | F | 75/48 | 47/33 | 80 | 80 | 226 | 263 |
| 14 | PAF | 1,2,3 | M | 182/103 | 70/51 | 71 | 96 | 152 | 172 |
| 15 | PAF | 2 | M | 150/79 | 61/44 | 58 | 55 | 32 | 42 |
| 16 | MSA | 2 | M | 152/94 | 92/70 | 53 | 101 | 209 | 1010 |
| 17 | PAF | 2 | M | 205/124 | 76/57 | 59 | 90 | 83 | 161 |
| 18 | PAF | 2 | M | 190/100 | 78/56 | 57 | 80 | 132 | 242 |
| 19 | PAF | 2 | M | 177/89 | 90/56 | 72 | 74 | 54 | 87 |
| 20 | PAF | 2 | M | 214/104 | 108/64 | 61 | 76 | 194 | 388 |
Study 1: Autonomic failure patients are more sensitive to NOS inhibition, suggesting increased endogenous NO function
AF patients were subjected to gradual head up tilt until a systolic blood pressure of about 110 mm Hg was reached, whereas healthy controls received trimethaphan to induce a transient autonomic failure. This paradigm resulted in similar “baseline” blood pressures (Figure 1A). L-NMMA was then infused at increasing doses individualized to increase systolic blood pressure to about 150 mm Hg. As expected, the changes in systolic blood pressure were very similar in both groups (from 108±2 to 152±4 and from 106±2 to 144±2 mm Hg in AF patients and controls, respectively). The cumulative dose of L-NMMA required to increase systolic blood pressure was 171±10 mg in patients with autonomic failure, whereas in healthy controls the dose needed was 512±24 mg (figure 1B, p= 0.001 by Mann-Whitney U test). This result suggests that instead of NO impairment, autonomic failure patients have a greater NO function. We also subdivided our autonomic failure patients between those with or without supine hypertension, to further determine if relative NO deficiency contributed to supine hypertension (figure 2). In nine patients