Abstract
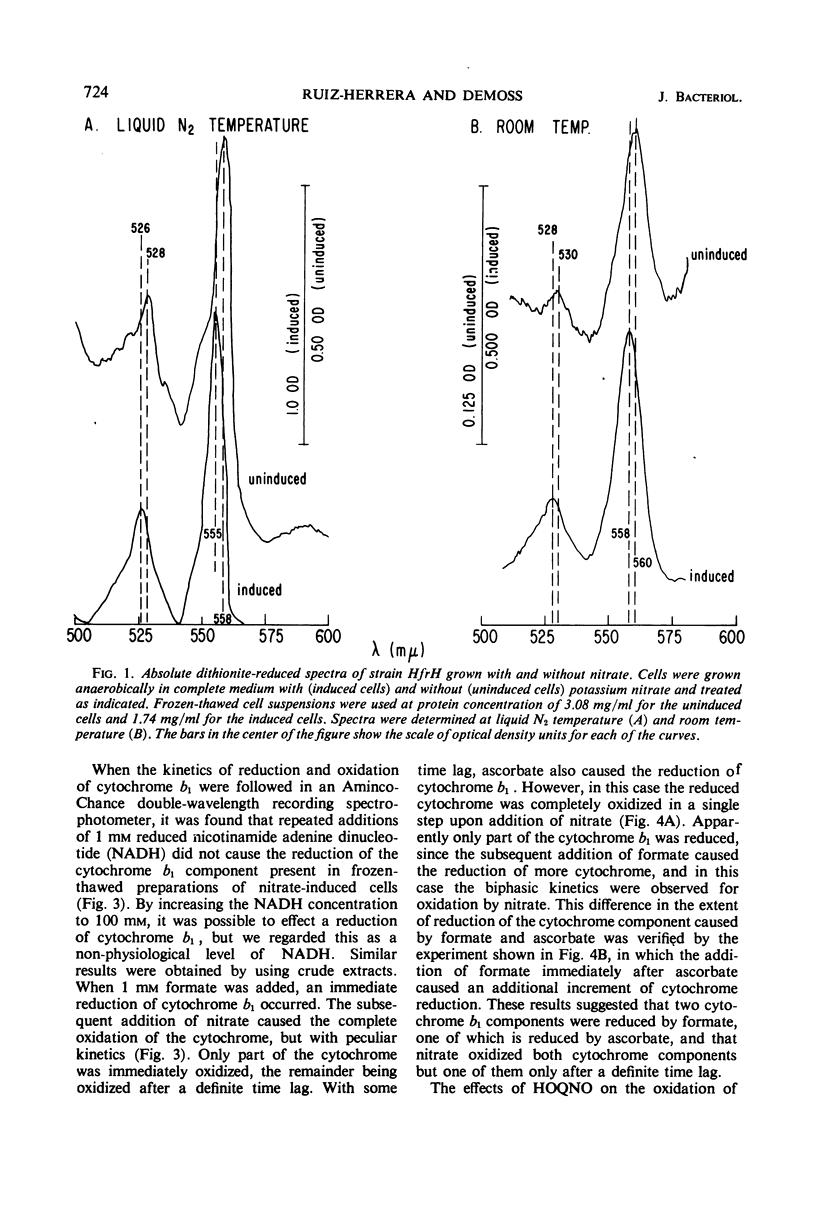
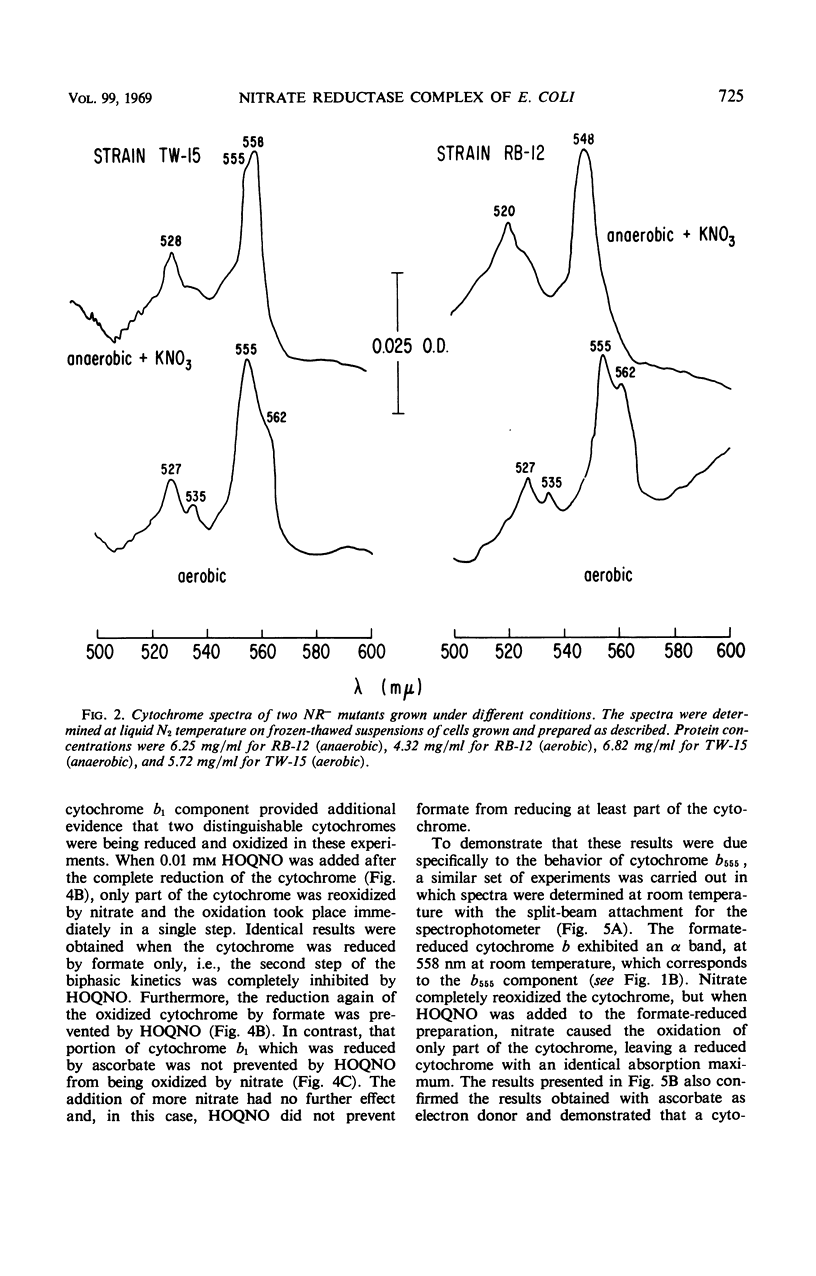
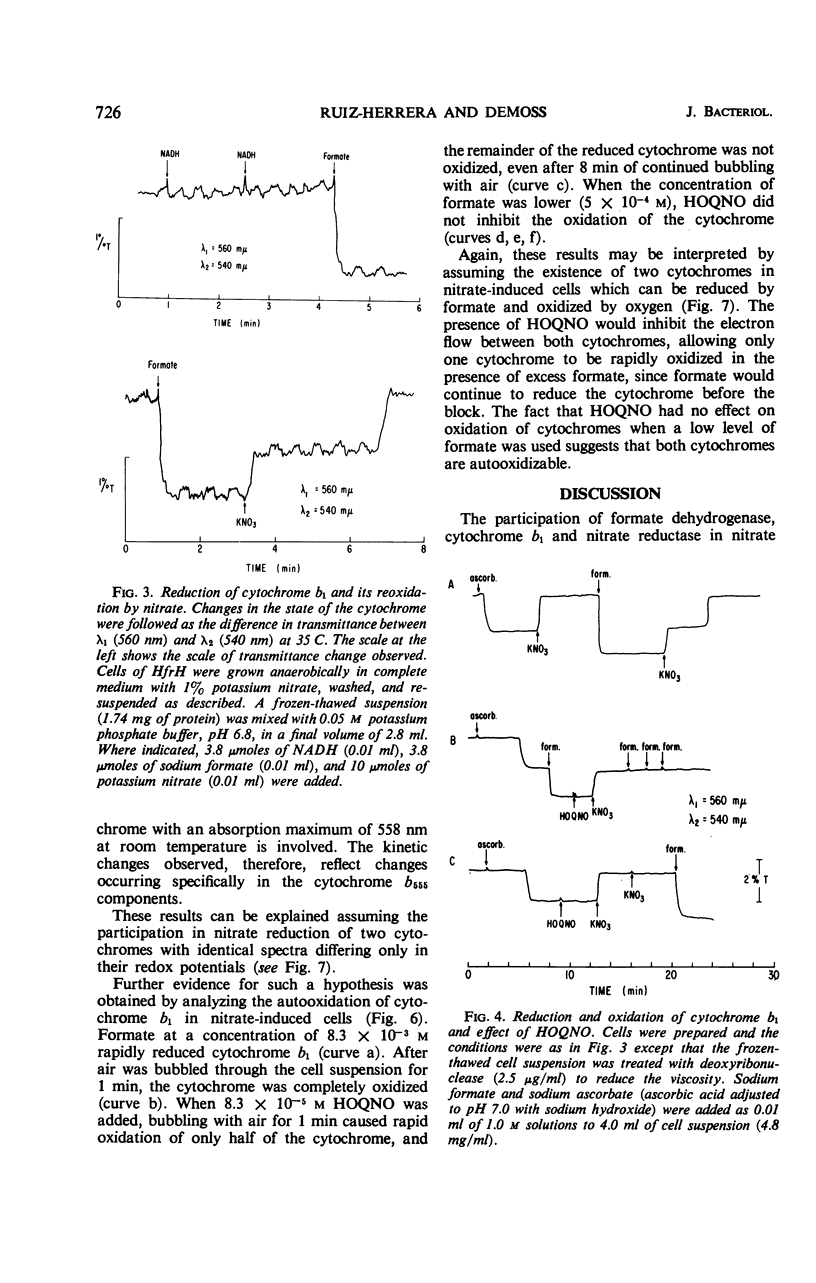
The participation of distinct formate dehydrogenases and cytochrome components in nitrate reduction by Escherichia coli was studied. The formate dehydrogenase activity present in extracts prepared from nitrate-induced cells of strain HfrH was active with various electron acceptors, including methylene blue, phenazine methosulfate, and benzyl viologen. Certain mutants which are unable to reduce nitrate had low or undetectable levels of formate dehydrogenase activity assayed with methylene blue or phenazine methosulfate as electron acceptor. Of nine such mutants, five produced gas when grown anaerobically without nitrate and