Abstract
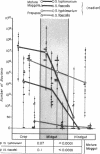
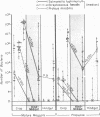
Survivorship of Salmonella typhimurium, Streptococcus faecalis, Proteus mirabilis, and Pseudomonas aeruginosa was studied in dibiotic and tribiotic interactions in vitro and in various regions of the digestive tract of the blow fly, Calliphora vicina. In dibiotic interactions, Salmonella typhimurium dominated Streptococcus faecalis and was dominated by P. mirabilis, but in neither case was it eliminated from the larval gut. In tribiotic interactions, there was synergic suppression and a definite trend toward elimination of Salmonella typhimurium from the gut. This trend approaches but does not match the total exclusion of S. typhimurium from the gut of maggots with a normal flora. Bacterial survival in the gut of the fly is discussed in relation to doubling time, sweep-out rate of the maggot and prepupal gut, and the midgut bactericide.
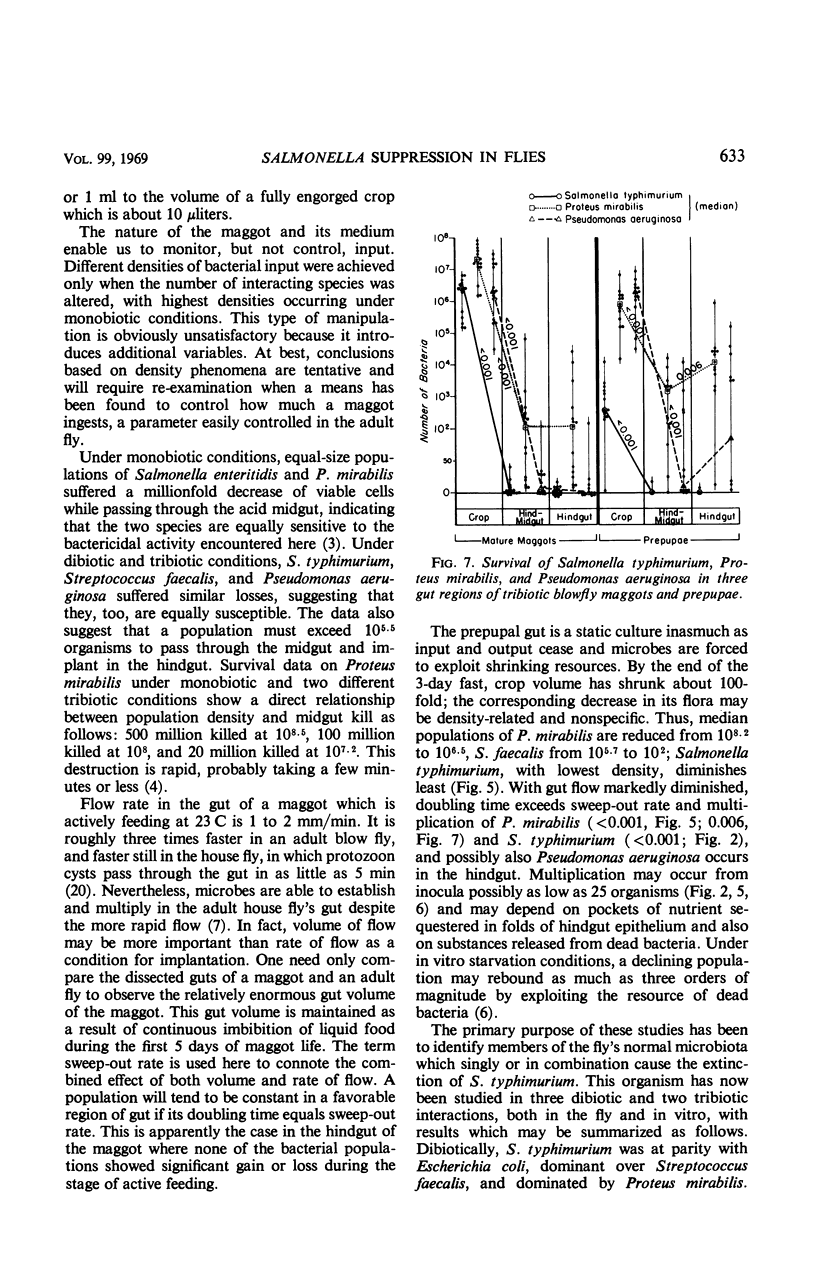
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- GREENBERG B., MIGGIANO V. Host-contaminant biology of muscoid files. IV. Microbial competition in a blowfly. J Infect Dis. 1963 Jan-Feb;112:37–46. doi: 10.1093/infdis/112.1.37. [DOI] [PubMed] [Google Scholar]
- Greenberg B. Model for destruction of bacteria in the midgut of blow fly maggots. J Med Entomol. 1968 Feb;5(1):31–38. doi: 10.1093/jmedent/5.1.31. [DOI] [PubMed] [Google Scholar]
- HAWLEY J. E., PENNER L. R., WEDBERG S. E., KULP W. The role of the house fly, Musca domestica, in the multiplication of certain enteric bacteria. Am J Trop Med Hyg. 1951 Sep;31(5):572–582. doi: 10.4269/ajtmh.1951.s1-31.572. [DOI] [PubMed] [Google Scholar]
- LOUCH C. D. Increased resistance to Trichinella spiralis in the laboratory rat following infection with Nippostrongylus muris. J Parasitol. 1962 Feb;48:24–26. [PubMed] [Google Scholar]