with supine hypertension (SBP=174±4 mm Hg) and five without supine hypertension (SBP=124±6 mm Hg), the cumulative total amount of L-NMMA needed to increase SBP up to 150 mm Hg was similar between groups (p=0.438 by Mann-Whitney U test). SBP increased from 107±2. to 154±5, and from 111±2 to 148±5 in patients with and without supine hypertension, respectively.
Figure 1. Autonomic failure patients required lower doses of L-NMMA to achieve similar increases in blood pressure compared to autonomically-blocked normal subjects .
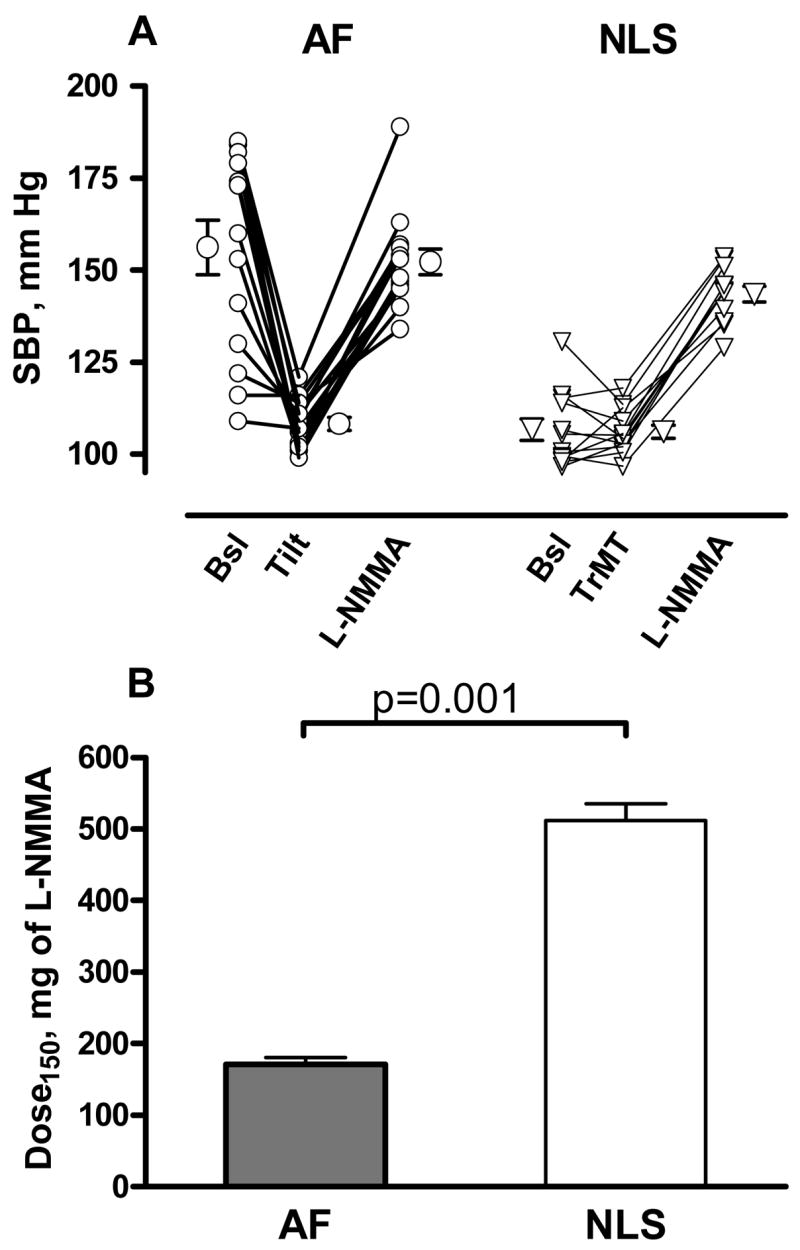
A shows individual values for systolic blood pressure (SBP) in patients with autonomic failure (AF, circles) and normal controls (NLS, triangles). At baseline, autonomic failure patients showed higher SBP values. They were subjected to graded head up tilt (Tilt) until a systolic blood pressure of about 110 mm Hg was reached, whereas autonomic blockade was induced in normal subjects with trimethaphan (TrMT). SBP were similar between groups after these interventions. The nitric oxide synthase inhibitor L-NMMA was then infused at increasing doses, individualized to reach a systolic blood pressure of 150 mm Hg. Panel B shows the cumulative total amount of L-NMMA required to increased systolic blood pressure to 150 mm Hg in autonomic failure patients (AF, gray bar) and normotensive controls (NLS, white bar).
Figure 2. NO inhibition with L-NMMA induces similar increases in systolic blood pressure in autonomic failure patients with or without supine hypertension.
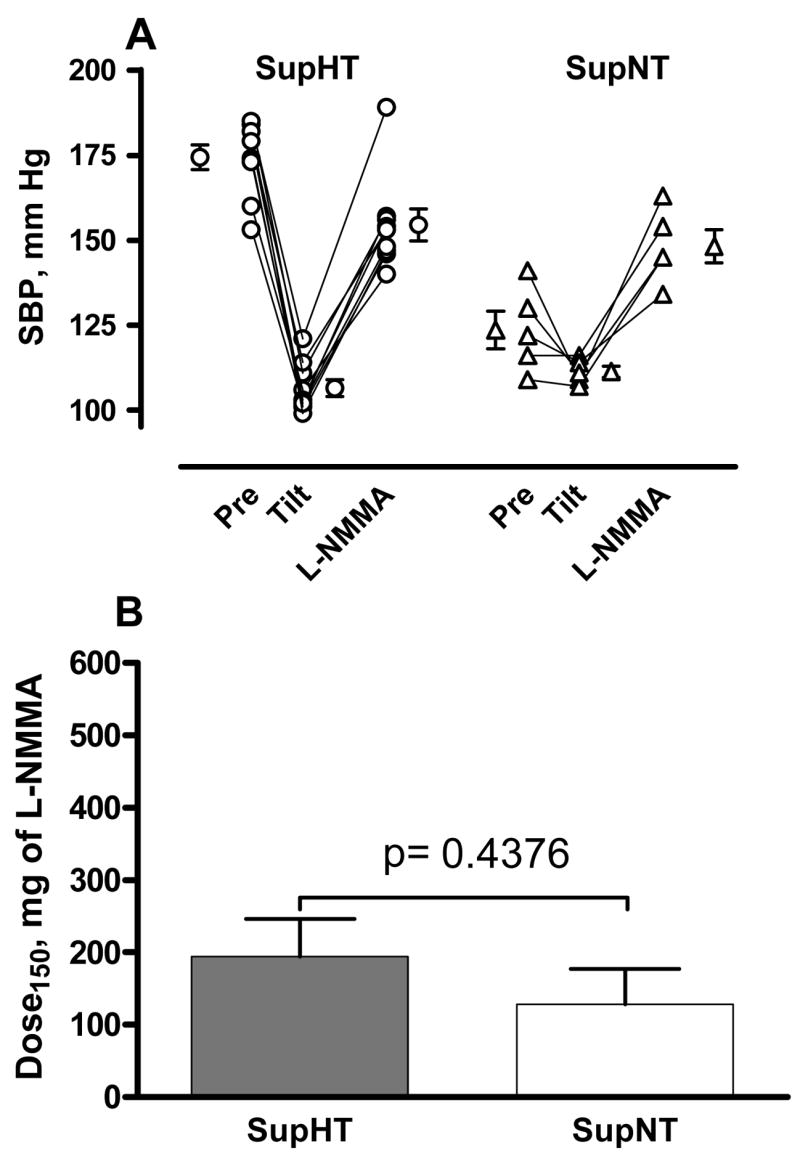
