Abstract
Sudden death resulting from ventricular fibrillation (VF) during acute myocardial ischaemia forms an important contribution to mortality associated with infarction. Its temporal distribution is not known, but 30% of mortality occurs within the first 60 minutes. Two distinct phases of arrhythmias have been demonstrated in laboratory animals subjected to coronary occlusion. The mechanism of the second, 1B phase (which is associated with more lethal events than the first, 1A phase) is largely unknown but appears to be related to cellular uncoupling, i.e. the closure of gap junctions.
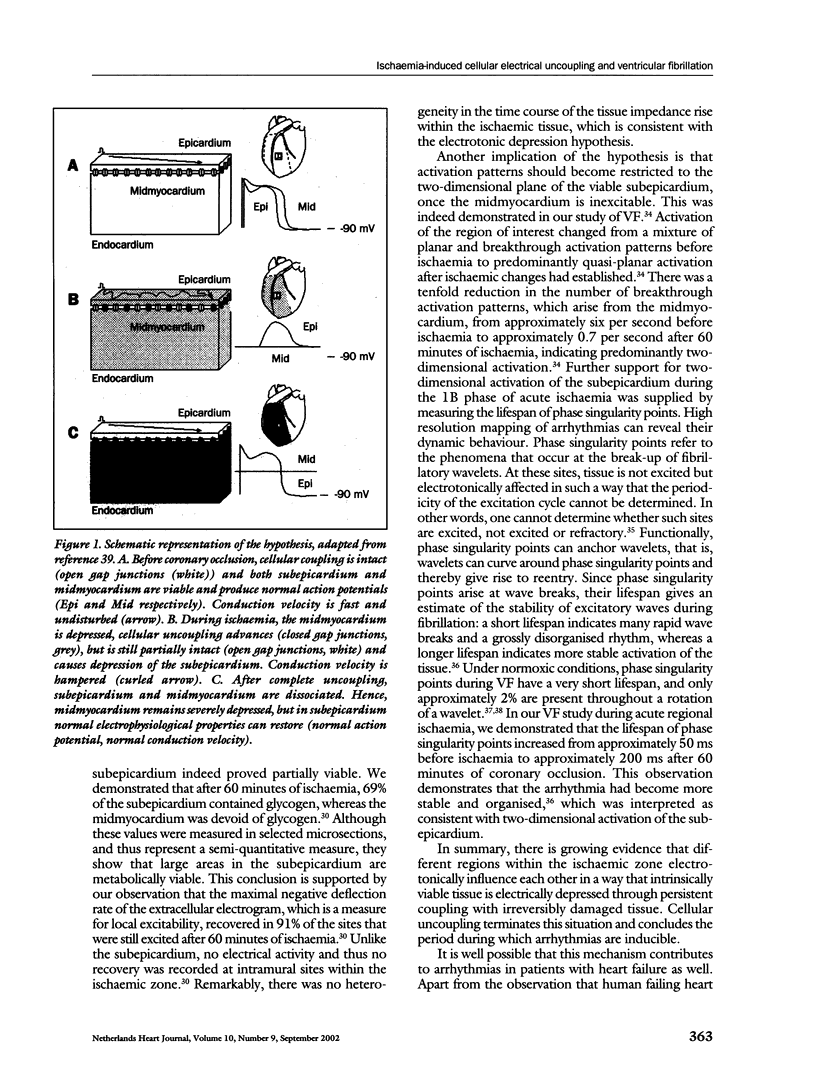
Gap junctions are intercellular communication channels that are permeable for ions and metabolites and are necessary for normal propagation of electrical activation. It has been suggested that closure of gap junctions results in a largely inhomogeneous substrate in which microreentry forms the electrophysiological mechanism for VF. However, there is growing support for the hypothesis that arrhythmias relate to the persistence of residual coupling rather than to the occurrence of uncoupling. With this, the ischaemic midmyocardium can depress the intrinsically viable tissue of the ischaemic subepicardium and subendocardium and cause conduction slowing and block leading to arrhythmias. Progression of uncoupling terminates this interaction and allows the subepicardium and subendocardium to recover. Indeed, electrophysiological properties recover subepicardially whereas the midmyocardial tissue becomes inexcitable. In addition, activation patterns during VF become restricted to the two-dimensional plane of the subepicardium. These observations support the hypothesis of residual coupling as an arrhythmogenic mechanism during the delayed phase of acute ischaemia. Whether this mechanism is equally important in patients with remodelled and failing hearts can at this time only be speculated upon. However, modifying intercellular coupling might turn out a new antiarrhythmic therapy.
