Abstract
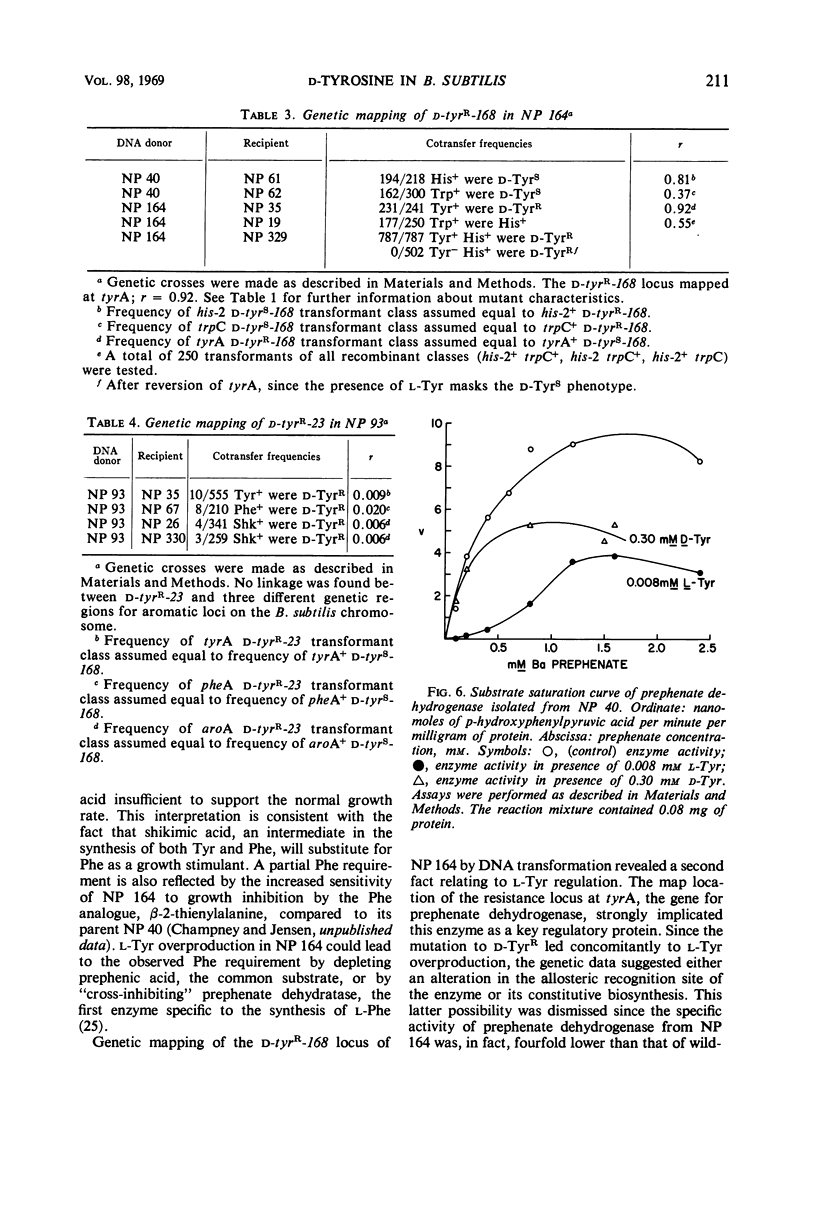
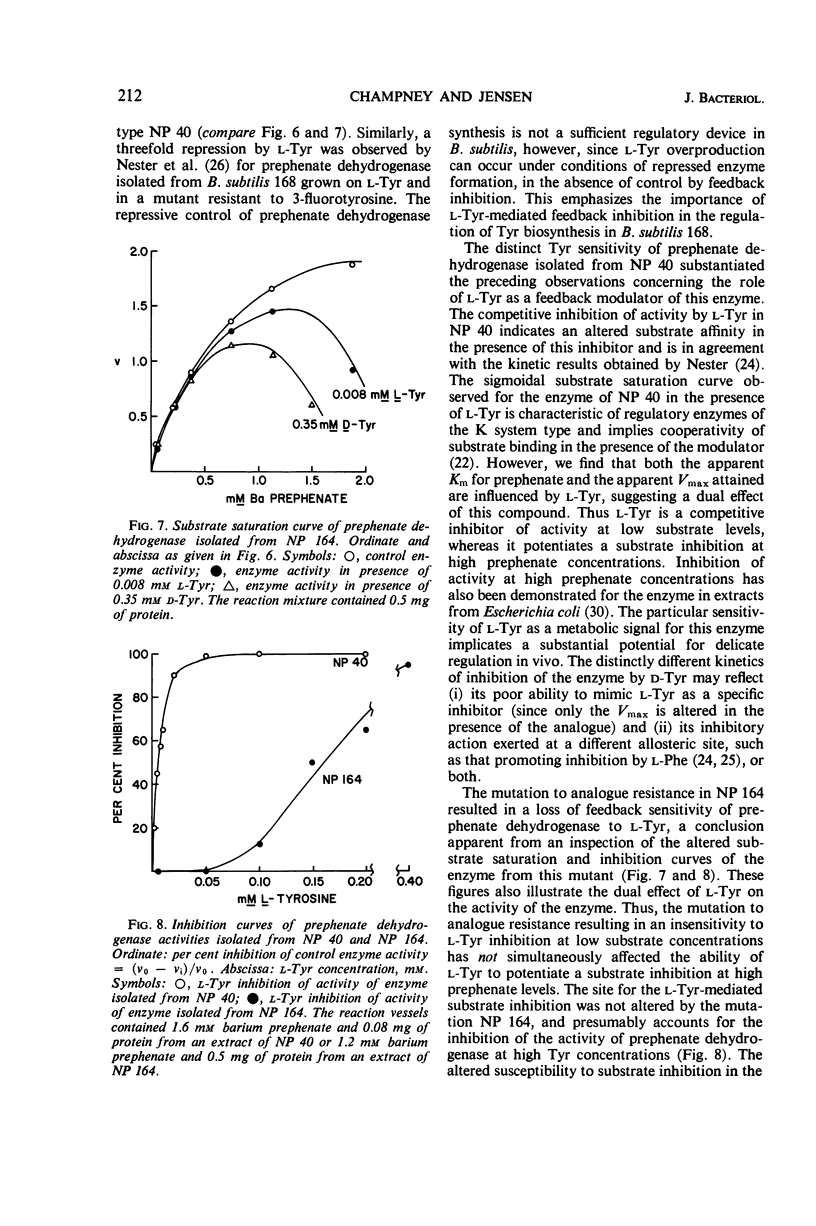
The d-isomer of tyrosine is a potent inhibitor of growth in transformable strain 168 of Bacillus subtilis. A d-tyrosine-resistant mutant of the inhibited strain was isolated which excreted l-tyrosine, had a diminished growth rate, and required l-phenylalanine to attain the growth rate of the wild-type parent. Mapping by deoxyribonucleate transformation located this resistance in the gene coding for prephenate dehydrogenase. This enzyme in the d-tyrosine-resistant mutant was insensitive to the usual feedback inhibition exerted by l-tyrosine in extracts of strain 168. In contrast, the growth of poorly transformable strain 23 of B. subtilis, as well as that of several other Bacillus species, was not affected by the analogue. Transformation mapping demonstrated no linkage of this latter “natural resistance” to several different aromatic markers. Prephenate dehydrogenase in extracts from strain 23 was as sensitive as that from strain 168 to feedback inhibition by l-tyrosine in vitro. The relationships of the latter results to the regulation of tyrosine biosynthesis and the possible nature of strain differences in d-tyrosine sensitivity are discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anagnostopoulos C., Crawford I. P. Le groupe des gènes régissant la biosynthèse du tryptophane chez Bacillus subtilis. C R Acad Sci Hebd Seances Acad Sci D. 1967 Jul 3;265(1):93–96. [PubMed] [Google Scholar]
- Aronson J. N., Wermus G. R. Effects of m-Tyrosine on Growth and Sporulation of Bacillus Species. J Bacteriol. 1965 Jul;90(1):38–46. doi: 10.1128/jb.90.1.38-46.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARAT M., ANAGNOSTOPOULOS C., SCHNEIDER A. M. LINKAGE RELATIONSHIPS OF GENES CONTROLLING ISOLEUCINE, VALINE, AND LEUCINE BIOSYNTHESIS IN BACILLUS SUBTILIS. J Bacteriol. 1965 Aug;90:357–369. doi: 10.1128/jb.90.2.357-369.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berberich R., Kaback M., Freese E. D-amino acids as inducers of L-alanine dehydrogenase in Bacillus subtilis. J Biol Chem. 1968 Mar 10;243(5):1006–1011. [PubMed] [Google Scholar]
- COTTON R. G., GIBSON F. THE BIOSYNTHESIS OF PHENYLALANINE AND TYROSINE; ENZYMES CONVERTING CHORISMIC ACID INTO PREPHENIC ACID AND THEIR RELATIONSHIPS TO PREPHENATE DEHYDRATASE AND PREPHENATE DEHYDROGENASE. Biochim Biophys Acta. 1965 Apr 12;100:76–88. doi: 10.1016/0304-4165(65)90429-0. [DOI] [PubMed] [Google Scholar]