Panel A shows individual values for systolic blood pressure (SBP) in 9 patients with autonomic failure and supine hypertension (SupHTN) and 5 patients without supine hypertension (SupNTN), Baseline (Pre) SBP values were obviously greater in AF patients with supine hypertension (174±3.7 vs. 124±5.6 mm Hg in those without supine hypertension). Subjects were then gradually tilted head up (Tilt) to induce a SBP of ~110 mm Hg, and then the nitric oxide synthase inhibitor L-NMMA was infused at increasing doses individualized to reach a systolic blood pressure of 150 mm Hg. The changes in SBP induced by L-NMMA infusion were very similar in both groups (from 107±2.4 to 154±4.7 and from 111±1.6 to 148±4.9 in patients with and without supine hypertension respectively). Panel B shows that similar cumulative total amounts of L-NMMA were required to increased systolic blood pressure to 150 mm Hg in autonomic failure patients with supine hypertension (SupHTN, white bar) and without supine hypertension (SupNTN, white bar, p=0.438).
Study 2: NO potentiation with sildenafil controls supine hypertension in autonomic failure
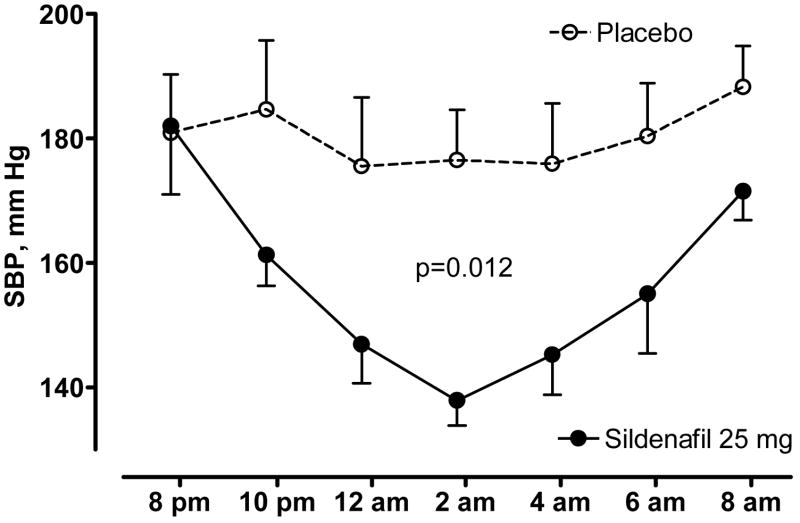
During the placebo night, SBP decreased from 181±9 at 8 p.m. to 177±8 mmHg at 2 a.m. Sildenafil produced a significantly larger decrease in SBP, from 182±11 to 138±4 mmHg (figure 3, p=0.012 for the differences in AUC between treatments by Wilcoxon signed-rank test). The maximal decrease in SBP was greater during sildenafil (52±18 mmHg vs.20±8 mm Hg for placebo, p=0.028 by Wilcoxon signed-rank test).
Figure 3. Potentiation of endogenous nitric oxide with sildenafil normalizes supine hypertension in autonomic failure.
Effect of sildenafil 25 mg PO administered at 8 p.m. on nighttime systolic blood pressure (SBP) in 8 autonomic failure patients with supine hypertension.