possessed a benzyl viologen-linked formate dehydrogenase activity, suggesting that distinct formate dehydrogenases participate in the nitrate reductase and formic hydrogenlyase systems. The other four mutants formed little gas when grown anaerobically in the absence of nitrate and lacked the benzyl viologen-linked formate dehydrogenase as well as the methylene blue or phenazine methosulfate-linked activity. The cytochrome b1 present in nitrate-induced cells was distinguished by its spectral properties and its genetic control from the major cytochrome b1 components of aerobic cells and of cells grown anaerobically in the absence of nitrate. The nitrate-specific cytochrome b1 was completely and rapidly reduced by 1 mm formate but was not reduced by 1 mm reduced nicotinamide adenine dinucleotide; ascorbate reduced only part of the cytochrome b1 which was reduced by formate. When nitrate was added, the formate-reduced cytochrome b1 was oxidized with biphasic kinetics, but the ascorbate-reduced cytochrome b1 was oxidized with monophasic kinetics. The inhibitory effects of n-heptyl hydroxyquinoline-N-oxide on the oxidation of cytochrome b1 by nitrate provided evidence that the nitrate-specific cytochrome is composed of two components which have different redox potentials but identical spectral properties. We conclude from these studies that nitrate reduction in E. coli is mediated by the sequential operation of a specific formate dehydrogenase, two specific cytochrome b1 components, and nitrate reductase.
