Abstract
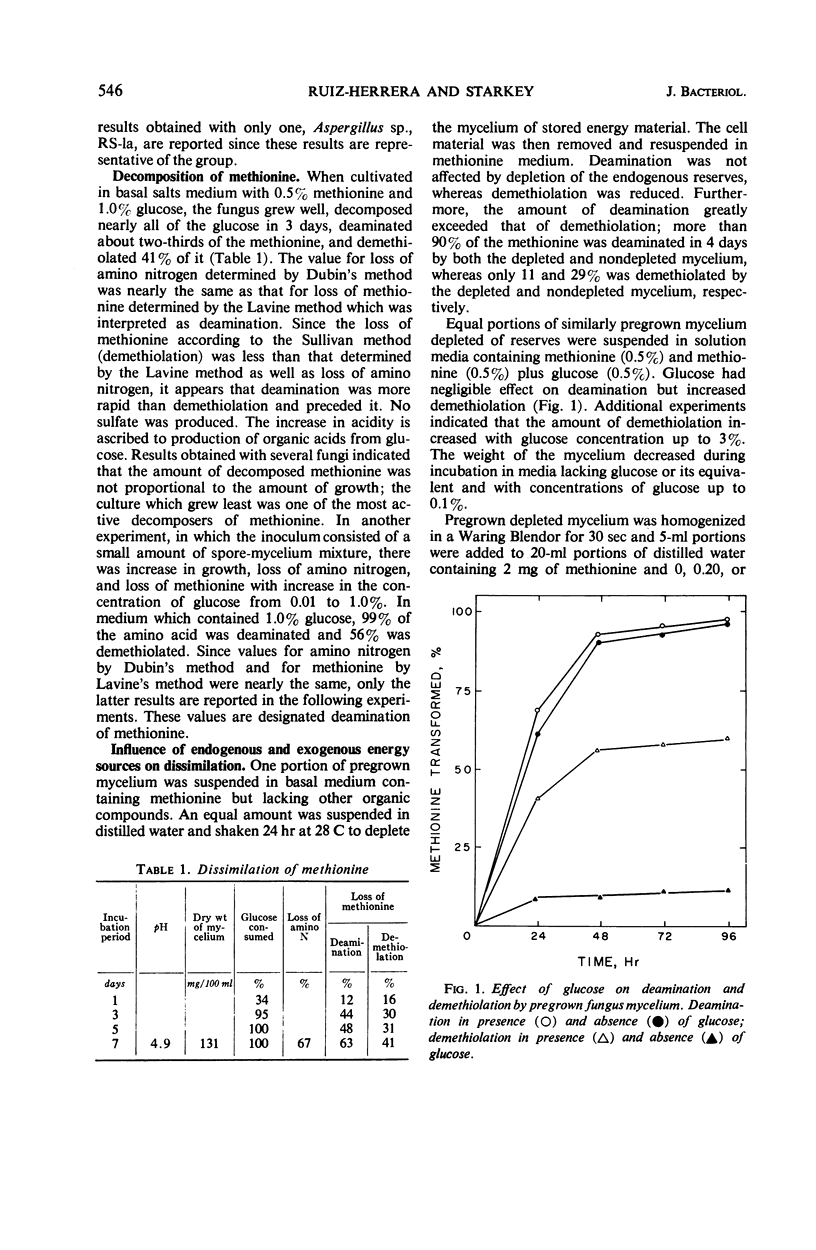
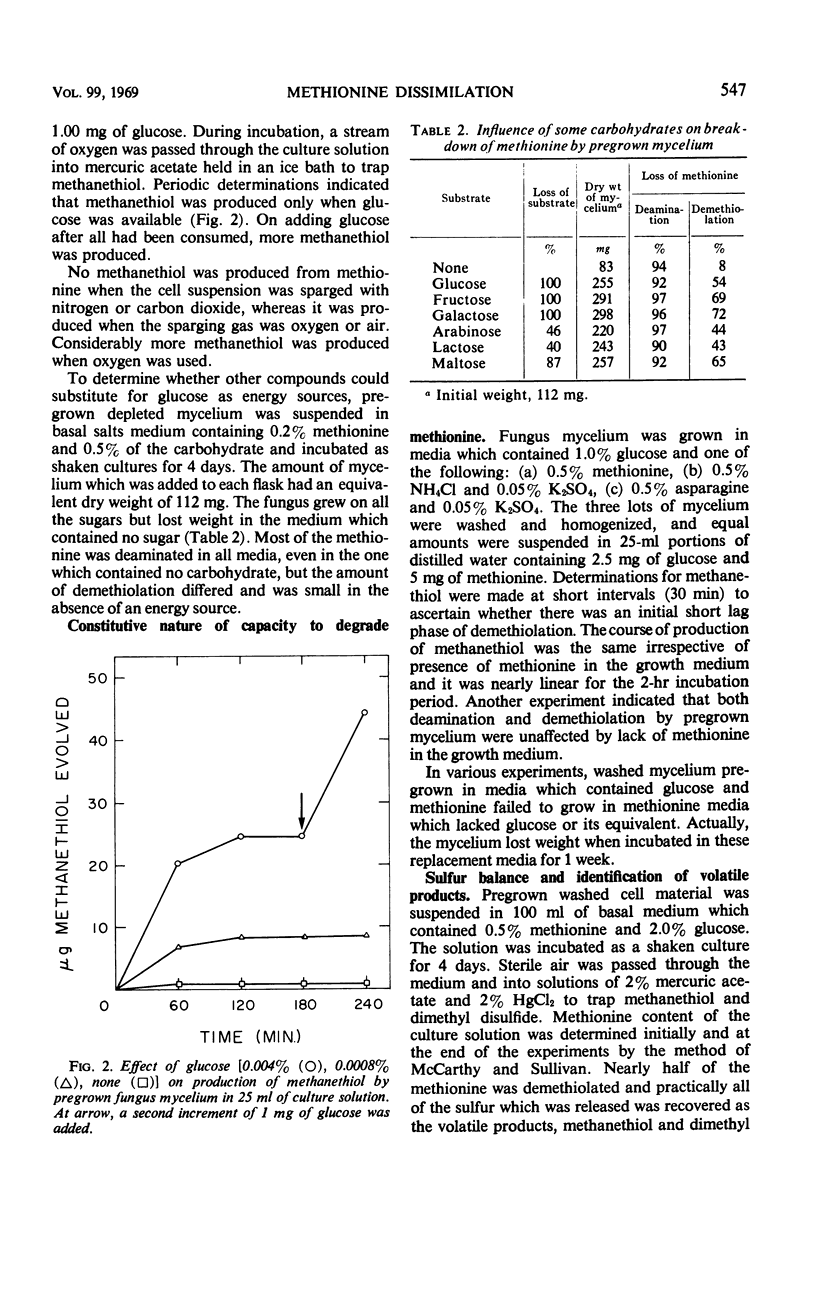
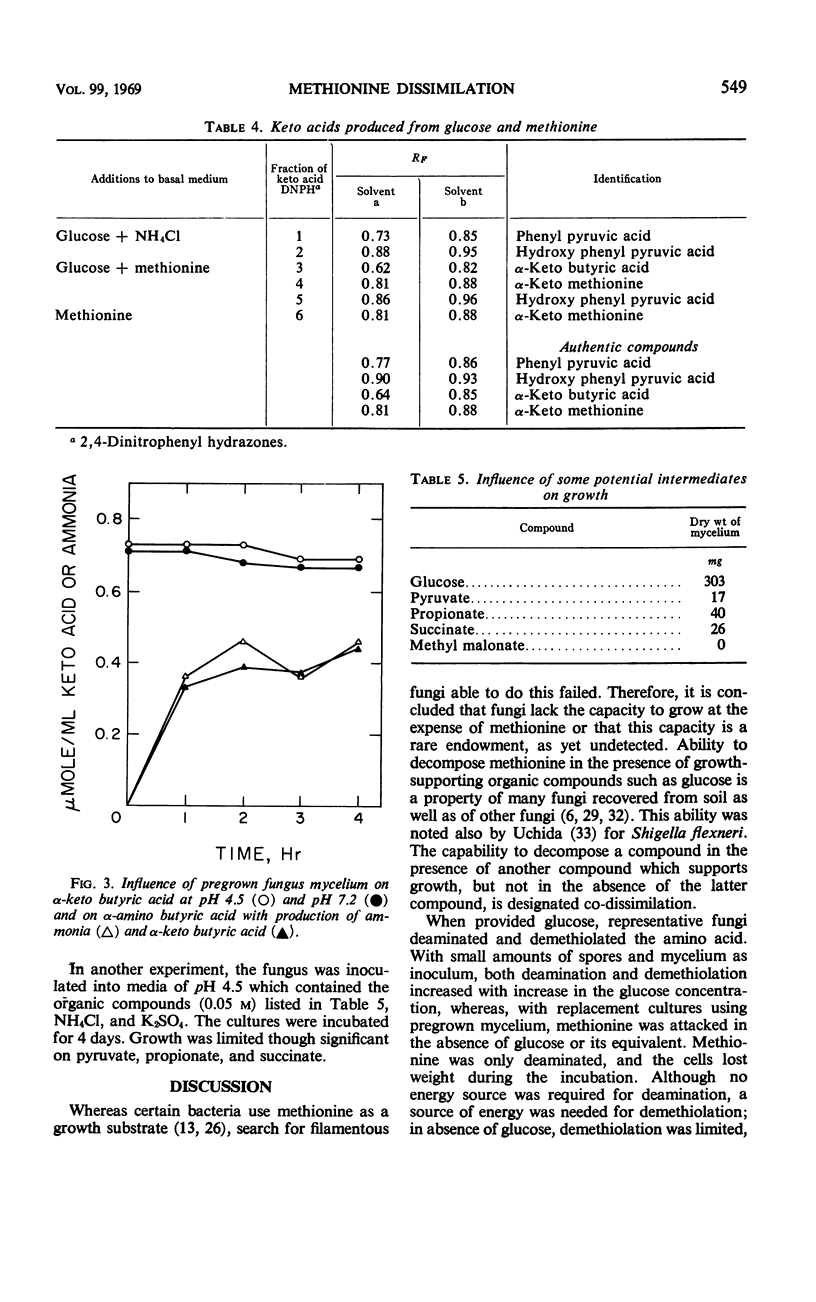
Soil fungi that attacked methionine required a utilizable source of energy such as glucose for growth. This is an example of co-dissimilation. Experiments with one of the fungi, representative of the group, are reported. In the absence of glucose, pregrown mycelium, even when depleted of energy reserves, oxidatively deaminated methionine with accumulation of α-keto-γ-methyl mercapto butyric acid and α-hydroxy-γ-methyl mercapto butyric acid. When glucose was provided, all of the sulfur of methionine was released as methanethiol, part of which was oxidized to dimethyl disulfide. No sulfate, sulfide, or hydrosulfide products were detected. Evidence was obtained that deaminase and demethiolase were constitutive. Deamination preceded demethiolation and α-keto butyric acid accumulated as a product of the two reactions. Other carbon