Study 3: Systemic NOS inhibition improves orthostatic tolerance in autonomic failure
Autonomic failure patients were subjected to graded head up tilt to presyncope or systolic blood pressure < 70 mm Hg, before and after systemic administration of L-NMMA or phenylephrine at doses titrated to achieve similar systolic blood pressures. On the L-NMMA day, tilt had to be stopped at 41±4 degrees at baseline, but during L-NMMA infusion patients were able to tolerate a higher head-up tilt (56±6 degrees, figure 4A, p=0.014). On the phenylephrine day orthostatic tolerance increased from 40±4 degrees to 59±5 degrees (figure 4B, p=0.005). No differences were found for the survival to tilt between phenylephrine and L-NMMA (p=0.9786 by the log-rank test).
Figure 4. Improvement of orthostatic tolerance after nitric oxide inhibition with L-NMMA in autonomic failure patients is similar to that achieved by a-stimulation with phenylephrine.
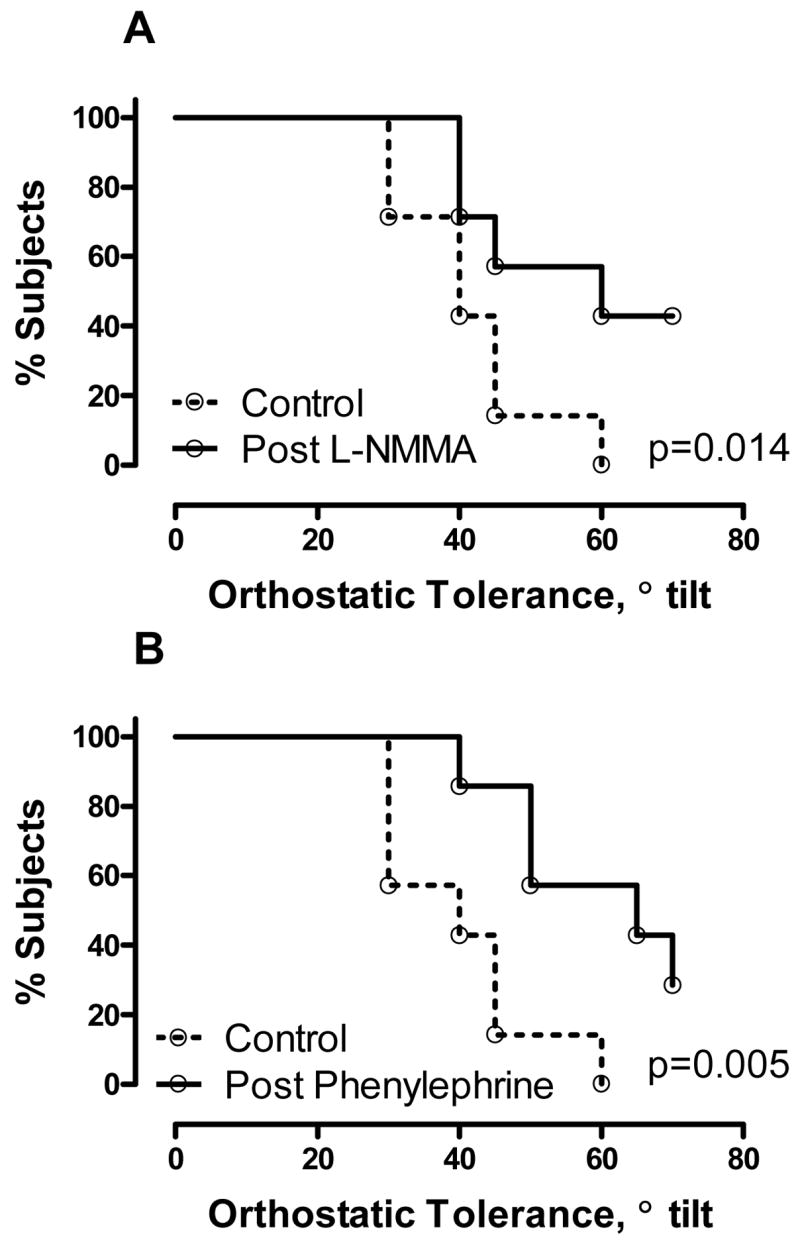
Autonomic failure patients were subjected to graded uptight tilt to presyncope or systolic blood pressure < 70 mm Hg before (dashed lines) and after L-NMMA (Panel A) or phenylephrine (Panel B) at doses titrated to achieve similar baseline systolic blood pressures (see text for details). Both drugs produced significant improvements in orthostatic tolerance, but no differences were found between phenylephrine and L-NMMA (p=0.9786).