Full text
PDF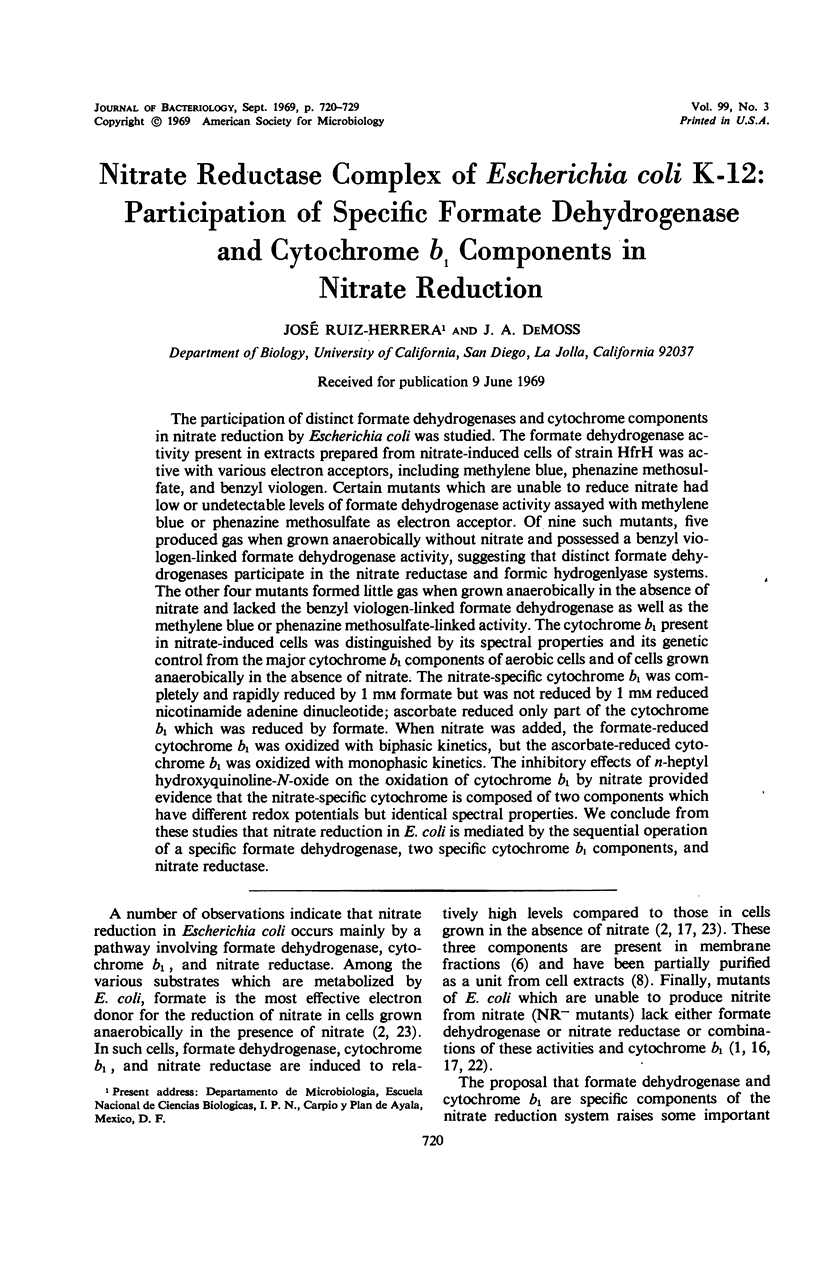





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azoulay E., Puig J., Pichinoty F. Alteration of respiratory particles by mutation in Escherichia coli K 12. Biochem Biophys Res Commun. 1967 Apr 20;27(2):270–274. doi: 10.1016/s0006-291x(67)80073-1. [DOI] [PubMed] [Google Scholar]
- Cole J. A., Wimpenny J. W. Metabolic pathways for nitrate reduction in Escherichia coli. Biochim Biophys Acta. 1968 Jul 16;162(1):39–48. doi: 10.1016/0005-2728(68)90212-0. [DOI] [PubMed] [Google Scholar]
- DEEB S. S., HAGER L. P. CRYSTALLINE CYTOCHROME B1 FROM ESCHERICHIA COLI. J Biol Chem. 1964 Apr;239:1024–1031. [PubMed] [Google Scholar]
- GRAY C. T., GEST H. BIOLOGICAL FORMATION OF MOLECULAR HYDROGEN. Science. 1965 Apr 9;148(3667):186–192. doi: 10.1126/science.148.3667.186. [DOI] [PubMed] [Google Scholar]
- Gray C. T., Wimpenny J. W., Hughes D. E., Mossman M. R. Regulation of metabolism in facultative bacteria. I. Structural and functional changes in Escherichia coli associated with shifts between the aerobic and anaerobic states. Biochim Biophys Acta. 1966 Mar 28;117(1):22–32. doi: 10.1016/0304-4165(66)90148-6. [DOI] [PubMed] [Google Scholar]
- ITAGAKI E., FUJITA T., SATO R. Solubilization and some properties of formic dehydrogenase from Escherichia coli. Biochem Biophys Res Commun. 1961 May 15;5:30–34. doi: 10.1016/0006-291x(61)90075-4. [DOI] [PubMed] [Google Scholar]
- Keilin D., Harpley C. H. Cytochrome system in Bacterium coli commune. Biochem J. 1941 Jun;35(5-6):688–692. doi: 10.1042/bj0350688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LINNANE A. W., WRIGLEY C. W. FRAGMENTATION OF THE ELECTRON TRANSPORT CHAIN OF ESCHERICHIA COLI. PREPARATION OF A SOLUBLE FORMATE DEHYDROGENASE-CYTOCHROME B1 COMPLEX. Biochim Biophys Acta. 1963 Nov 8;77:408–418. doi: 10.1016/0006-3002(63)90515-8. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- NICHOLAS D. J., NASON A. Diphosphopyridine nucleotide-nitrate reductase from Escherichia coli. J Bacteriol. 1955 May;69(5):580–583. doi: 10.1128/jb.69.5.580-583.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hara J., Gray C. T. Defects in formate hydrogenlyase in nitrate-negative mutants of Escherichia coli. Biochem Biophys Res Commun. 1967 Sep 27;28(6):951–957. doi: 10.1016/0006-291x(67)90072-1. [DOI] [PubMed] [Google Scholar]