Keywords: ischaemia-induced, ventricular fibrillation
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antzelevitch C., Shimizu W., Yan G. X., Sicouri S., Weissenburger J., Nesterenko V. V., Burashnikov A., Di Diego J., Saffitz J., Thomas G. P. The M cell: its contribution to the ECG and to normal and abnormal electrical function of the heart. J Cardiovasc Electrophysiol. 1999 Aug;10(8):1124–1152. doi: 10.1111/j.1540-8167.1999.tb00287.x. [DOI] [PubMed] [Google Scholar]
- Bevans C. G., Kordel M., Rhee S. K., Harris A. L. Isoform composition of connexin channels determines selectivity among second messengers and uncharged molecules. J Biol Chem. 1998 Jan 30;273(5):2808–2816. doi: 10.1074/jbc.273.5.2808. [DOI] [PubMed] [Google Scholar]
- Cinca J., Warren M., Carreño A., Tresànchez M., Armadans L., Gómez P., Soler-Soler J. Changes in myocardial electrical impedance induced by coronary artery occlusion in pigs with and without preconditioning: correlation with local ST-segment potential and ventricular arrhythmias. Circulation. 1997 Nov 4;96(9):3079–3086. doi: 10.1161/01.cir.96.9.3079. [DOI] [PubMed] [Google Scholar]
- Coronel R., Wilms-Schopman F. J., Dekker L. R., Janse M. J. Heterogeneities in [K+]o and TQ potential and the inducibility of ventricular fibrillation during acute regional ischemia in the isolated perfused porcine heart. Circulation. 1995 Jul 1;92(1):120–129. doi: 10.1161/01.cir.92.1.120. [DOI] [PubMed] [Google Scholar]
- Coumel P. The management of clinical arrhythmias. An overview on invasive versus non-invasive electrophysiology. Eur Heart J. 1987 Feb;8(2):92–99. doi: 10.1093/oxfordjournals.eurheartj.a062259. [DOI] [PubMed] [Google Scholar]
- De Mello W. C. Effect of intracellular injection of calcium and strontium on cell communication in heart. J Physiol. 1975 Sep;250(2):231–245. doi: 10.1113/jphysiol.1975.sp011051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekker L. R., Fiolet J. W., VanBavel E., Coronel R., Opthof T., Spaan J. A., Janse M. J. Intracellular Ca2+, intercellular electrical coupling, and mechanical activity in ischemic rabbit papillary muscle. Effects of preconditioning and metabolic blockade. Circ Res. 1996 Aug;79(2):237–246. doi: 10.1161/01.res.79.2.237. [DOI] [PubMed] [Google Scholar]
- Gray R. A., Pertsov A. M., Jalife J. Spatial and temporal organization during cardiac fibrillation. Nature. 1998 Mar 5;392(6671):75–78. doi: 10.1038/32164. [DOI] [PubMed] [Google Scholar]
- Horacek T., Neumann M., von Mutius S., Budden M., Meesmann W. Nonhomogeneous electrophysiological changes and the bimodal distribution of early ventricular arrhythmias during acute coronary artery occlusion. Basic Res Cardiol. 1984 Nov-Dec;79(6):649–667. doi: 10.1007/BF01908383. [DOI] [PubMed] [Google Scholar]
- Janse M. J., Wit A. L. Electrophysiological mechanisms of ventricular arrhythmias resulting from myocardial ischemia and infarction. Physiol Rev. 1989 Oct;69(4):1049–1169. doi: 10.1152/physrev.1989.69.4.1049. [DOI] [PubMed] [Google Scholar]
- Jongsma H. J., Wilders R. Gap junctions in cardiovascular disease. Circ Res. 2000 Jun 23;86(12):1193–1197. doi: 10.1161/01.res.86.12.1193. [DOI] [PubMed] [Google Scholar]
