Abstract
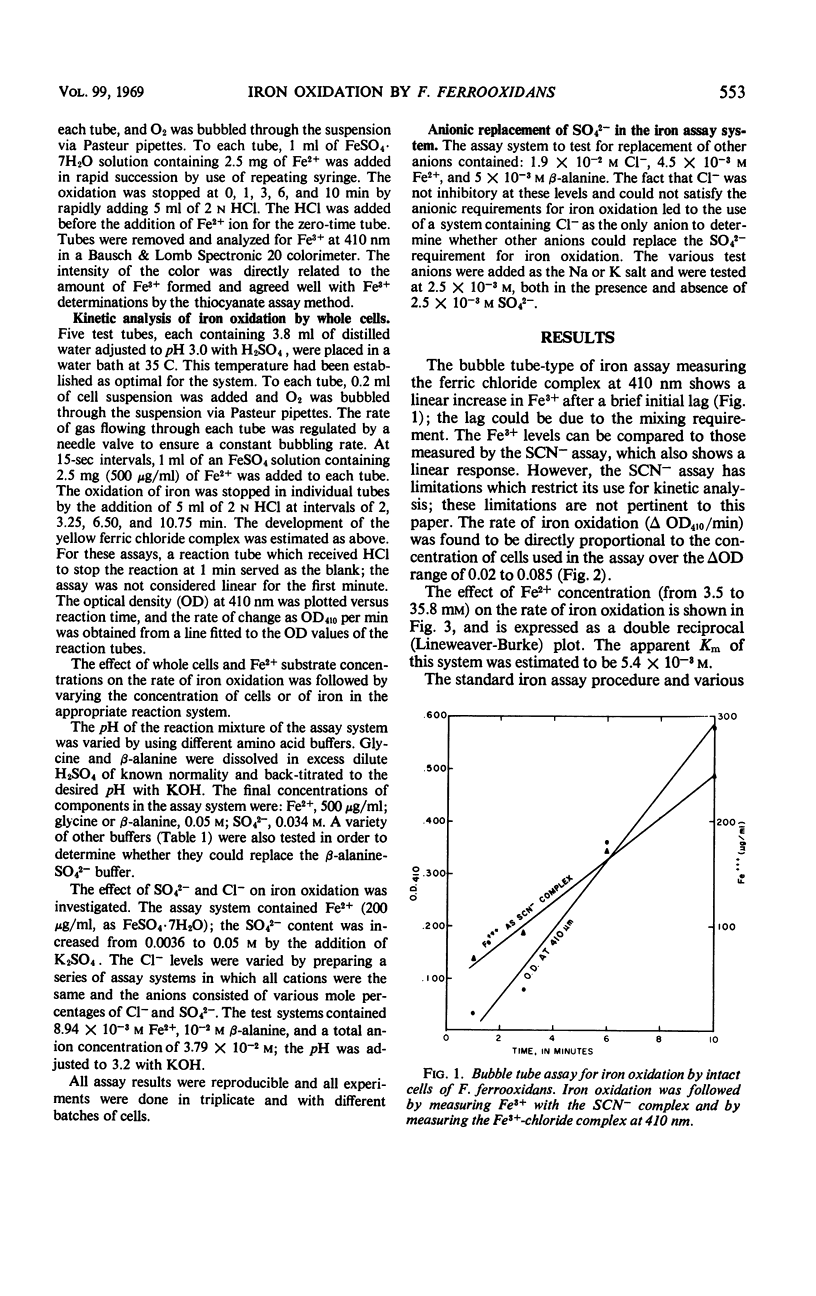
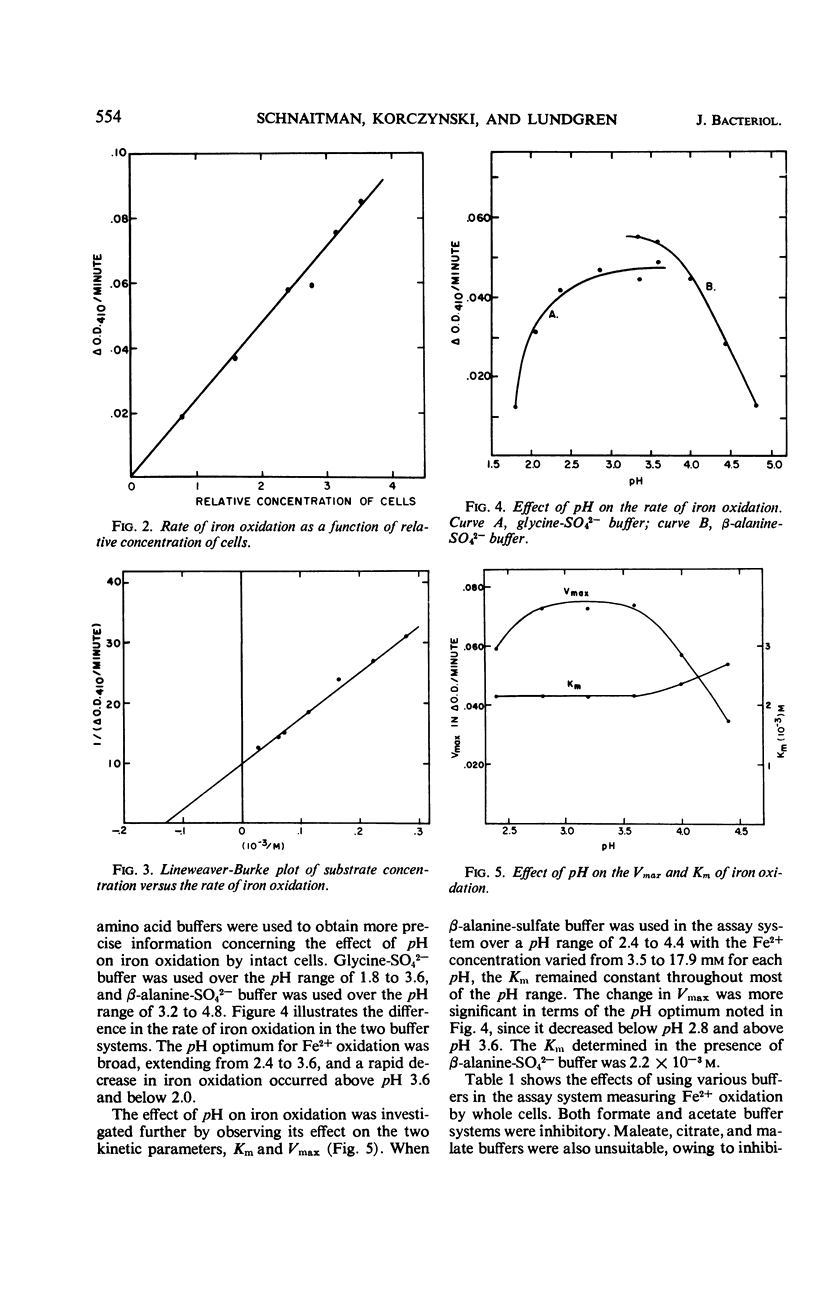
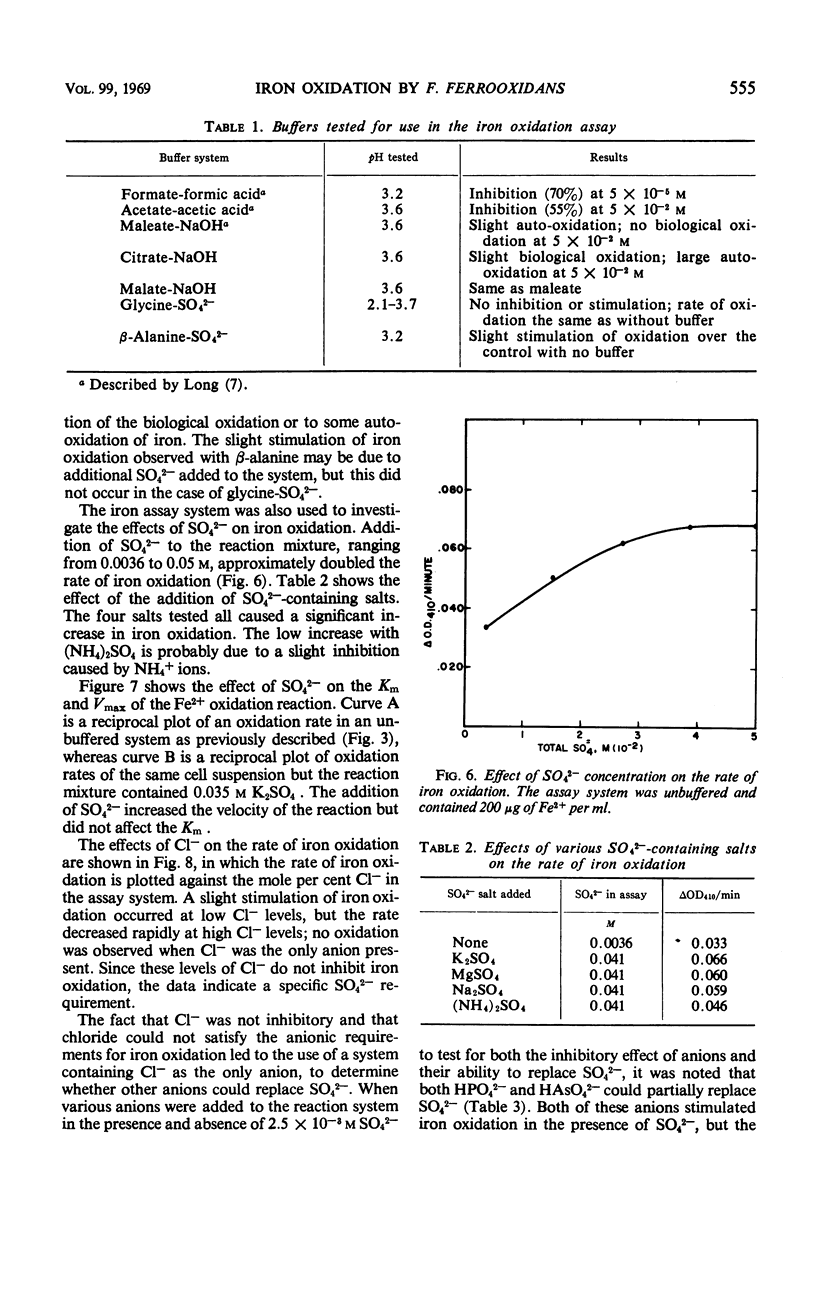
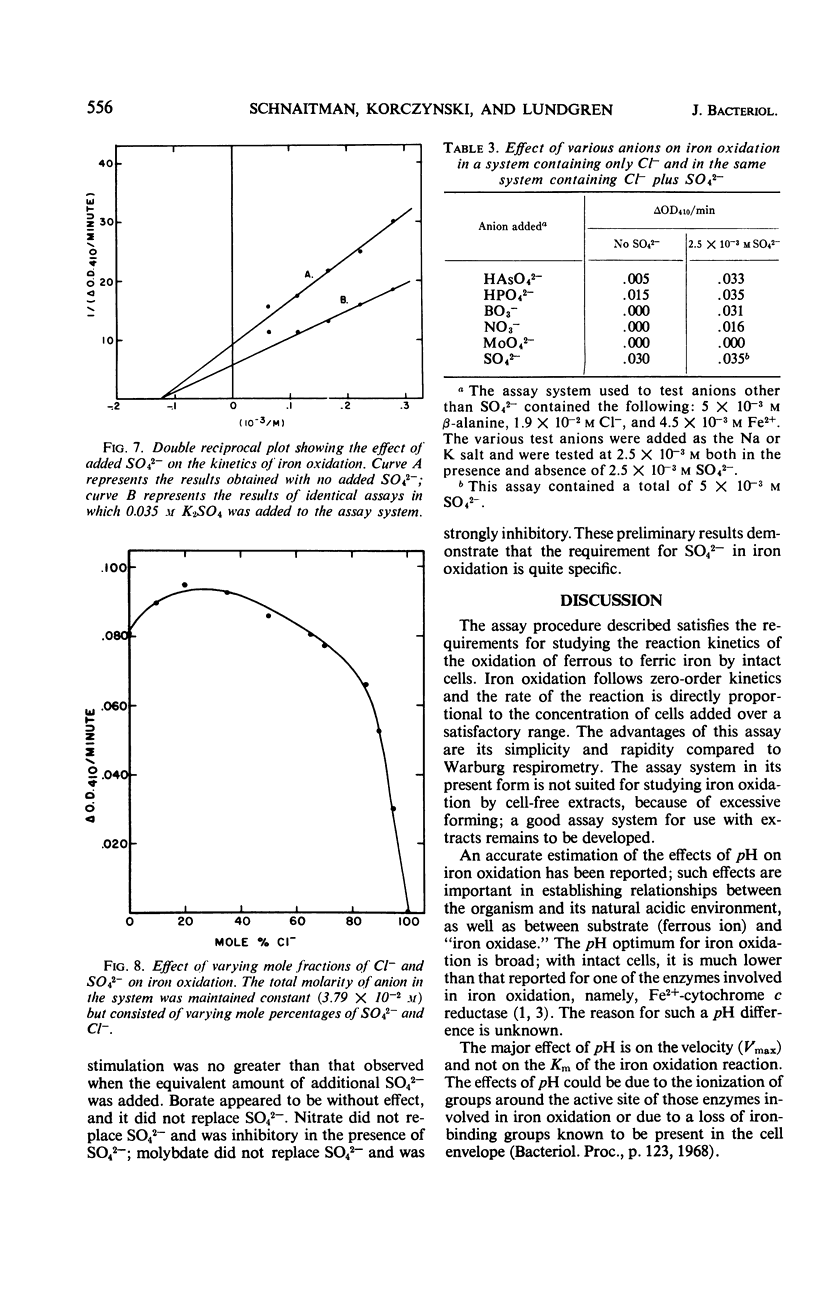
A colorimetric assay was developed for studying the kinetics of iron oxidation with whole cells of the chemoautotroph, Ferrobacillus ferrooxidans. The assay was more advantageous than the conventional method of Warburg manometry because of its simplicity, rapidity, and the small amount of cells required. The assay measured Fe3+ as a chloride complex which absorbs at 410 nm. Kinetic analysis showed the apparent Km for iron oxidation to be 5.4 × 10−3m in an unbuffered system and 2.2 × 10−3m in the presence of β-alanine-SO42− buffer. Glycine and β-alanine buffers were used in the measurement