- PECK H. D., Jr, GEST H. Formic dehydrogenase and the hydrogenlyase enzyme complex in coli-aerogenes bacteria. J Bacteriol. 1957 Jun;73(6):706–721. doi: 10.1128/jb.73.6.706-721.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puig J., Azoulay E. Etude génétique et biochimique des mutants résistant au Clo minus 3 (gènes chl A, chl B, chl C) C R Acad Sci Hebd Seances Acad Sci D. 1967 Apr 10;264(15):1916–1918. [PubMed] [Google Scholar]
- Ruiz-Herrera J., Showe M. K., DeMoss J. A. Nitrate reductase complex of Escherichia coli K-12: isolation and characterization of mutants unable to reduce nitrate. J Bacteriol. 1969 Mar;97(3):1291–1297. doi: 10.1128/jb.97.3.1291-1297.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH L. Bacterial cytochromes. Bacteriol Rev. 1954 Jun;18(2):106–130. doi: 10.1128/br.18.2.106-130.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Showe M. K., DeMoss J. A. Localization and regulation of synthesis of nitrate reductase in Escherichia coli. J Bacteriol. 1968 Apr;95(4):1305–1313. doi: 10.1128/jb.95.4.1305-1313.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sypherd P. S., Strauss N. CHLORAMPHENICOL-PROMOTED REPRESSION OF beta-GALACTOSIDASE SYNTHESIS IN ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1963 Mar;49(3):400–407. doi: 10.1073/pnas.49.3.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TANIGUCHI S., ITAGAKI E. Nitrate reductase of nitrate respiration type from E. coli. I. Solubilization and purification from the particulate system with molecular characterization as a metalloprotein. Biochim Biophys Acta. 1960 Nov 4;44:263–279. doi: 10.1016/0006-3002(60)91562-6. [DOI] [PubMed] [Google Scholar]
- Venables W. A., Wimpenny J. W., Cole J. A. Enzymic properties of a mutant of Escherichia coli K12 lacking nitrate reductase. Arch Mikrobiol. 1968;63(2):117–121. doi: 10.1007/BF00412166. [DOI] [PubMed] [Google Scholar]
- Wimpenny J. W., Cole J. A. The regulation of metabolism in facultative bacteria. 3. The effect of nitrate. Biochim Biophys Acta. 1967 Oct 9;148(1):233–242. doi: 10.1016/0304-4165(67)90298-x. [DOI] [PubMed] [Google Scholar]