Discussion
Our results indicate that, contrary to our original hypothesis, autonomic failure patients have increased nitric oxide function. This conclusion is based on two complementary observations; autonomic failure patients had a greater pressor response to nitric oxide synthase inhibition, and also had an exaggerated depressor response to NO function augmentation with sildenafil.
We found that lower doses of the NOS inhibitor L-NMMA were required to produce similar increases in blood pressure in autonomic failure compared to normal subjects in whom autonomic failure was induced with the ganglionic blocker trimethaphan. If autonomic failure patients had an impaired NO function, we would have seen the opposite response, a blunted pressor response to L-NMMA. This is precisely what we observed, using a very similar approach, in smokers,4 a patient population widely accepted to have an impaired nitric oxide function.13–16 The greater pressor effect of L-NMMA in AF patients was not due simply to their lack of baroreflex buffering because the baroreflex is similarly abolished by trimethaphan in normal subjects. A caveat of this interpretation is that our control group was younger than the autonomic failure patients. However, otherwise normal elderly are known to have reduced nitric oxide function, as assessed by a decrease in flow-mediated dilation.17 Consequently, if anything, our use of young normal controls may underestimate the increased nitric oxide function present in patients with autonomic failure. Therefore, we felt we were not justified in subjecting elderly volunteers to autonomic withdrawal with trimethaphan simply for the purpose of obtaining age-matched controls.
Our findings raise the possibility that sympathetic activation normally acts as a “brake” to the nitric oxide function, as this would be absent in our patients with autonomic failure. This concept is supported by the observation that acute activation of the sympathetic nervous system with lower body negative pressure induces an impairment in flow-mediated dilation18. Several conditions (e.g. obesity, congestive heart failure, awakening) are characterized by both sympathetic activation and NO deficiency. It is possible, therefore that in such cases sympathetic activation contributes to NO deficiency, but this hypothesis requires experimental validation. Autonomic failure patients also have parasympathetic impairment, and cholinergic blockade has been shown to markedly potentiate the pressor effects of L-NMMA19.
We also found that L-NMMA produced similar pressor responses in autonomic failure patients with or without supine hypertension. We conclude, therefore, that endogenous nitric oxide deficiency does not contribute to the supine hypertension of autonomic failure. On the contrary, AF patients have supine hypertension despite having excess NO function. Thus, increased nitric oxide function is not sufficient to prevent hypertension. Conversely, we and other have previously observed that nitric oxide deficiency as seen in heavy smokers is not sufficient to induce hypertension4,20,21. Thus, despite the potent effect endogenous nitric oxide has in the acute regulation of blood pressure, its role in sustaining hypertension in humans is not certain. We should note that we have only examined the NO function in patients with impaired autonomic function, and have not evaluated NO/autonomic interactions which could also contribute to hypertension.
The driving force for supine hypertension in pure autonomic failure remains unknown. On the other hand, we can use their nitric oxide function excess to our advantage in the treatment of supine hypertension. We found that potentiating NO signaling with the phosphodiesterase inhibitor sildenafil resulted in a significant lowering of nighttime blood pressure. Because sildenafil acts by enhancing endogenous NO function, the opposite would have been observed if patients had impaired NO function; e.g., sildenafil is less effective in improving endothelium-dependent vascular responses in smokers.16 Thus, the exaggerated hypotensive effect observed in our patients also supports the concept that autonomic failure is associated with increased nitric oxide function.
In contrast to the lack of effect of tonic NO in supine hypertension, inhibition of NO production with L-NMMA significantly improved orthostatic tolerance in AF patients. At baseline, none of our patients was able to tolerate a 70 degree head-up tilt, but 50% of patients were able to tolerate this after NOS blockade. This improvement in orthostatic tolerance was similar to that obtained by restoring noradrenergic receptor stimulation with phenylephrine.
Our results cannot differentiate between increased NO production or increased sensitivity to NO actions. The fact that similar baseline plasma NO values were seen in AF patients and controls raises the possibility that this increased NO function may be explained by an increased sensitivity to NO signaling. In this regard, autonomic failure patients also have an exaggerated depressor response to nitric oxide donors, such as nitroglycerine.1 A caveat to this conclusion is the known limitations in estimating nitric oxide production in vivo.
