Abstract
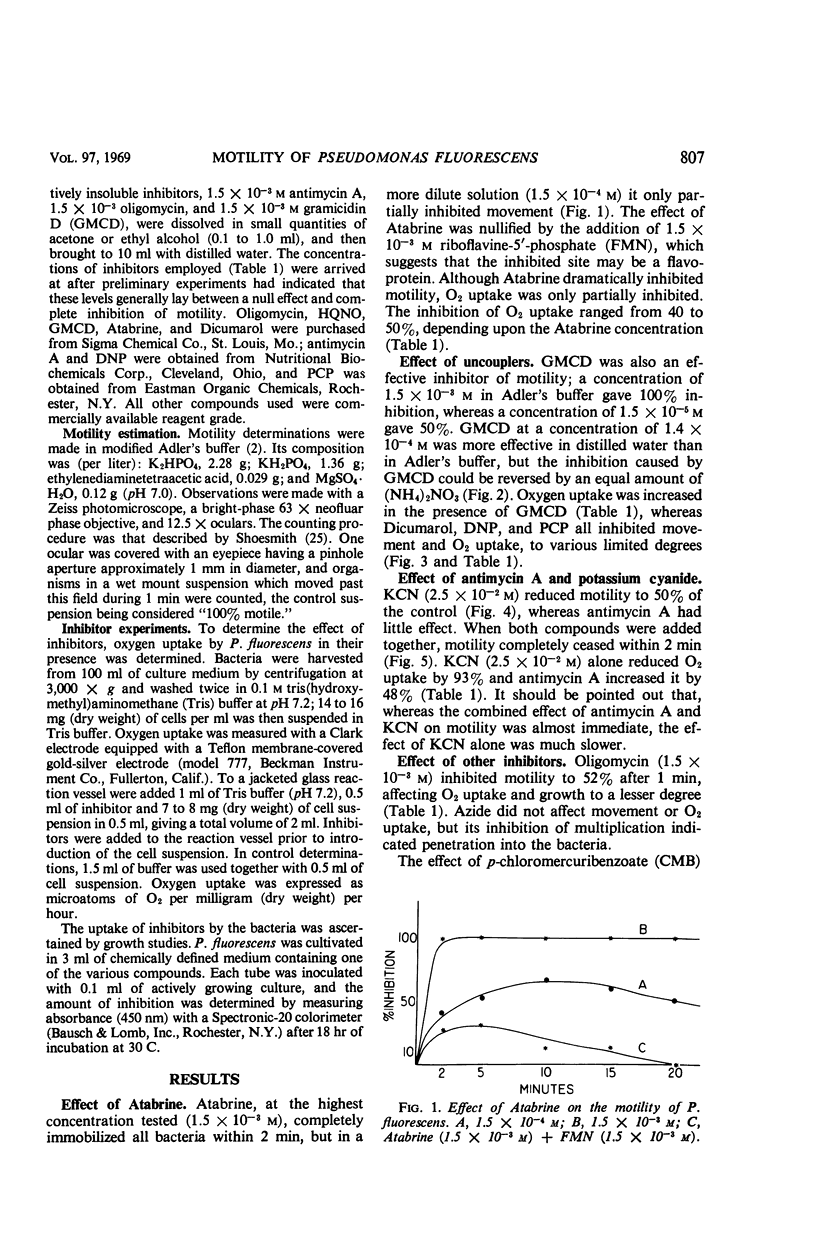
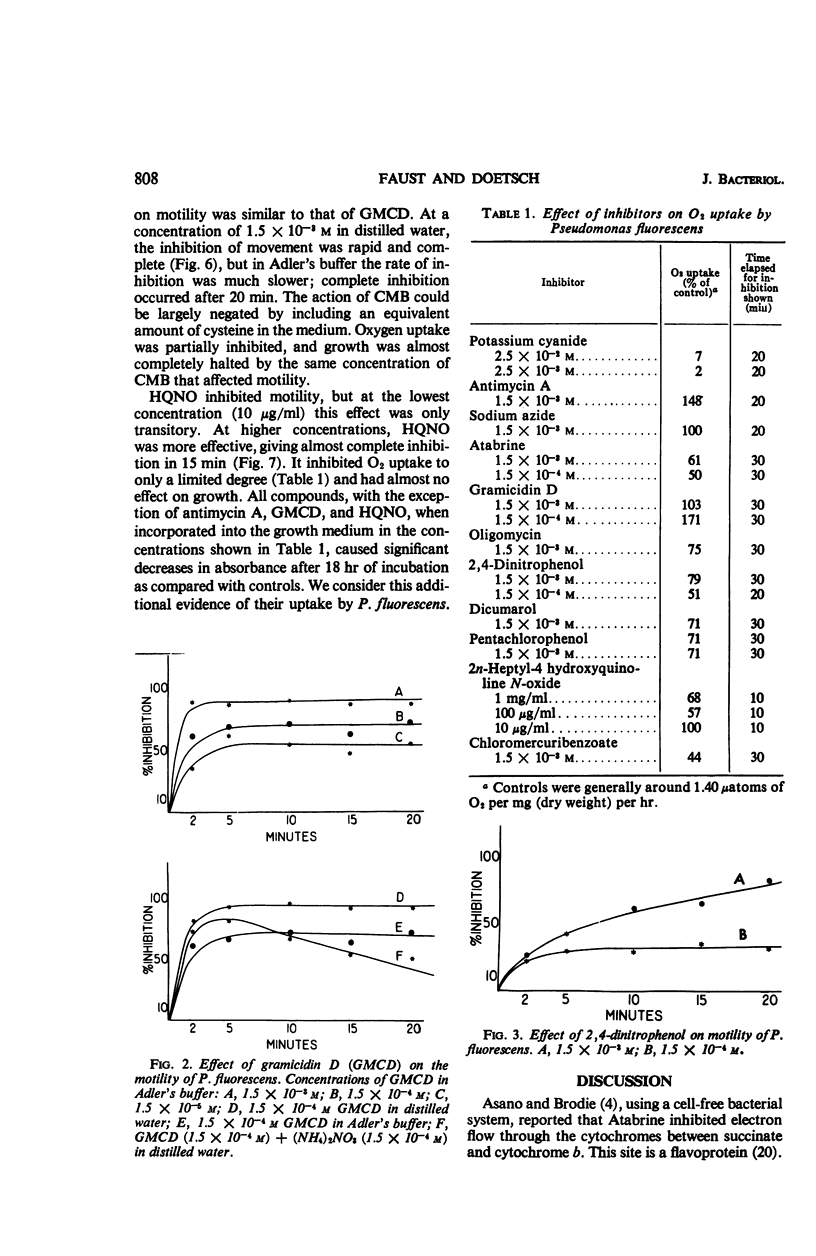
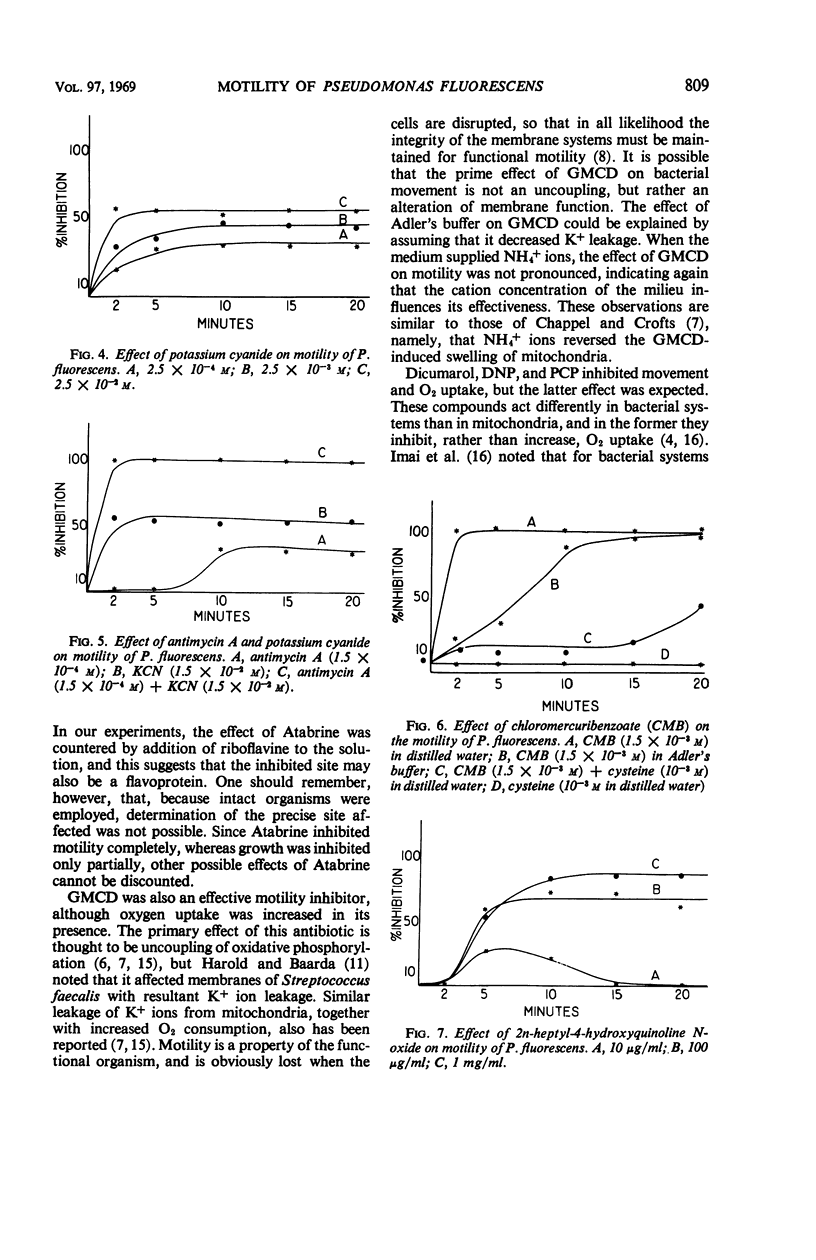
The translational motility of Pseudomonas fluorescens was weakly inhibited by oligomycin, Dicumarol, 2,4-dinitrophenol, 2n-heptyl-4-hydroxyquinoline N-oxide, and potassium cyanide. Atabrine and antimycin A together with potassium cyanide immediately immobilized this bacterium, but antimycin A alone was without effect. Gramicidin D also immobilized P. fluorescens, but its action was inhibited by K+ and NH4+ ions. In like manner, the effect of p-chloromercuribenzoate could be counteracted with cysteine, thereby suggesting the involvement of —SH groups in flagellar motility processes. It appears that the energy required for motility of P. fluorescens is generated by oxidative phosphorylation mediated by the cytochrome system.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMBLER R. P. THE PURIFICATION AND AMINO ACID COMPOSITION OF PSEUDOMONAS CYTOCHROME C-551. Biochem J. 1963 Nov;89:341–349. doi: 10.1042/bj0890341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ASANO A., BRODIE A. F. OXIDATIVE PHOSPHORYLATION IN FRACTIONATED BACTERIAL SYSTEMS. XIV. RESPIRATORY CHAINS OF MYCOBACTERIUM PHLEI. J Biol Chem. 1964 Dec;239:4280–4291. [PubMed] [Google Scholar]