- Kam Y., Kim D. Y., Koo S. K., Joe C. O. Transfer of second messengers through gap junction connexin 43 channels reconstituted in liposomes. Biochim Biophys Acta. 1998 Jul 17;1372(2):384–388. doi: 10.1016/s0005-2736(98)00075-3. [DOI] [PubMed] [Google Scholar]
- Kaplinsky E., Ogawa S., Balke C. W., Dreifus L. S. Two periods of early ventricular arrhythmia in the canine acute myocardial infarction model. Circulation. 1979 Aug;60(2):397–403. doi: 10.1161/01.cir.60.2.397. [DOI] [PubMed] [Google Scholar]
- Kléber A. G., Riegger C. B., Janse M. J. Electrical uncoupling and increase of extracellular resistance after induction of ischemia in isolated, arterially perfused rabbit papillary muscle. Circ Res. 1987 Aug;61(2):271–279. doi: 10.1161/01.res.61.2.271. [DOI] [PubMed] [Google Scholar]
- Lerner D. L., Yamada K. A., Schuessler R. B., Saffitz J. E. Accelerated onset and increased incidence of ventricular arrhythmias induced by ischemia in Cx43-deficient mice. Circulation. 2000 Feb 8;101(5):547–552. doi: 10.1161/01.cir.101.5.547. [DOI] [PubMed] [Google Scholar]
- Löwel H., Lewis M., Hörmann A. Prognostische Bedeutung der Prähospitalphase beim akuten Myokardinfarkt. Ergebnisse des Augsburger Herzinfarktregisters 1985-1988. Dtsch Med Wochenschr. 1991 May 10;116(19):729–733. doi: 10.1055/s-2008-1063671. [DOI] [PubMed] [Google Scholar]
- Menken U., Wiegand V., Bucher P., Meesmann W. Prophylaxis of ventricular fibrillation after acute experimental coronary occlusion by chronic beta-adrenoceptor blockade with atenolol. Cardiovasc Res. 1979 Oct;13(10):588–594. doi: 10.1093/cvr/13.10.588. [DOI] [PubMed] [Google Scholar]
- Ohara T., Ohara K., Cao J. M., Lee M. H., Fishbein M. C., Mandel W. J., Chen P. S., Karagueuzian H. S. Increased wave break during ventricular fibrillation in the epicardial border zone of hearts with healed myocardial infarction. Circulation. 2001 Mar 13;103(10):1465–1472. doi: 10.1161/01.cir.103.10.1465. [DOI] [PubMed] [Google Scholar]
- Peters N. S., Coromilas J., Severs N. J., Wit A. L. Disturbed connexin43 gap junction distribution correlates with the location of reentrant circuits in the epicardial border zone of healing canine infarcts that cause ventricular tachycardia. Circulation. 1997 Feb 18;95(4):988–996. doi: 10.1161/01.cir.95.4.988. [DOI] [PubMed] [Google Scholar]
- Peters N. S., Green C. R., Poole-Wilson P. A., Severs N. J. Reduced content of connexin43 gap junctions in ventricular myocardium from hypertrophied and ischemic human hearts. Circulation. 1993 Sep;88(3):864–875. doi: 10.1161/01.cir.88.3.864. [DOI] [PubMed] [Google Scholar]
- Rohr S., Kucera J. P., Kléber A. G. Slow conduction in cardiac tissue, I: effects of a reduction of excitability versus a reduction of electrical coupling on microconduction. Circ Res. 1998 Oct 19;83(8):781–794. doi: 10.1161/01.res.83.8.781. [DOI] [PubMed] [Google Scholar]
- Shaw R. M., Rudy Y. Ionic mechanisms of propagation in cardiac tissue. Roles of the sodium and L-type calcium currents during reduced excitability and decreased gap junction coupling. Circ Res. 1997 Nov;81(5):727–741. doi: 10.1161/01.res.81.5.727. [DOI] [PubMed] [Google Scholar]