In conclusion, contrary to our original hypothesis, AF patients do not have NO deficiency contributing to supine hypertension. On the contrary, they have increased NO function contributing to their orthostatic hypotension. Potentiation of NO could be used in the treatment of supine hypertension, and its inhibition offers a novel approach to improve the orthostatic intolerance of AF.
Perspectives
Patients with autonomic failure provide a unique model of a condition characterized by excess nitric oxide function. Autonomic failure patients with and without supine hypertension appear to have similar excess nitric oxide function, questioning the role of this autacoid in long-term blood pressure regulation. On the other hand, our finding that autonomic impairment is associated with greater NO function is in agreement with previous observations, suggesting that transient increases in sympathetic tone impair NO function. Taken together, these observations suggest modulation of NO by the autonomic nervous system.
Acknowledgments
We want to thank to all our patients and volunteers
Source(s) of Funding
This work was supported in part by National Institutes of Health grants 1RO1 HL67232, 1PO1 HL56693, RR00095 and M01 RR-00095. S.R.R. is supported by grant K23-RR020783.
Footnotes
Please refer to this study by ClinicalTrials.gov identifier NCT00178919 Study ID Numbers: 010876; NIH 1RO1HL71172 http://www.clinicaltrials.gov/ct/show/NCT00178919?order=1
Conflict(s) of Interest/Disclosure(s).- None
Reference List
- 1.Shannon J, Jordan J, Costa F, Robertson RM, Biaggioni I. The hypertension of autonomic failure and its treatment. Hypertension. 1997;30:1062–1067. doi: 10.1161/01.hyp.30.5.1062. [DOI] [PubMed] [Google Scholar]
- 2.Shannon JR, Jordan J, Diedrich A, Pohar B, Black BK, Robertson D, Biaggioni I. Sympathetically mediated hypertension in autonomic failure. Circulation. 2000;101:2710–2715. doi: 10.1161/01.cir.101.23.2710. [DOI] [PubMed] [Google Scholar]
- 3.Kronenberg MW, Forman MB, Onrot J, Robertson D. Enhanced left ventricular contractility in autonomic failure: assessment using pressure-volume relations. J Am Coll Cardiol. 1990;15:1334–1342. doi: 10.1016/s0735-1097(10)80023-3. [DOI] [PubMed] [Google Scholar]
- 4.Gamboa A, Shibao C, Diedrich A, Choi L, Pohar B, Jordan J, Paranjape S, Farley G, Biaggioni I. Contribution of endothelial nitric oxide to blood pressure in humans. Hypertension. 2007;49:170–177. doi: 10.1161/01.HYP.0000252425.06216.26. [DOI] [PubMed] [Google Scholar]
- 5.Panza JA, Quyyumi AA, Brush JE, Jr, Epstein SE. Abnormal endothelium-dependent vascular relaxation in patients with essential hypertension. N Engl J Med. 1990;323:22–27. doi: 10.1056/NEJM199007053230105. [DOI] [PubMed] [Google Scholar]
- 6.Treasure CB, Vita JA, Cox DA, Fish RD, Gordon JB, Mudge GH, Colucci WS, Sutton MG, Selwyn AP, Alexander RW. Endothelium-dependent dilation of the coronary microvasculature is impaired in dilated cardiomyopathy. Circulation. 1990;81:772–779. doi: 10.1161/01.cir.81.3.772. [DOI] [PubMed] [Google Scholar]
- 7.Kaufmann H. Consensus statement on the definition of orthostatic hypotension, pure autonomic failure, and multiple system atrophy. Clinical Autonomic Research. 1996;6:125. doi: 10.1007/BF02291236. [DOI] [PubMed] [Google Scholar]
- 8.Robertson D. Assessment of autonomic function. In: Baughman KL, Green BM, editors. Manual for House Officers. Baltimore: Williams and Wilkins; 1981. pp. 86–131. [Google Scholar]
- 9.Mosqueda-Garcia R. Evaluation of autonomic failure. In: Robertson D, Biaggioni I, editors. Disorders of the Autonomic Nervous System. London: Harwood Academic Press; 1995. pp. 25–59. [Google Scholar]