- Adler J. Chemotaxis in bacteria. Science. 1966 Aug 12;153(3737):708–716. doi: 10.1126/science.153.3737.708. [DOI] [PubMed] [Google Scholar]
- Bongers L. Phosphorylation in hydrogen bacteria. J Bacteriol. 1967 May;93(5):1615–1623. doi: 10.1128/jb.93.5.1615-1623.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAPPELL J. B., CROFTS A. R. GRAMICIDIN AND ION TRANSPORT IN ISOLATED LIVER MITOCHONDRIA. Biochem J. 1965 May;95:393–402. doi: 10.1042/bj0950393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE ROBERTIS E., PELUFFO C. A. Chemical stimulation and inhibition of bacterial motility studied with a new method. Proc Soc Exp Biol Med. 1951 Nov;78(2):584–589. doi: 10.3181/00379727-78-19148. [DOI] [PubMed] [Google Scholar]
- Doetsch R. N., Hageage G. J. Motility in procaryotic organisms: problems, points of view, and perspectives. Biol Rev Camb Philos Soc. 1968 Aug;43(3):317–362. doi: 10.1111/j.1469-185x.1968.tb00963.x. [DOI] [PubMed] [Google Scholar]
- Drapeau G. R., Matula T. I., MacLeod R. A. Nutrition and metabolism of marine bacteria. XV. Relation of Na+-activated transport to the Na+ requirement of a marine pseudomonad for growth. J Bacteriol. 1966 Jul;92(1):63–71. doi: 10.1128/jb.92.1.63-71.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREENWALT J. W., WEIBULL C., LOW H. The hydrolysis of adenosine triphosphate by cell fractions of Bacillus megaterium. II. Stimulation and inhibition of the enzymic activities. J Biol Chem. 1962 Mar;237:853–858. [PubMed] [Google Scholar]