of the pH optimum for iron oxidation; the optimum ranged from 2.5 to 3.8. The effect of pH was primarily on the Vmax while the Km remained constant. Added SO42− was found to stimulate iron oxidation by increasing the Vmax of iron oxidation by whole cells, but it did not affect the Km. Results of assays of iron oxidation in systems containing various mole percentages of SO42− and Cl− indicated that Cl− did not inhibit iron oxidation but that SO42− was required. Sulfate could be partially replaced by HPO42− and HAsO42− but not by BO3−, MoO42−, NO3−, or Cl−; formate and MoO42− inhibited iron oxidation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLAYLOCK B. A., NASON A. ELECTRON TRANSPORT SYSTEMS OF THE CHEMOAUTOTROPH FERROBACILLUS FERROOXIDANS. I. CYTOCHROME C-CONTAINING IRON OXIDASE. J Biol Chem. 1963 Oct;238:3453–3462. [PubMed] [Google Scholar]
- BRALEY S. A., Sr, KINSEL N. A., LEATHEN W. W. Ferrobacillus ferrooxidans: a chemosynthetic autotrophic Bacterium. J Bacteriol. 1956 Nov;72(5):700–704. doi: 10.1128/jb.72.5.700-704.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUGAN P. R., LUNDGREN D. G. ENERGY SUPPLY FOR THE CHEMOAUTOTROPH FERROBACILLUS FERROOXIDANS. J Bacteriol. 1965 Mar;89:825–834. doi: 10.1128/jb.89.3.825-834.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Din G. A., Suzuki I., Lees H. Ferrous iron oxidation by Ferrobacillus ferrooxidans. Purification and properties of Fe++-cytochrome c reductase. Can J Biochem. 1967 Oct;45(10):1523–1546. doi: 10.1139/o67-183. [DOI] [PubMed] [Google Scholar]
- Lazaroff N. SULFATE REQUIREMENT FOR IRON OXIDATION BY THIOBACILLUS FERROOXIDANS. J Bacteriol. 1963 Jan;85(1):78–83. doi: 10.1128/jb.85.1.78-83.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margalith P., Silver M., Lundgren D. G. Sulfur oxidation by the iron bacterium Ferrobacillus ferrooxidans. J Bacteriol. 1966 Dec;92(6):1706–1709. doi: 10.1128/jb.92.6.1706-1709.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SILVERMAN M. P., LUNDGREN D. G. Studies on the chemoautotrophic iron bacterium Ferrobacillus ferrooxidans. I. An improved medium and a harvesting procedure for securing high cell yields. J Bacteriol. 1959 May;77(5):642–647. doi: 10.1128/jb.77.5.642-647.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]