- Calendar R., Berg P. D-Tyrosyl RNA: formation, hydrolysis and utilization for protein synthesis. J Mol Biol. 1967 May 28;26(1):39–54. doi: 10.1016/0022-2836(67)90259-8. [DOI] [PubMed] [Google Scholar]
- Calendar R., Berg P. Purification and physical characterization of tyrosyl ribonucleic acid synthetases from Escherichia coli and Bacillus subtilis. Biochemistry. 1966 May;5(5):1681–1690. doi: 10.1021/bi00869a033. [DOI] [PubMed] [Google Scholar]
- Calendar R., Berg P. The catalytic properties of tyrosyl ribonucleic acid synthetases from Escherichia coli and Bacillus subtilis. Biochemistry. 1966 May;5(5):1690–1695. doi: 10.1021/bi00869a034. [DOI] [PubMed] [Google Scholar]
- Coats J. H., Nester E. W. Regulation reversal mutation: characterization of end product-activated mutants of Bacillus subtilis. J Biol Chem. 1967 Nov 10;242(21):4948–4955. [PubMed] [Google Scholar]
- Cotton R. G., Gibson F. The biosynthesis of tyrosine in Aerobacter aerogenes: partial purification of the T protein. Biochim Biophys Acta. 1967 Oct 23;147(2):222–237. doi: 10.1016/0005-2795(67)90401-1. [DOI] [PubMed] [Google Scholar]
- Gibson F. Chorismic acid: purification and some chemical and physical studies. Biochem J. 1964 Feb;90(2):256–261. doi: 10.1042/bj0900256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERBERT D., ELSWORTH R., TELLING R. C. The continuous culture of bacteria; a theoretical and experimental study. J Gen Microbiol. 1956 Jul;14(3):601–622. doi: 10.1099/00221287-14-3-601. [DOI] [PubMed] [Google Scholar]
- JENSEN R. A., NESTER E. W. THE REGULATORY SIGNIFICANCE OF INTERMEDIARY METABOLITES: CONTROL OF AROMATIC ACID BIOSYNTHESIS BY FEEDBACK INHIBITION IN BACILLUS SUBTILIS. J Mol Biol. 1965 Jun;12:468–481. doi: 10.1016/s0022-2836(65)80270-4. [DOI] [PubMed] [Google Scholar]
- Jensen R. A., Nasser D. S., Nester E. W. Comparative control of a branch-point enzyme in microorganisms. J Bacteriol. 1967 Nov;94(5):1582–1593. doi: 10.1128/jb.94.5.1582-1593.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lingens F. The biosynthesis of aromatic amino acids and its regulation. Angew Chem Int Ed Engl. 1968 May;7(5):350–360. doi: 10.1002/anie.196803501. [DOI] [PubMed] [Google Scholar]