- Harold F. M., Baarda J. R. Gramicidin, valinomycin, and cation permeability of Streptococcus faecalis. J Bacteriol. 1967 Jul;94(1):53–60. doi: 10.1128/jb.94.1.53-60.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins M. L. Release of sporangiospores by a strain of Actinoplanes. J Bacteriol. 1967 Sep;94(3):495–498. doi: 10.1128/jb.94.3.495-498.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai K., Asano A., Sato R. Oxidative phosphorylation in Micrococcus denitrificans. I. Preparation and properties of phosphorylating membrane fragments. Biochim Biophys Acta. 1967;143(3):462–476. doi: 10.1016/0005-2728(67)90052-7. [DOI] [PubMed] [Google Scholar]
- Jahn T. L., Bovee E. C. Movement and locomotion of microorganisms. Annu Rev Microbiol. 1965;19:21–58. doi: 10.1146/annurev.mi.19.100165.000321. [DOI] [PubMed] [Google Scholar]
- KERRIDGE D. The effect of inhibitors on the formation of flagella by Salmonella typhimurium. J Gen Microbiol. 1960 Dec;23:519–538. doi: 10.1099/00221287-23-3-519. [DOI] [PubMed] [Google Scholar]
- LIGHTBOWN J. W., JACKSON F. L. Inhibition of cytochrome systems of heart muscle and certain bacteria by the antagonists of dihydrostreptomycin: 2-alkyl-4-hydroxyquinoline N-oxides. Biochem J. 1956 May;63(1):130–137. doi: 