- 10.Goldstein DS, Polinsky RJ, Garty M, Robertson D, Brown RT, Biaggioni I, Stull R, Kopin IJ. Patterns of plasma levels of catechols in neurogenic orthostatic hypotension. Ann Neurol. 1989;26:558–563. doi: 10.1002/ana.410260410. [DOI] [PubMed] [Google Scholar]
- 11.Richard C, Gao J, LaFleur B, Christman BW, Anderson J, Brown N, Reese J. Patency of the preterm fetal ductus arteriosus is regulated by endothelial nitric oxide synthase and is independent of vasa vasorum in the mouse. Am J Physiol Regul Integr Comp Physiol. 2004;287:R652–R660. doi: 10.1152/ajpregu.00049.2004. [DOI] [PubMed] [Google Scholar]
- 12.Diedrich A, Jordan J, Tank J, Shannon JR, Robertson R, Luft FC, Robertson D, Biaggioni I. The sympathetic nervous system in hypertension: assessment by blood pressure variability and ganglionic blockade. J Hypertens. 2003;21:1677–1686. doi: 10.1097/00004872-200309000-00017. [DOI] [PubMed] [Google Scholar]
- 13.Higman DJ, Strachan AM, Buttery L, Hicks RC, Springall DR, Greenhalgh RM, Powell JT. Smoking impairs the activity of endothelial nitric oxide synthase in saphenous vein. Arterioscler Thromb Vasc Biol. 1996;16:546–552. doi: 10.1161/01.atv.16.4.546. [DOI] [PubMed] [Google Scholar]
- 14.Wiesmann F, Petersen SE, Leeson PM, Francis JM, Robson MD, Wang Q, Choudhury R, Channon KM, Neubauer S. Global impairment of brachial, carotid, and aortic vascular function in young smokers: direct quantification by high-resolution magnetic resonance imaging. J Am Coll Cardiol. 2004;44:2056–2064. doi: 10.1016/j.jacc.2004.08.033. [DOI] [PubMed] [Google Scholar]
- 15.Kiowski W, Linder L, Stoschitzky K, Pfisterer M, Burckhardt D, Burkart F, Buhler FR. Diminished vascular response to inhibition of endothelium-derived nitric oxide and enhanced vasoconstriction to exogenously administered endothelin-1 in clinically healthy smokers. Circulation. 1994;90:27–34. doi: 10.1161/01.cir.90.1.27. [DOI] [PubMed] [Google Scholar]
- 16.Kimura M, Higashi Y, Hara K, Noma K, Sasaki S, Nakagawa K, Goto C, Oshima T, Yoshizumi M, Chayama K. PDE5 inhibitor sildenafil citrate augments endothelium-dependent vasodilation in smokers. Hypertension. 2003;41:1106–1110. doi: 10.1161/01.HYP.0000068202.42431.CC. [DOI] [PubMed] [Google Scholar]
- 17.Donato AJ, Eskurza I, Silver AE, Levy AS, Pierce GL, Gates PE, Seals DR. Direct evidence of endothelial oxidative stress with aging in humans: relation to impaired endothelium-dependent dilation and upregulation of nuclear factor-kappaB. Circ Res. 2007;100:1659–1666. doi: 10.1161/01.RES.0000269183.13937.e8. [DOI] [PubMed] [Google Scholar]
- 18.Hijmering ML, Stroes ES, Olijhoek J, Hutten BA, Blankestijn PJ, Rabelink TJ. Sympathetic activation markedly reduces endothelium-dependent, flow-mediated vasodilation. J Am Coll Cardiol. 2002;39:683–688. doi: 10.1016/s0735-1097(01)01786-7. [DOI] [PubMed] [Google Scholar]
- 19.Lepori M, Sartori C, Duplain H, Nicod P, Scherrer U. Interaction between cholinergic and nitrergic vasodilation: a novel mechanism of blood pressure control. Cardiovasc Res. 2001;51:767–772. doi: 10.1016/s0008-6363(01)00325-x. [DOI] [PubMed] [Google Scholar]
- 20.Wennmalm A, Benthin G, Granstrom EF, Persson L, Petersson AS, Winell S. Relation between tobacco use and urinary excretion of thromboxane A2 and prostacyclin metabolites in young men. Circulation. 1991;83:1698–1704. doi: 10.1161/01.cir.83.5.1698. [DOI] [PubMed] [Google Scholar]
- 21.John U, Meyer C, Hanke M, Volzke H, Schumann A. Smoking status, obesity and hypertension in a general population sample: a cross-sectional study. QJM. 2006;99:407–415. doi: 10.1093/qjmed/hcl047. [DOI] [PubMed] [Google Scholar]