- MONOD J., WYMAN J., CHANGEUX J. P. ON THE NATURE OF ALLOSTERIC TRANSITIONS: A PLAUSIBLE MODEL. J Mol Biol. 1965 May;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- Nasser D., Nester E. W. Aromatic amino acid biosynthesis: gene-enzyme relationships in Bacillus subtilis. J Bacteriol. 1967 Nov;94(5):1706–1714. doi: 10.1128/jb.94.5.1706-1714.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nester E W, Schafer M, Lederberg J. Gene Linkage in DNA Transfer: A Cluster of Genes Concerned with Aromatic Biosynthesis in Bacillus Subtilis. Genetics. 1963 Apr;48(4):529–551. doi: 10.1093/genetics/48.4.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nester E. W. Cross pathway regulation: effect of histidine on the synthesis and activity of enzymes of aromatic acid biosynthesis in Bacillus subtilis. J Bacteriol. 1968 Nov;96(5):1649–1657. doi: 10.1128/jb.96.5.1649-1657.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nester E. W., Jensen R. A. Control of aromatic acid biosynthesis in Bacillus subtilis: sequenial feedback inhibition. J Bacteriol. 1966 Apr;91(4):1594–1598. doi: 10.1128/jb.91.4.1594-1598.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nester E. W., Jensen R. A., Nasser D. S. Regulation of enzyme synthesis in the aromatic amino acid pathway of Bacillus subtilus. J Bacteriol. 1969 Jan;97(1):83–90. doi: 10.1128/jb.97.1.83-90.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RICHMOND M. H. The effect of amino acid analogues on growth and protein synthesis in microorganisms. Bacteriol Rev. 1962 Dec;26:398–420. doi: 10.1128/br.26.4.398-420.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHWINCK I., ADAMS E. Aromatic biosynthesis. XVI. Aromatization of prephenic acid to p-hydroxyphenylpyruvic acid, a step in tyrosine biosynthesis in Escherichia coli. Biochim Biophys Acta. 1959 Nov;36:102–117. doi: 10.1016/0006-3002(59)90074-5. [DOI] [PubMed] [Google Scholar]
- Spizizen J. TRANSFORMATION OF BIOCHEMICALLY DEFICIENT STRAINS OF BACILLUS SUBTILIS BY DEOXYRIBONUCLEATE. Proc Natl Acad Sci U S A. 1958 Oct 15;44(10):1072–1078. doi: 10.1073/pnas.44.10.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKAHASHI I. Genetic transformation of Bacillus subtilis by extracellular DNA. Biochem Biophys Res Commun. 1962 Jun 4;7:467–470. doi: 10.1016/0006-291x(62)90337-6. [DOI] [PubMed] [Google Scholar]