residues were α-hydroxy butyric acid and α-amino butyric acid. Inability of the fungus to metabolize α-keto butyrate was responsible for its inability to utilize methionine as a source of carbon and energy. Several other fungi isolated from soil grew on α-amino butyrate but could not grow on methionine owing to inability to demethiolate it.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DUBIN D. T. The assay and characterization of amines by 2,4-dinitrofluorobenzene. J Biol Chem. 1960 Mar;235:783–786. [PubMed] [Google Scholar]
- GRUNERT R. R., PHILLIPS P. H. Sodium and its relation to alloxan diabetes and glutathione. J Biol Chem. 1949 Dec;181(2):821–827. [PubMed] [Google Scholar]
- KING T. E., CHELDELIN V. H. Pyruvic carboxylase of Acetobacter suboxydase. J Biol Chem. 1954 Jun;208(2):821–831. [PubMed] [Google Scholar]
- MEISTER A. Enzymatic preparation of alpha-keto acids. J Biol Chem. 1952 May;197(1):309–317. [PubMed] [Google Scholar]
- MITSUHASHI S. Decomposition of thioether derivatives by bacteria; methylmercaptan formation and the properties of the responsible enzyme. Jpn J Exp Med. 1949 Sep;20(2):211–222. [PubMed] [Google Scholar]
- REED L. J., LEACH F. R., KOIKE M. Studies on a lipoic acid-activating system. J Biol Chem. 1958 May;232(1):123–142. [PubMed] [Google Scholar]
- SENTHESHANMUGANATHAN S., NICKERSON W. J. Transaminase and D-amino acid oxidase of Trigonopsis variabilis. J Gen Microbiol. 1962 Mar;27:465–471. doi: 10.1099/00221287-27-3-465. [DOI] [PubMed] [Google Scholar]
- Segal W., Starkey R. L. Microbial decomposition of methionine and identity of the resulting sulfur products. J Bacteriol. 1969 Jun;98(3):908–913. doi: 10.1128/jb.98.3.908-913.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WIESENDANGER S., NISMAN B. La Lméthionine démercapto-désaminase: un nouvel enzyme à pyridoxal-phosphate. C R Hebd Seances Acad Sci. 1953 Oct 5;237(14):764–765. [PubMed] [Google Scholar]