- MEYNELL G. G. Antibacterial mechanisms of the mouse gut. II. The role of Eh and volatile fatty acids in the normal gut. Br J Exp Pathol. 1963 Apr;44:209–219. [PMC free article] [PubMed] [Google Scholar]
- Malmquist W. A., Fernelius A. L., Gutekunst D. E. Interference of bovine viral diarrhea virus by hog cholera virus in swine kidney cell cultures. Am J Vet Res. 1965 Nov;26(115):1316–1327. [PubMed] [Google Scholar]
- Mason M. M. A Comparison of the Maximal Growth Rates of Various Bacteria under Optimal Conditions. J Bacteriol. 1935 Feb;29(2):103–110. doi: 10.1128/jb.29.2.103-110.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POHJANPELTO P., COOPER P. D. INTERFERENCE BETWEEN POLIOVIRUSES INDUCED BY STRAINS THAT CANNOT MULTIPLY. Virology. 1965 Mar;25:350–357. doi: 10.1016/0042-6822(65)90054-1. [DOI] [PubMed] [Google Scholar]
- SCHNEIERSON S. S., SHORE B. ANTIBACTERIAL ACTIVITY OF HERPES SIMPLEX VIRUS GROWN IN TISSUE CULTURE. Nature. 1963 Aug 17;199:721–722. doi: 10.1038/199721a0. [DOI] [PubMed] [Google Scholar]
- SHINDALA A., BUNGAY H. R., 3rd, KRIEG N. R., CULBERT K. MIXED-CULTURE INTERACTIONS. I. COMMENSALISM OF PROTEUS VULGARIS WITH SACCHAROMYCES CEREVISIAE IN CONTINUOUS CULTURE. J Bacteriol. 1965 Mar;89:693–696. doi: 10.1128/jb.89.3.693-696.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHIRMAN G. A. Interaction of viruses in the intestinal tract of man. II. Interference between poliovirus vaccine strains. Acta Virol. 1961 Nov;5:359–366. [PubMed] [Google Scholar]
- SOMERSON N. L., COOK M. K. SUPPRESSION OF ROUS SARCOMA VIRUS GROWTH IN TISSUE CULTURES BY MYCOPLASMA ORALE. J Bacteriol. 1965 Aug;90:534–540. doi: 10.1128/jb.90.2.534-540.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]