- Smith W. T., 4th, Fleet W. F., Johnson T. A., Engle C. L., Cascio W. E. The Ib phase of ventricular arrhythmias in ischemic in situ porcine heart is related to changes in cell-to-cell electrical coupling. Experimental Cardiology Group, University of North Carolina. Circulation. 1995 Nov 15;92(10):3051–3060. doi: 10.1161/01.cir.92.10.3051. [DOI] [PubMed] [Google Scholar]
- Sugiura H., Toyama J., Tsuboi N., Kamiya K., Kodama I. ATP directly affects junctional conductance between paired ventricular myocytes isolated from guinea pig heart. Circ Res. 1990 Apr;66(4):1095–1102. doi: 10.1161/01.res.66.4.1095. [DOI] [PubMed] [Google Scholar]
- Taggart P., Sutton P. M., Opthof T., Coronel R., Trimlett R., Pugsley W., Kallis P. Inhomogeneous transmural conduction during early ischaemia in patients with coronary artery disease. J Mol Cell Cardiol. 2000 Apr;32(4):621–630. doi: 10.1006/jmcc.2000.1105. [DOI] [PubMed] [Google Scholar]
- Tan R. C., Joyner R. W. Electrotonic influences on action potentials from isolated ventricular cells. Circ Res. 1990 Nov;67(5):1071–1081. doi: 10.1161/01.res.67.5.1071. [DOI] [PubMed] [Google Scholar]
- Verkerk A. O., Veldkamp M. W., Coronel R., Wilders R., van Ginneken A. C. Effects of cell-to-cell uncoupling and catecholamines on Purkinje and ventricular action potentials: implications for phase-1b arrhythmias. Cardiovasc Res. 2001 Jul;51(1):30–40. doi: 10.1016/s0008-6363(01)00246-2. [DOI] [PubMed] [Google Scholar]
- Waalewijn R. A., de Vos R., Koster R. W. Out-of-hospital cardiac arrests in Amsterdam and its surrounding areas: results from the Amsterdam resuscitation study (ARREST) in 'Utstein' style. Resuscitation. 1998 Sep;38(3):157–167. doi: 10.1016/s0300-9572(98)00102-6. [DOI] [PubMed] [Google Scholar]
- Wilensky R. L., Tranum-Jensen J., Coronel R., Wilde A. A., Fiolet J. W., Janse M. J. The subendocardial border zone during acute ischemia of the rabbit heart: an electrophysiologic, metabolic, and morphologic correlative study. Circulation. 1986 Nov;74(5):1137–1146. doi: 10.1161/01.cir.74.5.1137. [DOI] [PubMed] [Google Scholar]
- Zheng Z. J., Croft J. B., Giles W. H., Mensah G. A. Sudden cardiac death in the United States, 1989 to 1998. Circulation. 2001 Oct 30;104(18):2158–2163. doi: 10.1161/hc4301.098254. [DOI] [PubMed] [Google Scholar]
- Zipes D. P., Wellens H. J. Sudden cardiac death. Circulation. 1998 Nov 24;98(21):2334–2351. doi: 10.1161/01.cir.98.21.2334. [DOI] [PubMed] [Google Scholar]
- de Bakker J. M., van Capelle F. J., Janse M. J., Tasseron S., Vermeulen J. T., de Jonge N., Lahpor J. R. Slow conduction in the infarcted human heart. 'Zigzag' course of activation. Circulation. 1993 Sep;88(3):915–926. doi: 10.1161/01.cir.88.3.915. [DOI] [PubMed] [Google Scholar]
- de Groot J. R., Wilms-Schopman F. J., Opthof T., Remme C. A., Coronel R. Late ventricular arrhythmias during acute regional ischemia in the isolated blood perfused pig heart. Role of electrical cellular coupling. Cardiovasc Res. 2001 May;50(2):362–372. doi: 10.1016/s0008-6363(01)00222-x. [DOI] [PubMed] [Google Scholar]