10.1042/bj0630130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOW H. On the participation of flavin in mitochondrial adenosine triphosphatase reactions. Biochim Biophys Acta. 1959 Mar;32(1):1–10. doi: 10.1016/0006-3002(59)90547-5. [DOI] [PubMed] [Google Scholar]
- MARQUIS R. E. NATURE OF THE BACTERICIDAL ACTION OF ANTIMYCIN A FOR BACILLUS MEGATERIUM. J Bacteriol. 1965 Jun;89:1453–1459. doi: 10.1128/jb.89.6.1453-1459.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOROWITZ H. J. The energy requirements for bacterial motility. Science. 1954 Feb 26;119(3087):286–286. doi: 10.1126/science.119.3087.286. [DOI] [PubMed] [Google Scholar]
- SHERRIS J. C., PRESTON N. W., SHOESMITH J. G. The influence of oxygen and arginine on the motility of a strain of Pseudomonas sp. J Gen Microbiol. 1957 Feb;16(1):86–96. doi: 10.1099/00221287-16-1-86. [DOI] [PubMed] [Google Scholar]
- Skou J. C., Hilberg C. The effect of sulphydryl-blocking reagents and of urea on the (Na+ + K+)-activated enzyme system. Biochim Biophys Acta. 1965 Nov 22;110(2):359–369. doi: 10.1016/s0926-6593(65)80043-1. [DOI] [PubMed] [Google Scholar]
- Smith J. L., Doetsch R. N. Motility in Pseudomonas fluorescens with special reference to survival advantage and negative chemotaxis. Life Sci. 1968 Aug 15;7(16):875–886. doi: 10.1016/0024-3205(68)90119-7. [DOI] [PubMed] [Google Scholar]


